ABSTRACT
Essential oils are distilled liquids generally extracted from the stems, leaves, flowers, or other parts of a plant. In this study, antifungal activity against Penicillium digitatum of black caraway (Carum carvi) and anise (Pimpinella anisum) essential oils was investigated at different concentrations (0, 200, 400, 600,and 800 μL.L−1) on blood orange (Citrus sinensis var. Moro) in vivo and in vitro. The in vitro experiment results showed that two used the essential oils had strongly fungicidal effects, so that, the growth of P. digitatum was completely inhibited by the application of black caraway and anise oils. Anise and black caraway oils completely inhibited P. digitatum growth at the concentrations of 600 μL.L−1. In vivo experiment resulted in the complete inhibition of P. digitatum growth by anise and black caraway essential oils on blood orange fruits at any applied concentrations compared to the controls. In addition, at higher concentrations of 600 μL.L−1 showed the positive effects of the two oils on fruit quality features, including total soluble solids, antioxidant and anthocyanin contents, titrable acidity, and pH and weight loss percentages. Therefore, the infections of blood orange fruits were inhibited by P. digitatum oils, and their storage life was increased as well. The antifungal effects of the essential oils applied both on the fruit postharvest in vivo and in vitro were corroborated by the results obtained in this research. Thus, application of these plant essential oils could be used as biodegradable and eco-friendly agent for disease management of green mold during blood orange fruit production.
Introduction
Fruits and vegetables are the most important sources for the healthy nutrition. Postharvest decay may happen during the different phases of the postharvest chain—during harvesting, packing processes, transport, and storing—which could result in significant economic loss (Prusky, Citation2011; Sivakumar and Bautista-Banos, Citation2014).
Table 1. Composition of black caraway and anise essential oils from Iran.
The incidence of postharvest diseases in a variety of fruits and vegetables can be well reduced via chemical controls. However, chemicals are somewhat problematic due to leaving risk fultoxic residues in the products and causing resistance of the adapted fungi (Sholberg and Conway, Citation2004). As an alternative, disease can be currently controlled by essential oils from plants for providing a rich source of bioactive chemicals (Isman, Citation2000). Antifungal effects of plant essential oils to control food spoilage fungi in vitro and in vivo were studied in apple (Amiri et al., Citation2008), mango (Dubey et al., Citation2008; Regnier et al., Citation2008), citrus (Du Plooy et al., Citation2009), tomato (Omidbeygi et al., Citation2007), Maluspumilo (Shahi et al., Citation2003), avocado (Sellamuthu et al., Citation2013), and plum (Aminifard and Mohammadi, Citation2013).
Citrus fruits are considered as the major tree crops in the world, with a total yield of more than 121 tons in 2014/2015 (FAO, Citation2015). Green mold disease caused by Penicillium digitatum fungus is a major disease for citrus fruits and postharvest losses caused by the development of green mold during the storage and distribution of harvested fruits are very high. Therefore, this work aimed at studying the effects of anise (Pimpinella anisum) and black caraway (Carum carvi) essential oils on the decay control as well as postharvest life and fruit quality of blood orange (Citrus sinensis var. Moro) during storage.
Materials and method
Plant materials and extraction of essential oils
The air-dried seeds of black caraway and anise were supplied from agricultural research fields of Ferdowsi University of Mashhad, Iran. After the plant seeds were authenticated, then 100 g of these medicinal plants was subjected to hydrodistillation for 3 h using a Clevenger-type apparatus. The oil was dried over anhydrous Na2SO4 and preserved in a sealed vial at 4°C for future analysis. Infected blood orange fruits were selected and collected from storage to isolate highly virulent P. digitatum. Isolate identity was confirmed using morphological and molecular criteria (Tiwari et al., Citation2011). The culture was maintained on potato dextrose agar (PDA) at 4°C.
Gas chromatography (GC)
The essential oils were analyzed by using a Shimadzu GC-9, a gas chromatograph equipped with a DB-5 fused silica column (J and W Scientific Corporation, Folsom, California, USA) (30 m × 0.25 mm i.d., film thickness 0.25 µm). Helium was used as the carrier gas with a linear velocity of 32 cm s−1, and then the percentages of compounds were calculated by the area normalization method without considering response factors.
Gas chromatography–mass spectroscopy (GC-MS)
GC–MS analyses were carried out in a Varian 3400 GC–MS system equipped with a DB-5 fused silica column (30 m × 0.25 mm i.d., film thickness 0.25 µm); oven temperature was 50–240°C at a rate of 4°C/min, transfer line temperature 260°C, carrier gas, helium, with a linear velocity of 31.5 cm/s, split ratio 1:60, ionization energy 70 eV, scan time 1 s, and finally mass range was 40–300 amu.
Component identification
The components of the oils were identified by comparison of their mass spectra with those of a computer library or with authentic compounds and confirmed by comparison of their retention indices, either with those of authentic compounds or with data published in the literature. The retention indices were calculated for all volatile constituents using a homologous series of alkanes (Davies, Citation1990).
Essential oil composition
The components identified in each essential oil are listed in . These were mainly terpenes and monoterpenes. The black caraway oil was found to contain mainly cumin aldehyde (50%), followed by peril aldehyde (21.8%). Anise oil contained trans-anethole as a major component (90.7%) followed by estragole (4.3%).
The first experiment: (in vitro experiment)
Antifungal effects of essential oils on mycelial radial growth in vitro conditions
Antifungal activity was studied by using a contact assay (in vitro), which produced hyphal growth inhibition. The method was used for essential oil treatment on PDA medium “solution method” (SM) (Ozden and Bayindirli, Citation2002). In this method, the essential oils were dissolved in Tween 80–water solution (5% v v−1) and the required amounts of the solutions/petri dish were added to each of the PDA plates containing 20 ml of agar at 45°C. Then, 0.5 mm disk of mycelium was located on PDA medium. The treated medium was incubated in 24°C and mycelium growth was determined daily. Inhibitory percentage was determined according to the following formula. IP = (dc–dt) ×100/dc, where IP = Inhibitory percent, dc = mycelium growth diameter in control, and dt = mycelium growth diameter in essential oil-treated Petri dish.
Spore germination assay
The effects of the essential oils on spore germination were tested in PDA. The used oils were added to a 10 ml glass tube containing 5 ml PDA to obtain the final concentrations (0, 200, 400, 600, and 800 μl L−1). A spore suspension (104 spores mL−1) of P. digitatum was prepared from an actively growing culture (8–9 days old) in distilled sterile water. At the same time, aliquots (1 ml) of spore suspensions of P. digitatum were added to each tube. In addition, after 5 days of incubation at 28 ◦C, a thin layer of mycelium was aseptically removed, placed in a drop of lacto phenol-cotton blue (0.1%, w/v) on a microscope glass slide, stained for 2 h, and then it was observed under a microscope (Olympus, Tokyo, Japan). For each treatment, five replicate plates were used (Xu et al., Citation2007).
The second experiment (in vivo experiment)
Effect of the essential oils on postharvest decay and quality factors of P. digitatum inoculation on orange fruits
The infected orange fruits were selected and collected from the storage to isolate P. digitatum. The culture was maintained on PDA at 4°C, and fresh cultures were grown on PDA plates before their use. Spore suspensions were prepared by removing the spores from the sporulation edges of a 8- to 9-day-old culture with a bacteriological loop, and suspending them in sterile distilled water. Spore concentration was determined with a hemocytometer, and adjusted as required with sterile distilled water (104 spores ml−1). Before infection, fruits were treated by sodium hypochlorite (100 μl L−1), then they were sprayed in prepared suspension and stored under room temperature for 2 h in order to fix fungal inoculation (Asghari Marjanlo et al., Citation2009). In this phase, SM was used as in vitro experiment. Fruits were treated by different required concentrations of black caraway and anise essential oils; then they were stored in separate packages. Treated and untreated (control) fruits were stocked in order to prevent loss of essential oils and moved into the cold storage (6°C) for 60 days.
Life storage fruit (decay rate)
The infection degrees of the fruits (decay score) were rated by using a 0–8 scale, each number of which stood for 0%, 0–10%, 10–20%, 20–30%, 30–40%, 40–50%, 50–65%, 65–80%, and 80–100% of infection, respectively.
pH, titrable acidity (TA), total soluble solids (TSSs)
The pH of fruit juices was measured at 20°C using a pH meter (Jenway 3320,Bibby Scientific, Staffordshire, UK). Titratable acidity (TA) was determined by titration with 0.1 N NaOH until pH reached 8.1 and was reported g 100 g–1 of malic acid fresh weight. Total soluble solids (TSSs) were determined at 20oC with a refractometer (model RFM340, Bellingham and Stanley, UK) and reported as oBrix (AOAC, Citation2000).
Weight loss percentage
In order to determine any weight loss during fruit storage, both treated and untreated fruits were weighed at the beginning and end of the storage period.
Total anthocyanin, antioxidant activity, ascorbic acid
Total anthocyanin contents were determined by the pH-differential method (Rapisarda et al., Citation2006). The antioxidant activity was determined by1,1-diphenyl-2-picrylhydrazyl free radical-scavenging activity (Brand-Williams et al., Citation1995). Ascorbic acid contents were measured by classical titration method using 2,6-dichlorophenol indophenol solution and expressed as mg per 100 g (AOAC, Citation2000).
Statistical analysis
Experiment was conducted in a completely randomized factorial design with four replications; each replication consisted of five fruits. Data were analyzed by using SASS 9.1. statistical software and means were compared with Duncan’s multiple range test at 5% level of confidence.
Results
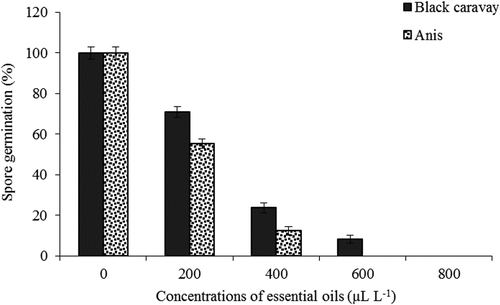
The effects of the different concentrations of the essential oils on the radial growth of P. digitatumare illustrated in . The two essential oils were found to inhibit P. digitatum growth in a dose-dependent manner. The highest radial growth was discovered in the control (without applying the essential oil), whereas the lowest radial growth was obtained with 400 μL.L−1anise and 600 μL.L−1 black caraway oils, respectively. Spore germination of P. digitatum was inhibited by black caraway and anise oils at all concentrations (). The two essential oils at 800 μL.L−1inhibited spore germination completely.
Figure 1. Effect of different concentrations of black caraway and anise essential oils on radial growth of P. digitatum.
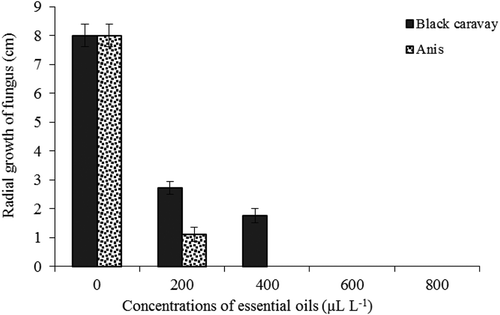
Figure 2. Effect of different concentrations of black caraway and anise essential oils on spore germination percent of P. digitatum.
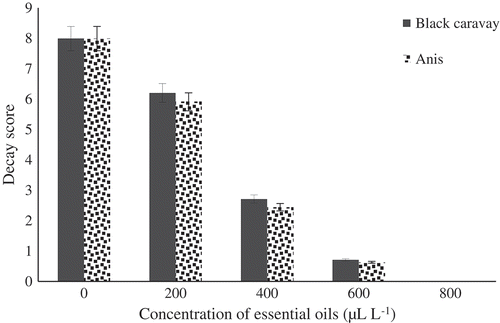
Better maintenance was achieved for the essential oil-treated fruits with lower decay scores than the control fruits, which showed maximum deterioration (). Fruits treated with two used oils had no significant difference together. The lowest decay score was related to the fruits treated at 800 μL.L−1.
Figure 3. Effect of different concentrations of black caraway and anise essential oils on decay score of blood orange fruit cv. Moro during storage.
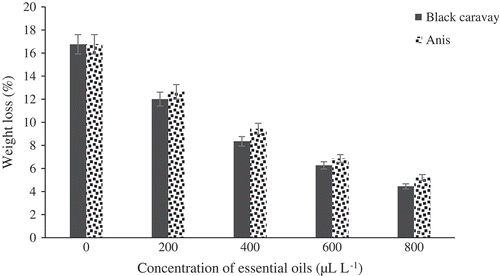
The effect of the five concentrations of the essential oils on TSS is portrayed in . Significant differences in TSS were observed between treated and control fruits. Fruits treated with black caraway and anise oils were not significantly different together. The fruits treated at 800 μL.L−1were found to have the highest TSS (12.5°Brix). There were significant differences in TA between treated and control fruits (). The best concentration of oil was 800 μL.L−1with TA content of 1.55 (g per 100 g of fresh weight). There were significant differences in pH between treated and control fruits (). Fruits treated at 800 μL.L−1 had the lowest pH (3.47). The weight loss percentage of the essential oil-treated fruits was considerably lower than that of the control fruits. Fruits treated at 800 μL.L−1of oils showed the lowest weight loss percentage (5.2%), while control fruits showed the highest weight loss percentages ().
Figure 4. Effect of different concentrations of black caraway and anise essential oils on TSS (°Brix) of blood orange fruit cv. Moro during storage.
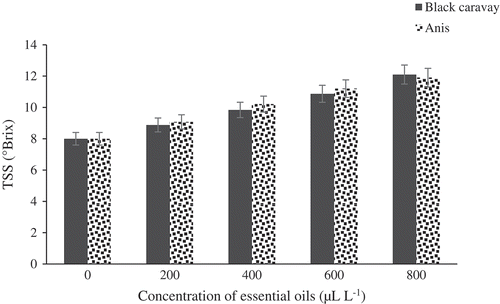
Figure 5. Effect of different concentrations of black caraway and anise essential oils on titrable acidity of blood orange fruit cv. Moro during storage.
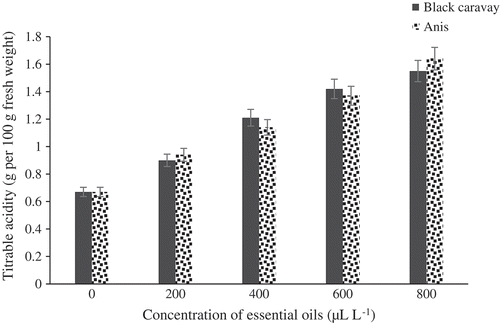
Figure 6. Effect of different concentrations of black caraway and anise essential oils on pH of blood orange fruit cv. Moro during storage.
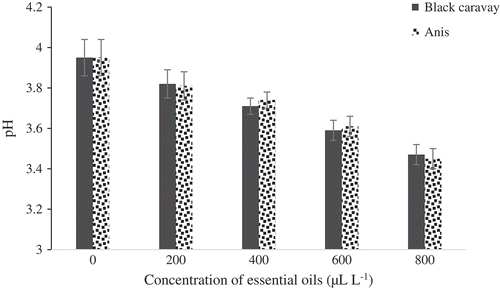
Figure 7. Effect of different concentrations of black caraway and anise essential oils on weight loss of blood orange fruit cv. Moro during storage.
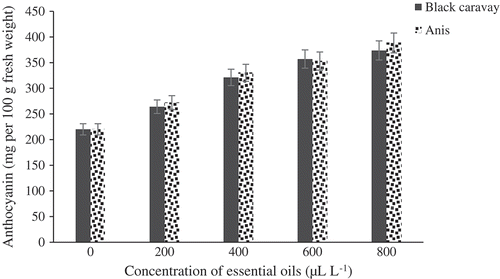
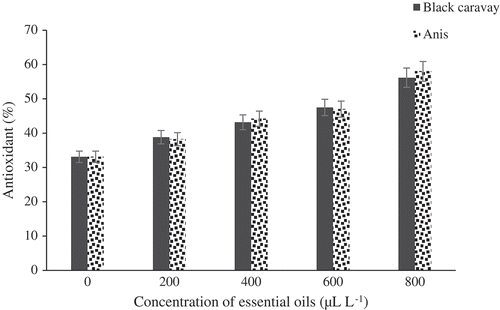
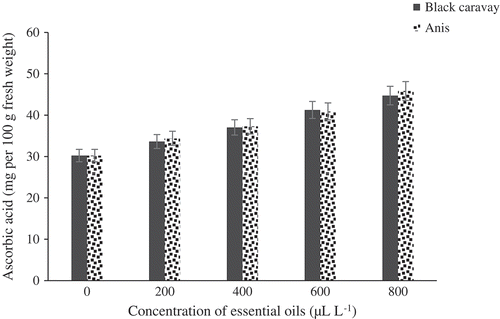
There was a significant difference between the treatments and the control based on ascorbic acid content (). The result revealed that the two oils used at 800 μL.L−1 had the highest ascorbic acid content compared to the control with 44.73 mg per 100 g of fresh weight. The anthocyanin contents of the fruits significantly differed among the concentration treatments (). The highest and lowest anthocyanin levels were found at 800 μL.L−1 of the two oil-treated fruits (373.67 mg per 100 g of fresh weight) and the control fruits (220 mg per 100 g of fresh weight), respectively. There was a significant difference between the varied treatments and the control based on antioxidant content (). The result showed that two used oils at 800 μL.L−1 were the highest content in antioxidant content than control with 56.17 mg per 100 g fresh weight.
Figure 8. Effect of different concentrations of black caraway and anise essential oils on ascorbic acid content of blood orange fruit cv. Moro during storage.
Discussion
Essential oils as natural antimicrobial agents can be applied in the food industry only when their releasing compounds over time and effects on the target plants and pathogens are well known and understood (Isman, Citation2000). In this research, the in vitro data were indicative of the fungicidal effects of the studied essential oils, especially anise oil at higher concentrations. Similarly, the growths of B. cinerea, Fusarium sp., and Clavibac termichiganensis subsp. michiganensis were completely inhibited by the essential oils extracted from dictamnus, marjoram, oregano, and thyme (Dimitra et al., Citation2003). Additionally, the mycelial growth of B. cinerea was reported by Bouchra et al. (Citation2003) to be inhibited by Thymus glandulosus and Origanum compactum essential oils. In the present study, essential oils were also seen to inhibit spore germination and germ tube elongation. Fung et al. (Citation1997) believed that the positive effects of essential oils on microbial growth might be caused by their phenolic compounds altering the permeability of microbial cells when interacting with membrane proteins. This in turn would lead to the deformation of cellular structure and function and subsequent loss of macromolecules from their interior (Pramila et al., Citation2012).
The present results demonstrated that the studied essential oils had positive effects on storage life, while reducing blood orange fruit decay and providing the longest storage life with a treatment of 800 μL.L−1 of black caraway and anise oils. The previous reports were indicative of reduced fruit decays by postharvest treatments of various products like kiwifruit and raspberry with volatile compounds (Wang, Citation2003; Williamson et al., Citation2007). Essential oils mainly conjugate to phenolic compounds, which accumulate in some plant cells, and have shown useful effects on these compounds for pathogen control (Plotto et al., Citation2003). It is well known that fungal growth and believingly apple scab fungus Venturiaina equalis are inhibited by phlorsidzinoxidation products having an o-dihidroxy phenol compound (Asghari Marjanlo et al., Citation2009). Fungal pectin as ehydrolyse pectin is an abundant cell-wall compound in the middle lamella, which has a role in cell adhesion. Hence, fungal ability to hydrolyze and invade plant cell walls are compromised by inhibiting pectinases (Vermeriss and Nicholson, Citation2006). Seemingly, phenolic compounds of essential oils play a similar role. Consequently, the probable positive effects of exogenous essential oils on the storage life of blood orange fruits and their decreased decays are revealed by these findings. The present research demonstrated the effectiveness of the studied essential oils on fruit quality maintenance. The fruits treated with black caraway and anise essential oils had higher TSS, TA, ascorbic acid, antioxidant, and anthocyanin contents than the control fruits, and their best treatments with the oils at 800 μL.L−1 were 12.5°Brix, 1.55 g per 100 g, 44.73 mg per 100 g of fresh weight, 56.17%, and 373.67 mg per 100 g of fresh weight for the mentioned contents, respectively. Our results were in line with those obtained by Asghari Marjanlo et al. (Citation2009), who reported that cumin oil enhanced strawberry infection with B. cinerea. The present results were indicative of the significant reduction of weight loss percentage by essential oil application and the fruits treated with anise and black caraway oils at 800 μL.L−1 showed the lowest weight loss percentage (5.2%). The benefits of decreased weight loss percentages of cherry and grape and plum by using natural antifungal compounds like thymol, eugenol, and menthol vapors were revealed in the previous experiments (Aminifard and Mohammadi, Citation2013; Rattanapitigorn et al., Citation2006; Serrano et al., Citation2005).
With regard to reduce dmycelial growth and germination of P. digitatum in vitro and decreased incidence of disease symptoms on essential oil-treated blood orange fruits and the increased storage life, it is concluded that anise and black caraway essential oils as bio-fungicides can be used against phytopathogenic fungi on blood orange fruits as an alternative to synthetic fungicides.
References
- Aminifard, M.H., and S. Mohammadi. 2013. Essential oils to control Botrytis cinerea in vitro and in vivo on plum fruits. J. Sci. Food Agric. 93:348–353.
- Amiri, A., R. Dugas, A.L. Pichot, and G. Bompeix. 2008. In vitro and in vivo activity of eugenol oil (Eugenia caryophylata) against four important postharvest apple pathogens. Int. J. Food Microbiol. 126:13–19.
- AOAC. 2000. Official methods of analysis. Association of Official Analytical Chemists, Washington, DC. p. 122–124.
- Asghari Marjanlo, A., Y. Mostofi, S. Shoeibi, and M. Fattahi. 2009. Effect of cumin essential oil on postharvest decay and some quality factors of strawberry. J. Med. Plants. 8:25–43.
- Bouchra, C., M. Achouri, L.I. Hassani, and M. Hmamouchi. 2003. Chemical composition and antifungal activity of essential oils of seven Moroccan labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 89(1):165–169.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Antioxidative activity of phenolic composition of commercial extracts of sage and rosemary. Lwt. 28:25–30.
- Davies, N.W. 1990. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and carbowax 20 M phases. Chromatogr. 503:1–24.
- Dimitra, J., N. Daferera, M. Ziogas, and G. Polissiou. 2003. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibactermi chiganensis subsp michiganensis. Crop Protect. 22:39–44.
- Du Plooy, W., T. Regnier, and S. Combrinck. 2009. Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol Technol. 53:117–122.
- Dubey, R.K., R. Kumar, J.P.N. Jaya Chansouria, and N.K. Dubey. 2008. Evaluation of Amomum subulatum Roxb oil as a source of botanical fungitoxicant for the protection of mango fruits from fungal rotting. J. Food Saf. 28:400–412.
- FAO. 2015. Report, pesticide residues in food, FAO plant production and protection paper 145, food and agriculture organization of the United Nations, Rome. http://www.fao.org/faostat/
- Fung, DYC., Taylor, S., and Kahan, J. 1997. Effects of butylated hydroxyanisole (BHA) and butylated hydroxitoluene (BHT) on growth and aflatoxin production of Aspergillus flavus. J. Food Saf. 1:39–51.
- Isman, B.M. 2000. Plant essential oils for pest and disease management. Crop. Protect. 19:603–608.
- Omidbeygi, M., M. Barzegar, Z. Hamidi, and H. Naghdibadi. 2007. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 18:1518–1523.
- Ozden, C., and L. Bayindirli. 2002. Effects of combinational use of controlled atmosphere, cold storage and edible coating applications on shelf life and quality attributes of green peppers. Eur. Food. Res. Technol. 21:320–326.
- Plotto, A., R.G. Roberts, and D.D. Roberts. 2003. Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). Acta Hort. 628:737–745.
- Pramila, D.M., R. Xavier, K. Marimuthu, S. Kathiresan, M.L. Khoo, M. Senthilkumar, K. Sathya, and S. Sreeramanan. 2012. Phytochemical analysis and antimicrobial potential of methanolic leaf extract of peppermint (Menthapiperita: lamiaceae). J. Med. Plants. Res. 6(2):331–335.
- Prusky, D. 2011. Reduction of the incidence of postharvest quality losses, and future. Prospects Food Secur. 3:463–474.
- Rattanapitigorn, P., M. Arakawa, and M. Tsuro. 2006. Vanillin enhances the antifungal effect of plant essential oils against Botrytis cinerea. Int. J. Aromather. 16:193–198.
- Rapisarda, P., Fanella, F., and Maccarone, E. 2006. Reliability of analytical methods for determining anthocyanin in blood orange juices. J. Agric Food Chem. 48:2249–2252.
- Regnier, T., W. Du Plooy, S. Combrinck, and B. Botha. 2008. Fungi toxicity of Lippia scaberrima essential oil and selected terpenoid components on two mango postharvest spoilage pathogens. Postharvest Biol. Technol. 48:254–258.
- Sellamuthu, P.S., D. Sivakumar, P. Soundy, and L. Korsten. 2013. Enhancing the defence related and antioxidant enzymes activities in avocado cultivars with essential oil vapours. Postharvest Biol. Technol. 81:66–72.
- Serrano, M., D. Martinez-Romero, S. Castillo, F. Guillen, and D. Valero. 2005. The use of the natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innovat. Food Sci. Emerg. Technol. 6:115–121.
- Shahi, S.K., M. Patra, A.C. Shukla, and A. Dikshit. 2003. Use of essential oil as botanical pesticide against postharvest spoilage in Malus pumilo fruits. Biocontrol. 48:223–232.
- Sholberg, P.L., and W.S. Conway. 2004. Postharvest pathology, p. 9. In: Gross K.C., Wang C.Y., and Saltveit M. (eds). Agricultural Handbook Number 66: the commercial storage of fruits, vegetables, and florist and nursery stocks. United States Department of Agriculture, Maryland, (USA).
- Sivakumar, D., and S. Bautista-Banos. 2014. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 64:27–37.
- Tiwari, K.L., S.K. Jadhav, and A. Kumar. 2011. Morphological and molecular study of different Penicillium species. Middle East J. Sci. Res. 7(2):203–210.
- Vermeriss, W., and R. Nicholson. 2006. Phenolic compound biochemistry. Springer, New York, NY. p. 273–274.
- Wang, C.Y. 2003. Maintaining postharvest quality of raspberries with natural volatile compounds. Postharvest Biol. Technol. 28:181–186.
- Williamson, B., B. Tudzynski, P. Tudzynski, and J. Van. 2007. Botrytis cinerea: the cause of gray mould disease. Mol. Plant Pathol. 8:561–570.
- Xu, W.T., K.L. Huang, F. Guo, W. Qu, J.J. Yang, Z.H. Liang, and Y.B. Luo. 2007. Postharvest grapefruit seed extract and chitosan treatments of table grapes to control Botrytis cinerea. Postharvest Biol. Technol. 46(1):86–94.