ABSTRACT
Grafting type and covering method of the graft union have significant effects on grafting success. Two experiments were conducted to determine the best method of grafting and the covering of graft union in walnuts during 2014 and 2015. In the first experiment, plants were grafted under a shade-house with 50% reduction of sunlight. Two methods of saddle and tongue grafting, and three covering methods of graft union including moist sawdust, moist mixed coverage (cocopeat + soil + manure) and plastic cover (control) were used. In the second experiment, two walnut cultivars, ‘Chandler’ and ‘Franquette’ were used as scions along with four grafting methods, including saddle, tongue, omega and V under outdoor conditions. In this experiment, graft unions were covered with moist sawdust. The results of the first experiment showed that, 4 weeks after grafting, the rate of graft-take was better in saddle grafting compared to tongue grafting (76.8 and 62%, respectively). Covering by moist sawdust was better than the other two covers. In the second experiment, the omega method caused the best quality of callus, the highest callused grafts (89.1%) and the most successful graft-take (81.6%). Meanwhile, V grafting caused the highest survival percentage (65.8%) and growth of scions (107.9 cm) after 6 months. Therefore, we recommend this method for grafting walnuts under outdoor conditions.
Introduction
One of the conventional methods for propagation is grafting. Grafting in walnut is still more difficult than in other fruit and nut trees, and it requires more attention in terms of the method, date and selection/handling of the scion and rootstock, particularly when we use Persian walnut as the rootstock (Vahdati, Citation2003; Rezaee and Vahdati, Citation2008). The results of investigations that have been conducted so far on walnut grafting can distinctly indicate several main factors that are potentially capable of causing a high percentage of success in grafting. These factors are the temperature (26°C) and relative humidity (80–95%) which remain effective for 3 to 4 weeks after grafting (Hartmann et al., Citation2014).
The acquired method of grafting has an undeniable effect on the graft success in walnuts. Various methods and different scion cultivars in walnuts have been reported and indicate different degrees of success under different climatic conditions (Avanzato and Atefi, Citation1997; Chandel et al., Citation2006; Dehghan et al., Citation2009). However, the effect of scion cultivar on graft success has mainly been negligible (Ozkan et al., Citation2001; Dehghan et al., Citation2009). On the other hand, maintaining sufficient humidity around the graft union at the wound site can play an important role in callus formation or the healing process in the graft site, and it can therefore improve graft survival (Dehghan et al., Citation2009). This could be attributed to the fact that covering the graft site with different materials can maintain different degrees of moisture for a successful reunion of the scion and rootstock. Air moisture levels below the saturation point have reportedly inhibited callus formation and the rate of desiccation of cells increased with the decrease in humidity percentage in the graft site (Wani, Citation2004).
The literature in this regard comprises various cases of research that have considered covering the graft union with different materials such as moist sawdust (Rezaee and Vahdati, Citation2008; Rezaee et al., Citation2008), paraffin wax and polyethylene film (Khalil et al., Citation2009), sand (Gandev, Citation2007), plant wax (Rongting and Pinghai, Citation1990), organic matter and peat moss (Vahdati, Citation2003), vermiculite, perlite (Dehghan et al., Citation2009) and black or white paints in cold and warm regions (Ramos, Citation1985; Vahdati, Citation2003). However, the majority of research on bench-grafting, hot callusing and heating techniques has been conducted under controlled conditions in bed (Atefi, Citation1997; Dehghan et al., Citation2010; Gandev, Citation2007; Paunovic et al., Citation2011). Meanwhile, the diverse effects of various methods of grafting and the use of various scion cultivars have been reported. However, these experiments need to be optimized in order to reach a level of commercialization in different regions and conditions.
Grafting walnuts under indoor and outdoor conditions has been studied in several countries. Ebrahimi et al (Citation2006), Vahdati (Citation2003) and Gautam (Citation1990) reported improved rates of budding/grafting success when the grafting was performed under controlled conditions, as compared to field (outdoor) conditions. Meanwhile, it is noteworthy to mention that the establishment of a greenhouse can be expensive, and walnut grafting is less successful in the field. Such problems prompted the design of the current research to create a convenient and inexpensive method that can cause a higher percentage of grafting success. Another objective is to particularize the desirable method for large-scale exploitation under field conditions. Using the right combination of grafting method and covering materials of the graft union can guarantee the grafting success. In general, the main objective of our research is to compare and optimize all of the success-related components in one package, i.e., a grafting method that can conform best in combination to a covering material, when grafting walnuts under indoor and outdoor conditions. Even though previous studies have used various grafting methods and covering materials, they have not used both factors as reciprocal combinations in one experimental setup. This research employs combinations of covering materials and grafting methods to reach an optimal trade-off between both factors.
Materials and methods
Two experiments were carried out. The first experiment was performed at the Department of Horticulture, College of Aburaihan, University of Tehran, Pakdasht, Tehran, under a shade house. The second experiment was performed in Alashtar, Lorestan, located in the west of Iran, and the grafting took place outdoors.
In the first experiment, scions of the ‘Chandler’ were grafted (using tongue and saddle grafting methods) with different covering materials of graft union including plastic cover, moist mixed coverage (cocopeat + soil + manure in a 1:1:1 ratio) and moist sawdust (). The grafting was performed on two-year-old half-sib seedling rootstocks under the shade house. Sawdust and mixed covers were also sterilized with 0.3 and 0.4% Captan®, respectively. In this treatment, the shade-house tunnels reduced 50% of the sunlight. A mist system was employed to spray water, from above, on the grafted seedlings every half an hour for 30 s from 9 am to 7 pm. One nozzle per meter was installed with a vertical distance of 2.5 meters from the plants. The soil texture was silty loam, and had a pH value of 7.3.
Figure 1. Covering of graft union with polyethylene film (A), moist mixed covering (B), moist sawdust (C) and 4 weeks after Covering with moist sawdust (D).

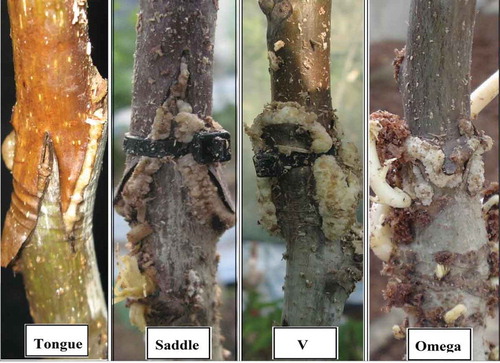
In the second experiment, two cultivars of ‘Chandler’ and ‘Franquette’ were used as scions along with four methods of saddle, tongue, omega and V on two-year-old seedling rootstocks. The graft union in this experiment was wrapped by the plastic cover filled with moist sawdust (). Seedling rootstocks were produced by planting seeds in a nursery with a soil of sandy loam texture and a pH value of 6.8.
Scions
The scions of ‘Chandler’ and ‘Franquette’ were taken from East Azarbaijan in North-West of Iran on 1 February. Only two scion cultivars were used because the literature suggests that the effect of scion cultivar on graft success is mainly negligible (Ozkan et al., Citation2001; Dehghan et al., Citation2009). Mother stock trees were severely pruned to stimulate vigorous one-year-old shoots for all of the experiments. Scions (dormant shoots) were cut to 30–40 cm in length. Then they were wrapped in a damp paper, placed in a plastic cover bag and stored in a refrigerator at 2 ± 2°C until they were used for the multiple grafting methods. Before storage and grafting, the scions were soaked in 0.3% concentration of Captan® fungicide for disinfection.
Grafting methods
Saddle and V grafting were performed by a machine (Carlo A. Manares Co., Italy). We used this machine to cut rootstocks and scions in two different ways. In the first way, we made saddle grafting in which the scion became V shaped and in the second way we made V grafting in which the rootstock became V shaped. Tongue grafting was done with hand using a sharp knife, and omega grafting was done using an omega hand-held machine (Zhangjiagang Xingxing Tools Co., China). All of the grafting methods were conducted in the first half of April 2014, and they were repeated in 2015.
In the first experiment, graft union was covered with three different methods including plastic cover, moist mixed coverage (coco-peat + soil + manure) and moist sawdust. In the second experiment, we only used moist sawdust because it yielded satisfactory results in our preliminary evaluations (Data not shown).
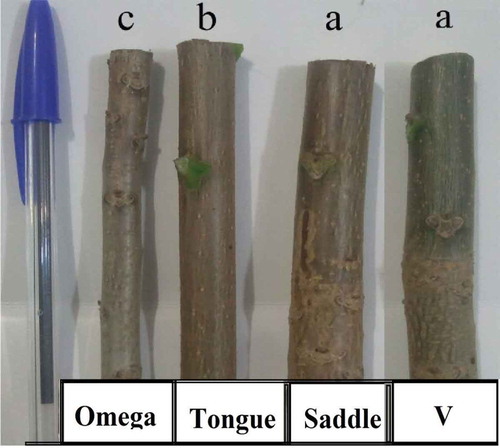
After removing the covers (4 weeks after grafting), data were recorded for callus quality (based on a visual scale of 1 to 4 in which 1 = low, 2 = medium, 3 = high and 4 = very high callusing (). Callused grafts (%) and graft-take were assessed 28 days after grafting based on the percentage of the scions which retained their green color and plumpness, and their buds did not desiccate. Subsequently, the percentage of graft survival and scion growth () were measured 6 months after the grafting date for each treatment at the end of the season (October). The measurements were performed according to the methodology described by Rezaee and Vahdati (Citation2008), Dehghan et al. (Citation2009) and Dehghan et al. (Citation2010).
Data analysis
First experiment
The experiments were performed as factorial, based on completely randomized design (CRD) with three replications, and six grafts were considered as a replication.
Second experiment
This experiment was performed as factorial, based on a CRD with three replications, and 10 grafts per replication.
Since both experiments were carried out during a 2-year period in 2014 and 2015, the average values of data pertaining to both years are reported in the results section.
Data were analyzed using SAS software (Version 1.6) and means were compared using Duncan’s multiple range tests (DMRT) at P ≤ 0.05.
Results and discussion
First experiment
Saddle grafting resulted in the highest value for all of the measured traits (callus quality, callused grafts, graft take, survival and scion growth). The lowest values of the traits were obtained in tongue grafting. The highest percentage of survival among plants was obtained in saddle grafting (63.8%) followed by tongue grafting (55.5%) (). These results are in agreement with Ozkan and Gumus (Citation2001), Ozkan et al. (Citation2001), Vahdati and Zarei (Citation2006) and Dehghan et al. (Citation2009) who reported that the method of grafting is very effective in grafting success. Covering the graft union with sawdust resulted in the highest values of all traits (callus quality, callused grafts, graft take, survival and scion growth) and the lowest success was obtained by polyethylene films (). In saddle and tongue grafting, wrapping the graft site with moist sawdust resulted in the highest values of all the measured traits ().
Table 1. Effect of methods of grafting on callus quality, callused grafts (%), graft-take (%), graft survival (%), and scion growth (cm) of Persian walnut.. Also all values are the means of the two years data (2014 and 2015).
Table 2. Effects of different covering materials for banding graft union on callus quality, callused grafts, graft-take (%), graft survival (%), and scion growth of Persian walnut. Also all values are the means of the two years data (2014 and 2015).
Table 3. Interaction between year, different methods of grafting and different covering materials on callus quality, callused grafts, graft-take (%), graft survival (%), and scion growth (cm) of Persian walnut.
Adequate aeration and care of the graft union can play an important role in callus formation and the success of grafting (Hartmann et al., Citation2014; Rezaee and Vahdati, Citation2008; Rongting and Pinghai, Citation1990). However, in covering the graft union with polyethylene film, many scions failed to continue growth and did not survive by the end of the growing season. This failure may be a result of low callus quality until 4 weeks after covering () in the grafted walnuts (Dehghan et al., Citation2009; Vahdati, Citation2003).
Second experiment
The analysis of variance of the four grafting methods in 2014 and 2015 for all measured traits showed significant results (), but for the two scion cultivars the results were non-significant in the second experiment, except for callus quality (). In this experiment, omega grafting resulted in the highest quality of callus (i.e., average rating of 2.7 out of a maximum of 4.0). The tongue grafting had the lowest quality of callus (with 2.0 out of a minimum of 4.0). Furthermore, the highest and the lowest rate of scion growth were achieved by V and omega methods, respectively, which were 104.4 cm and 80 cm (). In this study, the V method (with 65.8% success rate) was the best method, compared to omega and tongue methods (which had a success rate of 49.1%) ().
Table 4. Effect of different methods of grafting on callus quality, callused grafts, graft-take (%), graft survival (%), and scion growth (cm) of Persian walnut Also all values are the means of the two years data (2014 and 2015).
We obtained different data (callus quality, percentage of callused grafts, graft-take, graft survival and scion growth), under different grafting methods, which is in agreement with reports by other researchers (Dehghan et al., Citation2009; Ierahim et al., Citation1978; Soleimani et al., Citation2010). The main advantage of the present study is to provide a grafting success rate in the open air and a vast area, while incurring the minimum cost compared to the greenhouse. The differences observed in the scion growth were likely due to the grafting methods and covering materials, since half-sib seedlings were used as rootstocks in this experiment, and when the experimental population is large, minor differences between various degrees of rootstock vigor become negligible.
A higher grafting success was obtained using Carlo A. Manares Machine Co. (Italy) (which assisted the saddle and V grafting) compared to the tongue graft (which was performed by hand) and the omega graft performed by Omega-Hand Machines (Zhangjiagang Xingxing Tools Co. China). The higher success could be related to more cambial contact in the saddle and V grafting. Again, since the experimental population, including the number of rootstocks, was sufficiently large in this experiment, it can be said that the differences in graft success are less related to rootstock vigor.
The V and saddle grafts were performed when seedling rootstocks were between 20 to 55 mm in diameter, while tongue graft was performed when seedlings were 20 to 40 mm and omega ≤ 20 mm in diameter. In this experiment, V and saddle grafting resulted in higher success rates, compared to other studied methods, because of using the Carlo A. Manares machine grafting for thick/large diameter rootstocks compared to the tongue graft that was done by hand. Soleimani et al. (Citation2009) and Rezaee et al. (Citation2008) also reported increased rates of grafting success in low versus high vigorous seedlings. Two-year old vigorous seedlings also yielded more success compared to one-year-old, low vigorous seedlings (Lantos, Citation1990; Solar et al., Citation2001; Vahdati and Zarei, Citation2006; Yildiz et al., Citation2005). This can be attributed to the rapid healing of the wound in two-year-old seedlings (Vahdati and Zarei, Citation2006). The percentage of graft-take in all of the grafting methods used in the preliminary trial was good (64.1–81.6%), indicating a healthy start of growth. However, many scions failed to continue growth and did not survive six months after grafting. This failure may be a result of warm and dry summers or an inadequate rootstock/scion connection that produces vessels. This failure may not be a result of high root pressure (sap bleeding) of walnut rootstocks, because sap bleeding is absorbed by sawdust in the graft union. The higher success of the V method is consistent with the findings of Solar et al. (Citation2001) and Dehghan et al. (Citation2009). Also, the higher success of the V method compared with the omega is consistent with the findings of Soleimani et al. (Citation2010), and may be a result of it being performed by machines, leading to a greater physical overlap between the cambial layers of the rootstock and the scion, a requirement for successful grafting in difficult-to-graft species (Hartmann et al., Citation2014; Vahdati, Citation2003).
The lowest level of graft survival was achieved by tongue and omega grafting. In this experiment, however, the omega grafting resulted in the highest quality of callus, callused grafts and graft-take versus other studied methods, but graft survival and scion growth were very low due to the disability of the omega-hand machine when grafting thick/large diameter rootstocks, compared to the other grafting methods ().
However, graft success rate is affected by machines used for grafting (Dehghan et al., Citation2010; Solar et al., Citation2001), grafting method (Avanzato and Atefi, Citation1997; Chandel et al., Citation2006; Dehghan et al., Citation2010), rootstock vigor (Rezaee et al, Citation2008) and scion genotype (Dehghan et al., Citation2010; Ozkan et al., Citation2001). Nonetheless, grafting is more successful by machines, compared to grafting by hand, when considering the efficiency of grafting thick/large rootstocks. The higher graft survivals of V and saddle methods, when grafting on thick/large rootstock, are in agreement with the findings of Lantos (Citation1990), Solar et al. (Citation2001), and may be a result of using big rootstocks (which contain adequate amounts of carbohydrates, water and nutrients) necessary for grafting success. Also, Vahdati and Zarei (Citation2006) showed that 2-year-old seedlings (i.e., vigorous), compared to 1-year-old seedlings (i.e., less vigorous), can produce more callus and can cause an earlier healing in the graft site. Comparisons of mean values of the two scion genotypes (‘Chandler’ and ‘Franquette’) for all of the traits were non-significant, except for callus quality ().
Table 5. Effects of genotypes scion on callus quality, callused grafts, graft-take (%), graft survival (%), and scion growth (cm) of Persian walnut Also all values are the means of the two years data (2014 and 2015).
The results also showed that scion cultivars have a weak effect on the studied factors (), and this is in agreement with previous results reported by Ozkan et al. (Citation2001), Dehghan et al. (Citation2009). In this regard, Ozkan et al. (Citation2001) reported that the effect of different scion genotypes on graft survival is 5%. Some articles have reported that the effects of scion on grafting success are high, which do not confirm the results of this research (Khalil et al., Citation2009; Rezaee et al., Citation2008; Ertürk, Citation2011). The interaction between year of grafting, grafting method and scion cultivar was significant for all combinations and measurements (). Also in this experiment, the highest and lowest graft-take (83.3 % vs. 60%) were observed in Omega grafting × ‘Franquette’ and in tongue × ‘Chandler’, respectively. The highest and lowest graft-survival (66.6% vs. 46.6%) was observed in V grafting × ‘Franquette’ and in Omega × ‘Chandler’, respectively (). According to the results of other researchers, graft-take percentage usually varies in different walnut selections (Ebrahimi et al., Citation2006), which is consistent with our results. Nonetheless, graft survival varied from 53.75 to 57.08% () in different scion cultivars. The different values attributed to grafting success could be explained by the physiological status of the mother stock (scion), rather than its genetic structure (Rezaee and Vahdati, Citation2008).
Table 6. Interaction of year, different method of grafting and scion cultivar (‘Chandler’ and ‘Franquette’) on callus quality, callused grafts, graft-take, graft survival, and scion growth (cm) of Persian walnut.
Conclusions
In the first experiment, we improved grafting success using economic, convenient and inexpensive methods in the field under a semi-arid climate using a shade-house tunnel. Covering by moist sawdust yielded more optimum results compared to the mixed coverage and the control group, in terms of callus quality, callused grafts, graft-take, survival rate and growth of the scion. In the second experiment, we used moist sawdust for covering the graft union under field conditions. In this experiment, the best quality of callus, the highest callused grafts (89.1%), and the highest success of graft-take (81.6%) were observed in omega grafting. Meanwhile, the V grafting had the highest survival percentage (65.8%) and growth of scions (107.9 cm) after 6 months. Therefore, we recommend the V method for grafting walnuts outdoors. Even though this study examined graft success and survival, the scion growth still deserves further experimental enquiry. Future studies can assess scion vigor by more detailed values, such as scion diameter, number of leaves, branches or sprouting and the branch length where the scion is situated.
Acknowledgments
Thanks to Mr Amir Rezaei for his assistance in data analyses.
Additional information
Funding
References
- Atefi, J. 1997. Comparison of hypocotyle and hot callus cable graft with traditional grafting method. Acta Hortic 442:309–313.
- Avanzato, D., and J. Atefi. 1997. Walnut grafting by heating the graft point directly in the field. Acta Hortic 442:291–294.
- Chandel, J.S., D.R. Guatam, and N.C. Sharma. 2006. Chip budding an excellent method of propagation of walnut (Juglans regia L.). Acta Hortic 705:335–340.
- Dehghan, B., K. Vahdati, D. Hassani, and R. Rezaee. 2010. Bench-grafting of Persian walnut as affected by pre-and post-grafting heating and chilling treatments. Hortic. Sci. Biotechnol 85(1):48–52.
- Dehghan, B.K., R. Vahdati., D. Rezaee., Hassani, and A. Papachatzis. 2009. Top-working of mature walnut trees as affected by different bleeding control methods and scion cultivars. Vol. XIV, (XLX).
- Ebrahimi, A.K., Vahdati, and E. Fallahi. 2006. Improved success of Persian walnut grafting under environmentally controlled conditions. Int. J. Fruit Sci 6:3–12.
- Ertürk, U. 2011. Effect of cultivar on successful grafting and development of nursery trees of potted walnut. In II Balkan Symposium on Fruit Growing, Pitesti, Romania, 981, 443–447. Abstract
- Gandev, S. 2007. Budding and grafting of the walnut (Juglans regia L.) and their effectiveness in Bulgaria (Review). Bulgarian J. Agric. Sci 13:683–689.
- Gautam, D.R. 1990. Studies on the winter and summer vegetative propagation techniques of walnut (Juglans regia L.). Acta Hortic 284:27–32.
- Hartmann, H.T., D.E. Kester, F.T. Davies, and R. Geneve. 2014. Plant propagation: principles and practices. Pearson Prentice Hall, New Jersey, Usa 8(14):453-456.
- Ierahim, M., C.M. Sadiq, and C.M. Idris. 1978. Experiment on comparative studies of different propagation techniques in English walnut (Juglans regia L.). Agric. Res 16(2):205–209. Pakistan
- Khalil, U.A., K. Navab., B. Amjad., Jehan, and A. Riaz. 2009. Response of various indigenous walnut genotypes to graft-take success. Sarhad J. Agric 253:399–403.
- Lantos, A. 1990. Bench grafting of walnut. Acta Hortic 284:53–57.
- Ozkan, Y., and A. Gumus. 2001. Effects of different applications on grafting under controlled conditions of walnut (Juglans regia L.). Acta Hortic 544:515–520.
- Ozkan, Y., Y. Edizer, and Y. Akca. 2001. A study on propagation with patch budding of some walnut cultivars (Juglans regia L.). Acta Hortic 544:521–525.
- Paunovic, S.M., R. Miletic, M. Mitrovic, and D. Jankovic. 2011. Effect of callusing conditions on grafting success in walnut (Juglans regia L.). Journal of Fruit and Ornamental Plant Research 19(2):5–14.
- Ramos, D. E. 1985. Walnut orchard management. Cooperative Extension, University of California, Division of Agriculture and Natural Resources, USA. 38-46.
- Rezaee, R., and K. Vahdati. 2008. Introducing a simple and efficient procedure for topworking of Persian walnut trees. J. Am. Pomological Scociety 62:21–26.
- Rezaee, R., K. Vahdati, V. Grigoorian, and M. Valizade. 2008. Walnut grafting success and bleeding rate as affected by different grafting methods and seedling vigor. J. Hortic. Sci. Biotechnol 83:94–99.
- Rongting, X., and D. Pinghai. 1990. Theory and practice of walnut grafting. Acta Hortic 284:69–89.
- Solar, A., F. Stampar, M. Trost, J. Barbo, and S. Avsec. 2001. Comparison of different propagation methods in walnut (Juglans regia L.) made in Slovenia. Acta Hortic 544:527–531.
- Soleimani, A., V. Rabie, D. Hassani, and M.E. Amiri 2009. Effect of rootstock and cultivar on propagation of Persian walnut (Juglans regia L.) using hypocotyl grafting. Seed and Plant Production 25 (2): 93-101 (in Persian).
- Soleimani, A.V., Rabiei, and D. Hassani. 2010. Effect of different techniques on walnut (J. regia L.) grafting. J. Food, Agric. Environ 8(2):544–546.
- Vahdati, K. 2003. Nursery management and grafting of walnut. Khaniran Publisher, Tehran, Iran, 113.
- Vahdati, K., and R. Zarei. 2006. Evaluation of side stub and hypocotyle grafting efficency for Walnut propagation in Iran. Acta Hortic 705:347–351.
- Wani, G.M. 2004. Studies on vegetative propagation of walnut as influenced by biochemical and environmental factors under hot callusing technique. PhD Thesis. University of Agricultural Sciences and Technology. Kashmir. India.
- Yildiz, K.F., Koyuncu, and H. Yilmaz. 2005. Effect of root pruning on xylem exudation of seedling and graft success of walnut. ANADOLU, J, of AARI 15(1):44–48.