ABSTRACT
Kokum (Garcinia indica) tree is endemic to Western Ghats region of India. Commercially, Kokum fruits are well-known for anti-obesity factor hydroxy citric acid as well as for their UV protecting activity. Locally, it is also used for culinary purposes and preparing healthy beverages. Still, due to endemicity and unreliable fruit production, this plant is largely underutilized. In the current study, a controlled pollination experiment was performed on three sets of female flowers to determine the need of pollination for fruit set. The set of flowers bagged without pollination did not show any fruit set, suggesting the need of pollen stimulus for the fruit setting. In artificially and naturally pollinated flowers, a well-developed female gametophyte was seen after 12 days from anthesis, but zygotic embryo or endosperm was not detected suggesting apomictic fruit set. The possibility of apomixis was also studied at the molecular level for a known male and two female plants and their F1 progenies with the help of ISSR markers. The majority of F1 progenies showed a banding pattern identical to the female parent indicating apomixis, while a few progenies showed different banding patterns, suggesting sexual reproduction. Therefore, it appears that the G. indica reproduces via facultative apomixis.
Introduction
The genus Garcinia comprises of 400 species. It is the largest genus of family Clusiaceae (Cox, Citation1976). The genus is distributed across tropical regions of Asia, Africa, and Polynesia. Garcinia indica is polygamodioceous plant endemic to Western Ghats region of India. This plant is commercially important as its fruit rinds are rich with hydroxy citric acid (HCA) which is a known anti-obesity, anti-cholesterol substance. Traditionally, fruit rinds of G. indica are used for culinary purposes and as a condiment to flavor fish curry in coastal region of India. Fruits of G. indica are also used for the preparation of pleasant and attractive beverage with bilious action (Patil, Citation2005).
Although G. indica is commercially very important, due to its highly endemic nature it is underutilized and understudied. Thatte et al. (Citation2012) reported the variations in height, branching pattern, conopy shape, and fruit morphology of these plants at various locations in Maharashtra. It was necessary to ascertain whether the observed variability is due to environmental impact or it exists at genetic level as well. Therefore, various populations of G. indica were screened with molecular markers (ISSR, RAPD). Most of the studied population [Dapoli-(4–10%), Chiplun (5–20%)] showed very less variability and almost all plants in this locality exhibited monomorphic banding pattern (Thatte et al., Citation2012). Such a low degree of polymorphism is alarming because it will threaten the existence of this highly endemic plant. The reason for low levels of polymorphism might be in its unique mode of reproduction through apomixis. Various species in genus Garcinia exhibit different modes of reproduction. Garcinia bracillienasis reproduces sexually, while G. mangostana and G. hombroniana showed sporophytic apomixis also known as adventive embryony; whereas G. parvifolia reproduces by gametophytic apomixis. In G. indica, the mode of reproduction is still not confirmed. Thus, in the present investigation, we decided to undertake a systematic study of the fertility of pollen grains in different floral types, phenology, pollination mechanism, and formation of embryo. The zygotic or apomictic progeny can be differentiated using molecular markers.
Materials and Methods
Collection of Plant Materials
The flowers required for the study were collected from the orchard of Dr. Balasaheb Sawant Agricultural University, Dapoli, District Ratnagiri, Maharashtra, India during the year’s 2013–2016. The orchard hosts about 250 plants of G. indica which includes thoroughly identified male, female and bisexual plants.
Study of Flower Morphology
Various floral types from identified male, female, and bisexual plants of G. indica from the orchard were collected according to their flowering seasons. The flowering season for the male plants is from November to January, whereas for the female plant it falls in the period of December to February.
Pollen Viability by Trypan Blue Staining Method
Representative sample of pollen grains was prepared from freshly dehisced anthers and drop of 1% trypan blue dye in phosphate buffered saline (PBS) was placed on each sample on a glass slide and covered with cover slip. After 15 min, the number of stained and unstained pollen grains was noted, and percentage viability was calculated.
Histological Studies
For the embryological studies, five female flowers were fixed in FAA (formalin: acetic acid: 90% ethanol in 1: 1: 9) at each stage of development viz. 5 days before anthesis, 3 days before anthesis, and on the day of anthesis. To study the post-fertilizational changes in female gametophytes, the flowers from two female trees (F159 and F244) were artificially pollinated with pollen grains collected from male plant M214. At an interval of 24 h, for 12 days, five flowers were fixed to study the changes occurred in female gamatophyte to detect embryo formation. The fixed flowers were dehydrated in a graded series of TBA (tertiary butyl alcohol) and embedded in paraffin wax. Serial sections of 6–8 µm thickness were cut on a Reichert 2020 microtome and processed for staining with “Fast Green” stain or with “Toluidine Blue O” stain (O’Brien et al., Citation1964). The stained sections were observed under light microscope.
Controlled Breeding Experiment
The controlled pollination experiment was conducted during the flowering seasons of year 2014–2016. A male tree with high viability of pollen grains (M214) and two female trees (F159 and F244) were selected for breeding experiment. For performing breeding experiment, flowers were divided into three sets. The first set of 100 flowers was bagged using butter paper bags without pollination in order to study the chances of apomictic fruit formation. The second set of flowers was artificially pollinated with freshly collected pollen grains from male plant M214. Further, these flowers were also protected with the help of butter paper bags to avoid natural pollination. The third set of flowers was left open to pollinate naturally.
In all breeding conditions, the fruits were set completely in 15 days from anthesis and the fruits were collected post-maturation. The seeds derived from natural and artificial pollinations were germinated in pots filled with cocopeat and sand mixture (1:1). The leaves of these F1 progenies (seedlings) derived from seeds collected after breeding experiments were used for DNA extraction.
Extraction of Genomic DNA
All the chemicals were purchased from Merck® (Bengaluru, India) and Qualigens® (Mumbai, India). Tender leaves of parent plants (F159, F244, and M214) and from F1 progenies were used for DNA extraction. The DNA was extracted by slightly modifying SDS method originally standardized by Doyle and Doyle (Citation1987). The quality and purity of extracted DNA was checked on 0.8% Agarose gel.
PCR Amplification Using ISSR Markers
The 17mer primers (UBC-kit 09) were obtained from Bangalore Genei Pvt. Ltd., Bangalore, India. PCR amplification reactions were carried out in 25-µL volume containing 40 ng of template DNA, 2.5 µL of 10X Assay buffer (Tris with 15-mM MgCl2), 25 µM each of dNTP’s, 0.4 µM of ISSR primer, and 1.2U of Taq Polymerase (Bangalore Genei Pvt. Ltd., Bangalore, India). The amplification was carried out in a thermal cycler (Applied Biosystem ThermoFischer Sci. Cop., Waltham, MA, USA). The first denaturation took place at 94°C for 5 min. In total, 45 cycles were repeated with initial denaturation at 94°C for 30 s. Primer annealing temperature varied from 50°C to 56°C for 45 s, and extension at 72°C for 2 min. Final extension step was carried out at 72°C for 7 min. The amplification products were electrophoresed on 2.5% agarose gel in 1X TAE buffer. The DNA banding pattern was observed in UV light after staining with ethidium bromide (0.1 µg/mL of gel solution) and photographed in Gel-Documentation system (Alpha-innotech, San Leandro, USA).
Results
The peculiarity of genus Garcinia is its “dioecy”, which is uncommon sexual system in angiosperms (~6%). The unisexuality aroused by inhibition or suppression of alternative sexual whorl and may have formed from hermaphrodites through evolutionary pathways such as androdioecy or gynodioecy, etc (Wu and Cheung, Citation2000).
Types of Flowers
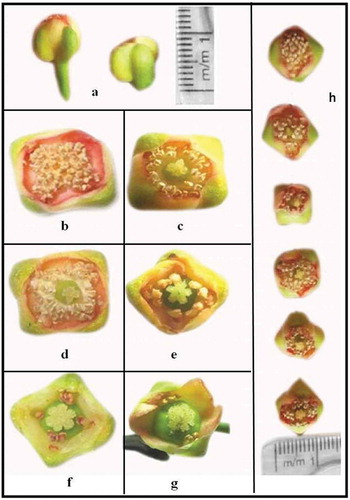
Garcinia indica is polygamodioecious with three types of plants: no fruit yielding or male, high fruit yielding or functional females, and low fruit yielding bisexual. Male plants bear flowers in the month of November–January. These flowers possess elongated receptacle: four petals and four sepals which are reddish cream in color (). Stamens are numerous, fertile, and cohere at the base forming anthophore. Carpels are absent or small and rudimentary pistils are observed (,). Female plants produce flowers slightly late, in the period from December–February. The flowers on female plants are comparatively broader with short pedicel than male flower (): four sepals and pale yellowish-green corolla. These flowers have fewer stamens or staminodes which are arranged in two, four, and eight tufts surrounding pistils (,). Ovary of the flower is larger with four or eight functional ovules on axile placenta. Bisexual plants bear the typical female flowers as well as flowers with a ring of stamens or staminodes around carpel. The carpel of the flower is fertile, and its petals are reddish in color (, ). These plants produce spindle-shaped fruits but rather in very low yields.
Figure 1. Various types of flowers of G. indica; (a) difference in length of receptacle of male and female flower. (b, c) Staminate flowers found on male plants. (f, g) Various types of flowers found on female plant. (d, e) Types of flowers found on bisexual plant. (h) Flowers arranged with increase in complexity of carpel and decreasing no. of stamens.
Pollen Morphology
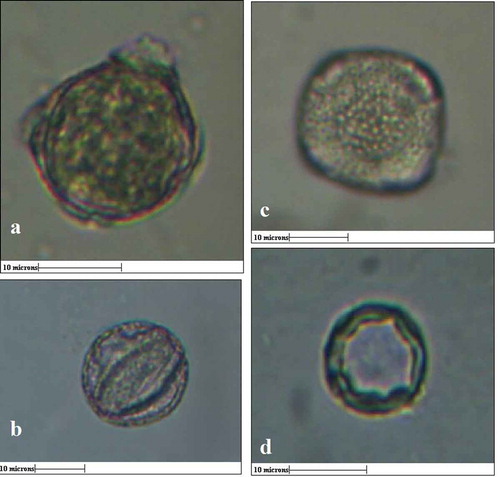
In the case of G. indica, the pollen grains from male flowers were spheroidal with about 20-µM diameter, tetrazonocolporate. , represents the polar and equatorial view of pollen grains from male flowers. The exine is coarsely granulated ornate with thickness of 2 µm (). The pollen grains from female flowers were much smaller in size, i.e. about 10 µM in diameter, exine was much thicker (3 µm), and the apertures were inconspicuous ().
Pollen Viability
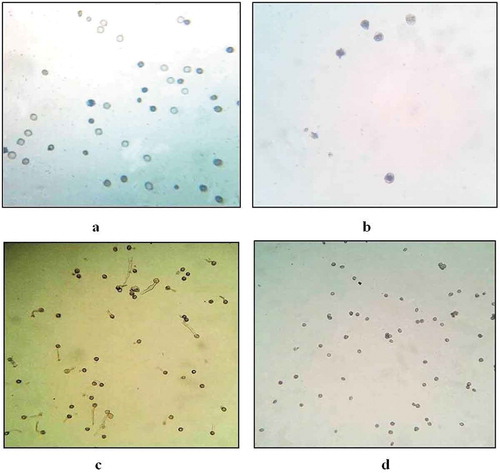
Pollen viability of various floral types was determined by “Trypan blue” method. The viability of pollen grains from male flowers was about 63–66%, whereas in the case of female flowers it was about 1–2% only ().
In Vitro Pollen Germination
To study in-vitro pollen germination, pollen grains from various floral types were transferred to 1% sucrose solution in humidity chamber. After 24 h, the pollen germination from male flower was found to be 65–70% (), whereas the pollen grains from female flowers were not germinated (). Pollen fertility was found to be dependent on size of carpel present in the male flowers. As the size of carpel increased, the number of viable pollen grains decreased. In the case of male flowers, with no carpel, 65% germination ability was observed, whereas in the case of flowers with carpel size about 1 mm and 2 mm, the pollen germination ability was decreased to 45% and 25%, respectively.
Structure of Carpel in Female Flower
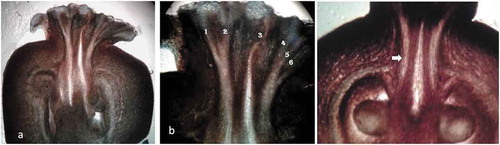
In female flower, ovary was globular, having 5–7 locules with ovules on axile placenta. Stigma is sessile, lobed (), and each lobe consists of stigmatic canal (), which leads to stylar canal. Stylar canals were hollow and were surrounded by vascular tissues ().
In Vivo Pollen Germination on Stigmatic Surface
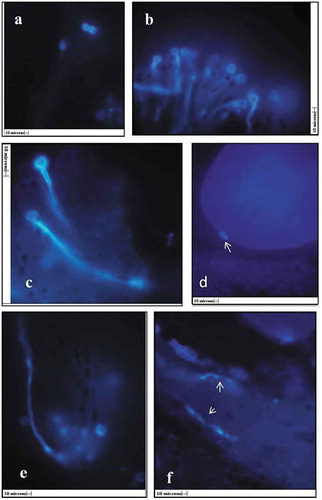
In female flowers, the series of events of traveling of the pollen tube toward ovule was traced by using the fluorescent microscopy (). The L. S. of flowers were stained with decolorized aniline blue fluorescent dye and observed under fluorescent microscope. The pollen adhesion, hydration, germination, and penetration of pollen tube into the stigma were completed within 3 h after anthesis. Profuse pollen germination was observed on papillate stigmatic surface within 5–6 h of anthesis (). During the next 6 h, the pollen tube traveled through stigmatic tissue and led toward the stylar canal (,,). Further, it appears to be reaching the ovule in about 24 h of anthesis ().
Figure 5. In vivo pollen germination a) pollen grains on stigmatic surface, b) stigmatic surface showing germinated pollen grains (10x), c,e) Pollen tube entering into stigmatic canals (45x), f) Pollen tube traveling through stylar canal (arrows) (45x), d) pollen tube entering into ovule (arrow), (All scales 10µ).
Histological Studies and Embryo Development
Formation of Female Gametophyte and Post Anthesis Changes in Ovule
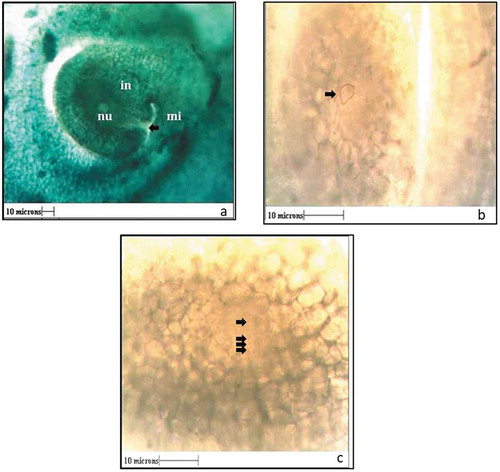
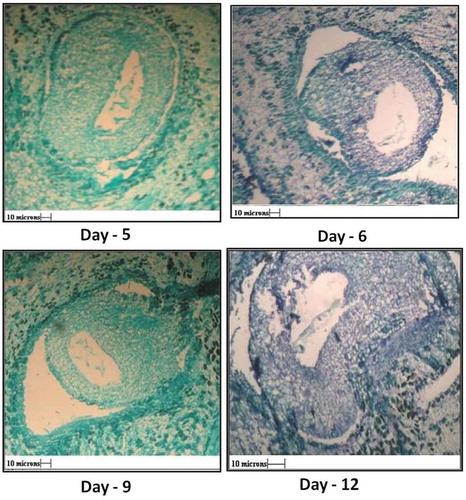
The embryological events were studied by taking hand cut as well as paraffin block vertical sections of ovary. In G. indica, ovules are anatropous (). About 5 days before anthesis, the archesporial cell with dense cytoplasm appeared in the nucellus (). This cell gave rise to a “linear tetrad” 3 days before anthesis (). The lowermost cell of the tetrad enlarged and developed to form the female gametophyte. By the time of anthesis, the ovule is ready with fully developed female gametophyte. Structures such as synergids, egg cell, and antipodal cells were not clearly visible in the female gametophyte.
Post Anthesis Events
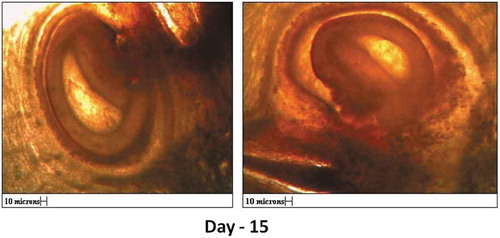
After anthesis, various developmental stages in ovule were studied daily for 12 days. As seen in , the embryo sac goes on enlarging after anthesis, but it did not show signs of development of endosperm or the formation embryo (, 5th, 7th, 9th, and 12th day).
Figure 7. V. S. of ovule 5th, 6th, 9th and 12th day after anthesis showing shriveled embryo sac. Parthenogenic development of seed in absence of embryo formation, wall of integument increases.
About 24–48 h after anthesis, in the female gametophyte few prominent cells were seen (Marked by arrow, ,). During the observation period of 12 days, these cells did not undergo any division to form pro-embryos ().
Figure 8. Various stages of embryogenesis after anthesis. a) 24 hours after anthesis, b) 48 hours after anthesis and c) 72 hours after anthesis.
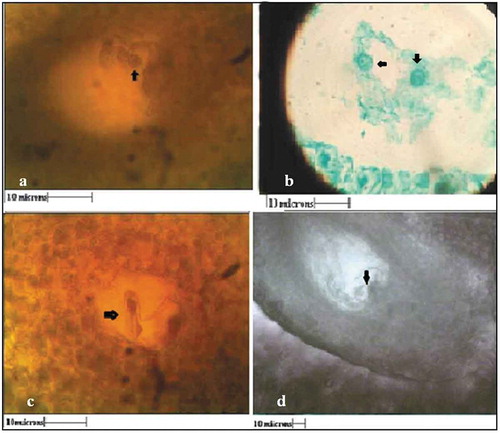
The integuments later developed into seed coat after about 15 days (). Therefore, it appears that the seeds of G. indica were formed by parthenogenesis without development of embryo and endosperm.
Use of Molecular Markers for Detection of Apomixis
Breeding Experiment
To confirm or rule out the possibility of apomixis or sexual reproduction, male and female plants with polymorphic banding pattern were selected. Although G. indica shows only 4–10% polymorphism (Thatte et al., Citation2012), out of 60 plants screened with 11 ISSR markers only two females F159 and F244 and a male M214 showed different banding patterns for female and male plants.
In this experiment, the flowers of F159 and F244 female plants were divided into three sets. The first set of flowers was bagged with butter paper bags without pollination to study the possibility of apomixis. The second set of flowers was artificially pollinated with pollen grains from known male plant M214. Further to avoid natural pollination, these flowers were also protected with butter paper bags. In the third set, the flowers were left open to pollinate naturally.
In the case of the first set, i.e., flowers bagged without any pollination, none of the 100 flowers could set fruit and dropped down after 5–6 days. This observation suggests that the plant is not obligate apomictic. In artificially and naturally pollinated flowers, fertilization and further fruit sets were observed. From a total 100 artificially pollinated flowers of plant F159, 35% could set fruit, while in naturally pollinated flowers of the same plant showed 55% fruit set. In the case of plant F244, 25% fruit set was observed for artificially pollinated flowers, whereas for naturally pollinated flowers the fruit set percentage was 40% ().
Table 1. Percentage fruit set in artificially and naturally pollinated flowers of Garcinia indica.
The fruits formed by artificial and natural pollination were collected and their seeds were germinated. The plantlets obtained after germination were used for further molecular analysis. If the F1 progeny is derived by apomixis, it should show exactly similar banding pattern to that of the female parent, whereas if it is derived via sexual reproduction, its banding pattern will be different from that of the female parent.
Molecular Fingerprinting for Detection of Apomixis
(a) Molecular fingerprinting of F159 using UBC841 for artificially pollinated progenies
represents the banding pattern produced by primer UBC 841 for parents F159 and M214. In this figure, lane L represents the marker DNA, lane M represents banding pattern of the male parent M214, and lane F corresponds to banding pattern of the female parent F159. Lanes H1 to H7 are the F1 progenies obtained through artificial pollination with M214.
Figure 10. Molecular fingerprinting of F159 progenies resulting through a. artificial pollination using ISSR primer UBC 841, b. naturally pollinated, using ISSR primer UBC 841. (L- marker DNA, M- male parent (M214), F- female parent (F159), H1-H7- artificially pollinated F1 progenies and N1 to N8- naturally pollinated F1 progenies).
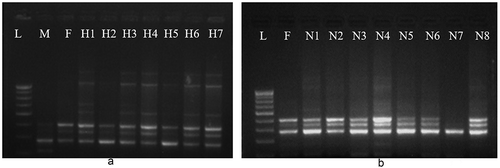
As seen in the figure, UBC 841 produces a 210-bp polymorphic band in lane M which is missing in female parent and F1 progenies obtained through artificial pollination. All progenies H1 to H7 showed identical banding pattern to maternal parent, which indicates that they were produced via apomixis.
(b) Molecular fingerprinting of F159 using UBC841 for naturally pollinated progenies
represents the PCR profile of naturally pollinated plant progeny produced by the primer UBC 841. In this figure, lane L represents the marker DNA of 100 bp. Lane F represents the banding pattern of the female parent F159 and lanes N1 to N8 correspond to its F1 progenies obtained by natural pollination. In the figure, lanes N1 to N6 and lane 8 of F1 progenies showed very similar banding pattern to female parent which confirms that they are reproduced by apomixis. Lane N7 showed a polymorphic banding pattern with primer UBC 841, which is distinct from its maternal parent. This observation proves that progeny N7 was resulted through sexual reproduction.
(c) Molecular fingerprinting of F244 using UBC 809 for artificially pollinated progenies
represents the PCR banding pattern produced by primer UBC 809 for female parent F244. Lane L represents ladder DNA of 100 bp, lane M represents male parent M214, and lane F corresponds to female parent F244. Lanes H1 to H7 represent F1 progenies obtained through artificial pollination. In this figure, lanes H1, H2, H3, and H7 show a banding pattern identical to female parent which indicates that these progenies were resulted through apomixis. Progenies H4, H5, and H6 showed a polymorphic banding pattern indicating of sexual reproduction.
Figure 11. Molecular fingerprinting of F244 progenies resulting through a. Artificial pollination using ISSR primer UBC 809, b. Naturally pollinated, using ISSR primer UBC 809. (L- marker DNA, M- male parent (M214), F- female parent (F244), H1-H7- artificially pollinated F1 progenies and N1 to N8- naturally pollinated F1 progenies).
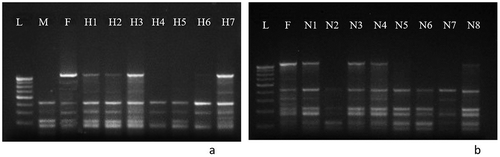
(d) Molecular fingerprinting of F244 using UBC 809 for naturally pollinated progenies
represents the PCR analysis of female F244 and its F1 progenies produced by natural pollinations. In this figure, in this case, progenies N1, N3, N4, and N8 showed a banding pattern similar to the maternal parent indicating reproduction by apomixis. Lanes N6 and N7 showed a distinct banding pattern which indicates sexual reproduction. Therefore, it can be concluded that G. indica reproduced via both apomictic and sexual pathways.
Discussion
Some species of Garcinia are androdioecious, where male and hermaphrodite plants are present in the same population. Such condition was found in G. brasiliensis. In the case of G. brasiliensis, staminate flowers, androecium consists of free stamens while pistillate flowers show pistillodes. Although the morphologically perfect flowers are pistillate due to absence of pollen, Leal et al. (Citation2013) characterized this species as “cryptic dioceous”. Garcinia atroviridis is gynodioecious and produces hermaphrodite and female (pistillates) plants. Hermaphrodites produce flowers with long stamens and abundant fertile pollen grains. The female plants produce pollen-less anthers and bear large number of fruits than hermaphrodites (Pangsuban, Citation2007). Karink (Citation1978) in his master’s dissertation performed a detailed study of flowers and fruits in kokum with the help of beautiful diagrammatic sketches. He described 11 floral types in G. indica which can be arranged in increasing functionality of stamens or decreasing functionality of carpel. He reported presence of bisexual plant in the G. indica species for the first time. He found the natural sex distribution of these plants to be 37% of male plants, 55% of female, and about 8% of the population to be bisexual. Our observations of population distribution in G. indica were also in line with the one reported by Karnik.
Engler (Citation1925) divided genus Garcinia into 23 subgenera based on morphology of androciceae. Maheshwari (Citation1964) studied Indian species of Garcinia and provided keys to identify them. Both papers reported studied on the diversity of androcieous plants and structure of pollen grain which were influenced by geographical distribution of the plant. Seetharam (Citation1989) reported diversity in pollen morphology of several species of Garcinia from Malaysia, Australia, Africa, Madagascar, and India. However, he did not elaborate on pollen grains of G. indica. Recently, pollen morphology of some of the Nigerian Clusiaciae members has been discussed by Nnamani and Nwosu (Citation2012) which includes the detailed structure of G. Kola. The pollen grains from male flowers are spherical, amb is circular and tetrazonocolporate. Pollen ornamentation was psilate with thick exine. While in G. indica, the pollen grains from male flowers were spheroidal, tetrazonocolporate. The exine is coarsely granulated ornate. The pollen grains from female flowers were much smaller in size while the exine was much thicker with inconspicuous apertures. Te Chato (Citation2007) studied pollen viability in some Garcinia species. According to his studies, the viability of pollen grains from male flowers of G. mangostana, G. speciosa, G. cowa and G. schomburgkianawas was very high (93–100%), whereas the viability of pollen grains in pistillate flowers of the same species was very low. In the case of G. dulcis, the pollen grains from female flowers were found to be non-viable. In our studies on G. indica, we found that the pollen grains from the male flowers were more viable (up to 70%) than the viability of pollen grains from pistillate flowers (1–2% only).
Although the reproductive behavior of many species of Garcinia viz. G. pariflora, G. hombroniana (Richards, Citation1990a, Citation1990b), and G. atroviridis (Pangsuban et al., Citation2009) were studied, none of them studied the pollen germination in stylar canal in artificially or naturally pollinated flowers.
Patil et al. (2013) explained pollen pistil interaction in Abelmochus esculentus. In their studies, they used “aniline blue” staining method to visualize the pollen–pistil interaction with the help of fluorescent microscopy (Karnik, Citation1978). The authors observed pollen tube traveling through stigma, style and reaching the ovule which resulted in fruit setting. Later, Geetha et al. (2012) observed pollen–pistil interaction in Commiphora wightii (Guggul). According to their studies, they found a unique case of pollen–pistil incompatibility, which allowed only one pollen tube to enter the style and initiate the fertilization in sexual plants. In G. indica flowers, the traveling of pollen tube toward ovule was traced with fluorescent microscopy by staining the L. S. of flowers with decolorized aniline blue fluorescent dye. The pollen tube seen traveling through stigmatic tissue, leading toward the stylar canal and further it appears to be reaching the ovule.
Ha et al. (Citation1988) made a detailed report on reproduction of G. parvifolia (Ha et al., Citation1988). In this specie, male trees are not found, and no viable pollen grains were observed on the staminode of hermaphrodite plant. Thus, the hermaphrodite plants are functionally females. Similar observations were made by Lim et al. (Citation1984) in the case of G. mangostana (Lim, Citation1984). Recently, reproductive system in G. brasiliensis was studied in detail by Leal et al. (Citation2013) where the plant is male biased. The male plants bear staminate flowers and female individuals are morphologically perfect but bear no pollen grains. In this species, all indications of sexual reproduction were recorded including development of megasporocyte in nucellus, dyad and tetrad formation, megasporogenesis, maturation of embryo sac and formation of zygotic embryo. Richards Citation1990a,Citation1990b reported the occurrence of both gametophytic and sporophytic agamospermy in G. hombroniana, which is unusual phenomenon. He studied both unpollinated and cross-pollinated ovaries and, in both cases, the proembryos were developed. However, in the case of G. indica, the histological studies suggested that the pre-fertilization events were normal, but the embryonic development was incomplete. The archesporial cell with dense cytoplasm appeared in the nucellus which later gave rise to a “linear tetrad”. The lowermost cell of the tetrad enlarged and formed the female gametophyte. We also did not observe formation of structures such as synergids, egg cell, and antipodal cells in female gametophyte. The embryo sac enlarged gradually, but development of endosperm or embryo was not observed. Therefore, it appears that the seeds of G. indica were formed by parthenogenesis without development of embryo and endosperm.
Thus, genus Garcinia exhibits all types of reproductions pathways. Normal sexual reproduction was observed in G. bracilliensis. In the case of plants like G. mangostana and G. hombroniana, where the male plant populations are missing, and hermaphrodite plants bear staminodes, the apomictic proembryos develop from the walls of nucellus or integument. These species reproduce by sporophytic apomixis with adventitious embryony. In G. parvifolia, male plants are absent, and the female gametophytes are developed without any meiosis and form embryos, i.e., this species reproduces through gamatophytic apomixis.
Garcinia indica is unique where male plants develop viable pollen grains which reach the stigmatic surface and successfully germinate. The ovules showed the presence of female gametophyte, but the zygotic embryos were not formed. Instead, parthenocarpic seeds were formed. These observations are in line with the one reported by Malik et al. (Citation2005) based on seed morphology concluding G. indica as an “apomictic” plant species.
In genus Garcinia, molecular markers have been employed to differentiate apomictic and sexual progenies only in the case of G. atroviridis (Pansgsuban et al. 2009). The authors studied three female trees and a set of 25 offspring from each female using six RAPD primers to distinguish the apomictic and zygotic progenies. They found some of the seedlings formed by apomixis as indicated by banding pattern identical to that of the maternal parent, whereas remaining seedlings had polymorphic banding pattern indicating sexual reproduction. Therefore, they concluded that G. atroviridis follows facultative apomixis for reproduction.
In our studies on G. indica, set of two females, one male and their naturally and artificially pollinated offspring were analyzed with comparatively more reliable ISSR markers. Out of the two plants, female F159 when artificially pollinated produced 100% of the F1 progenies by apomixis. However, the same plant when allowed to pollinate naturally produced 87% of apomictic progenies. The other female plant, F244, upon artificial pollination produced 57.14% of apomictic F1 progenies. While in the case of the naturally pollinated flowers, the apomixes were found to be 66.6%. Rest of the progenies in each scenario were derived via sexual reproduction. The percentage of apomixes might be dependent on the morphology of female flower, the fertility of the stamens, and size of the carpel. The plants showing 100% apomictic or true to type progeny can be used for clonal propagation. Therefore, with the evidence found in the histological studies and in the molecular analysis, we concluded that G. indica reproduces through facultative apomixis.
Acknowledgments
We are thankful to K.E. T’s V. G. Vaze College of Arts, Science and Commerce, Mulund, Mumbai for providing laboratory facilities, Dr. N. B. Gokhale, Dr. B. S. K. K. V., Dapoli, Dist. Ratnagiri, for allowing us to perform experiments in Garcinia indica orchard, Dr. Deepak Modi, NIRRH, Mumbai for fluorescent microscopy.
References
- Cox, J.E.K. 1976. Garcinia mangostana – Mangosteen, p. 361–375. In: R.J. Garner and S.A. Chaudhari (eds.). The propagation of tropical fruit trees. Horticulture Review No. 4. Commonwealth Bureau of Horticulture and plantation crops, England.
- Doyle, J.J., and J.L. Doyle. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19:11–15.
- Engler, A. 1925. Guttiferae: Nat. Planzenfamilien 21:154–237.
- Ha, C.O., V.E. Sands, E. Soepadmo, and K. Jong. 1988. Reproductive patterns of selected understory trees in the Malaysian rain forest: The apomictic species. Bot. J. Linn. Soc. 97:317–331. doi:10.1111/j.1095-8339.1988.tb01586.x.
- Karnik, A.R. 1978. Studies on flowering and fruiting in Kokum (Garcinia indica choisy). Konkan Agricultural University, Dapoli, Maharashtra, India, M. Sc. Thesis, pp. 38–42.
- Leal, D.O., C.R. Benevides, R.C.P. Silva, L.D.R. Santiago-Fernandes, B. Sa-Haiad, and H.A. Lima. 2013. Garcinia brasiliensis: Insights into reproductive phenology and sexual system in a Neotropical environment. Plant Syst. Evol. 173(2):172–183.
- Lim, A.L. 1984. The embryology of Garcinia mangostata L. (Clusiaceae). Gard. Bull (Singapore) 37:93–103.
- Maheshwari, J.K. 1964. Taxonomic studies on Indian Guttiferae III. The genus Garcinia linn. Bull. Bot. Surv. India 6:126–144.
- Malik, S.K., R. Chaudhary, and Z. Abraham. 2005. Seed morphology and germination characteristics in three Garcinia species. Seed Sci. Technol. 33:595–604. doi:10.15258/sst.2005.33.3.07.
- Nnamani, C.V., and M.O. Nwosu. 2012. Pollen morphology of some members of Nigerian clusiaceae and its Taxonomic significance. IOSR J. Pharm. Biol. Sci. 3:14–19. doi:10.9790/3008-0331419.
- O’Brien, T.P., N. Feder, and M.E. Mc Cully. 1964. Polychromatic staining of plant cell walls by Toluidine Blue O Protoplasma. 59:367–373.
- Pangsuban, S., N. Bamroongrugsa, K. Kanchanapoom, and C. Naulsri. 2007. An evaluation of the sexual system of Garcinia atroviridis L. (Clusiaceae), based on reproductive features. Songklanakarin J. Sci. Technol. 29:1457–1468.
- Pangsuban, S., N. Bamroongrugsa, K. Kanchanapoom, and C. Nualsri. 2009. Facultative apomixis in Garcinia atroviridis (Clusiaceae) and effects of different pollination regimes on reproductive success. Trop. Life Sci. Res. 20:89–108.
- Patil, B.P. 2005. Everything you wanted to know about Kokum (Garcinia family) - Botany forum. Kokum, Brochure, Western Ghats Kokum foundation, Goa.
- Richards, A.J. 1990a. Studies in Garcinia, dioecious tropical forest trees: Agamospermy. Bot. J. Linn. Soc. 103:233–250. doi:10.1111/boj.1990.103.issue-3.
- Richards, A.J. 1990b. Studies in Garcinia, dioecious tropical forest trees: The phenology, pollination biology and fertilization of G. hombroniana pierre. Bot. J. Linn. Soc. 103:251–256. doi:10.1111/j.1095-8339.1990.tb00187.x.
- Seetharam, Y.N. 1989. Diversity of androcium and pollen grains in the genus Garcinia L. And it’s bearing on Geographical distribution and evolution. Proc. Indian Acad. Sci (Plant Sci.). 92:107–115.
- Te Chato, S. 2007. Floral and fruit morphology of some species in Garcinia Spp. J. Sci. Technol. 29:245–252.
- Thatte, K.S., R.G. Khandekar, and M.A. Deodhar. 2012. Assesment of diversity in Garcinia indica (Dupetit-thouars) using morphological and molecular markers. J. Trop. Agric. 50:30–36.
- Wu, H., and A. Cheung. 2000. Programmed cell death in plant reproduction. Plant Mol. Biol. 44:267–281. doi:10.1023/A:1026536324081.