ABSTRACT
Water stress problems including drought stress and flooding are the most important factors limiting in citrus growth, development and production. The main purpose of this study was to investigate the effects of putrescine on reducing negative effect of flooding of Carrizo Citrange (Citrus sinensis Osb. × Poncirus trifoliata Raf.) and Volkameriana (Citrus volkameriana) rootstocks under flooding condition. Three concentrations of putrescine [0.0, 1.0 and 2.0% (w/v)] were sprayed on all the leaves of two mentioned rootstocks in a factorial experiment in a completely randomized design under flooding and field capacity conditions. In comparison to the field capacity condition, growth vegetative indicators, RWC, SPAD unit, stomatal conductance (gs), chlorophyll a and b content, chlorophyll fluorescence yield (Fv/Fm), and CO2 assimilation rate (AcO2) decreased in the flooded seedlings whereas intercellular CO2 concentration (Ci), SOD, and CAT antioxidant enzymes activities increased. Putrescine foliar application particularly in 2 mM concentration, resulted in a decrease in the intercellular CO2 concentration and an increase in the other study values. Overall, putrescine could help seedlings against flooding stress through increasing growth, improving physiological statuses, and reducing oxidative damages.
Introduction
Flooding is a result of climate discrepancy in which soil is exposed to a prolonged period of irrigation, erratic and unpredictable rainfall, or poor drainage (Colmer and Voesenek, Citation2009; Partiya et al., Citation2018) so that some parts of plants stem are submerged and their photosynthesis phenomena are hampered. However, waterlogging is a condition of land in which the largest pore spaces of the soil are saturated with the water and accompanied by anaerobic conditions. Flooding is one of the crucial stress factors disrupts many physiological and biochemical agents in agriculturally essential plants (Ashraf, Citation2003) and affects the soil through structural changes, oxygen depletion, carbon dioxide accumulation, and induction of anaerobic decomposition of organic material followed by iron and manganese revival (Kozlowski, Citation1997).
Confining root development and thereby decreasing aerobic respiration is the main effect of flooding stress, which promotes developing a set of anatomical, morphological, and biochemical alterations (Domingo et al., Citation2002). These changes can also cause the plant to react to the flooding condition by diminishing the leaf water potential and stomatal conductance (Islam et al., Citation2003; Li et al., Citation2007). In general, citrus has no anatomical and physiological adaptation to the flooding stress (Ruız-Sanchez et al., Citation1996). Therefore, when the flooding period persists, the gas exchanges and the carbon dioxide assimilation rates are decreased in citrus leaf (Gimeno et al., Citation2012). Reducing the root hydraulic conductivity causes a reduction in the water absorbance, as well (Ruız-Sanchez et al., Citation1996). Root growth reduction followed by the leaf chlorosis and blight diseases (Yelenoski et al., Citation1995), which are higher than that in shoots, are commonly observed under flooding detrimental stress (Kozlowski, Citation1997).
When plants are exposed to a variety of environmental stresses, they confront with oxidative stresses (Arbona et al., Citation2008) during which the mechanisms of energy evacuation of photosystems, such as light breathing and production of hydrogen peroxide (H2O2) and molecular oxygen (O2−) are increased (Gimeno et al., Citation2012). Further, flooding is an abiotic stress factor in the agglomeration of acetaldehyde and other compounds taken from anaerobic metabolism, which can be decomposed and lead to the hydrogen peroxide production (Blokhina et al., Citation2003). As a morphological response toward flooding, plants typically produce several antioxidant compounds to overcome the oxidation stress by shifting their metabolism (Lin et al., Citation2006).
Polyamines include aliphatic amines commonly exist in plants, microorganisms, plants, and animals nature. Putrescine, spermine, and spermidine are the main polyamines in plants, which interfere in the formation of diverse proceedings like, growth, cell proliferation, mofogenesis, distinction, and cell-mediated death (Yiu et al., Citation2009). Polycationic nature of the polyamines stimulates them to react with nucleic acids, membrane phospholipids, proteins, and cell wall components in physiological pH and eventuate to the activity or stability of these molecules (Kusano et al., Citation2007). The first scientific document belongs to the putrescine accumulation in response to the levels, which are lower than the normal potassium level in the atmosphere (Richards and Coleman, Citation1952).
The physiological gravity of putrescine in plants is due to the effects of non-living stresses comprising low pH (Young and Galston, Citation1983), potassium deficiency (Watson and Malmberg, Citation1996), cadmium toxicity (Weinstein et al., Citation1986), and osmotic pressure (Flores and Galston, Citation1982). In fact, the putrescine was successfully applied to tolerate the salinity (Verma and Mishra, Citation2005; Ndayiragije and Lutts, Citation2006), cold climate (Nayyar and Chander, Citation2004; Nayyar, Citation2005), drought condition (Amri and Shahsavar, Citation2010), and flooding in plants (Arbona et al., Citation2008, Citation2017).
However, several analytical techniques have been recommended to tolerate flooding condition in fruit trees each of which in turn is effective, one of the most significant and practical solutions is the utilization of growth regulators such as polyamines. When plants are exposed to the flooding condition the internal surface of polyamines increase in many plants, therefore polyamines are found to be effective in plant flood-tolerant. Several authors argued about the incredible diverse results of polyamines application in different plant species (Amri and Shahsavar, Citation2010; Fu et al., Citation2014; Sen et al., Citation2018).
The Carrizo Citrange (Citrus sinensis Osb. × Poncirus trifoliata Raf.) and Volkameriana (Citrus Volkameriana) rootstocks are the most important and popular rootstocks planted in north and south of Iran, respectively. Accordingly, in this study morphological, physiological and biochemical effects of flooding were first studied. Afterward, the influence of putrescine foliar application was investigated on reducing the stress consequences.
Material and Methods
Sample Preparation
The current research was held in the Laboratory of Plant Physiology at Department of Horticultural Science, Bu-Ali Sina University, Hamedan, Iran and Genetic and Agricultural Biotechnology Institute of Tabarestan associated with Sari University of Agricultural Sciences and Natural Resources, Sari, Iran. One-year-old seedlings of Carrizo Citrange and Volkameriana rootstocks were provided from the greenhouse, which was approved by the Institute of Seed and Plant Certification in Iran. The seedlings with the same diameter, age, and size were singly planted in 10-liter pots containing garden soil and sand with a ratio of 1: 3. This research was carried out as a factorial experiment consisting of three factors in a completely randomized design with three replications during two growth years. The research independent parameters were putrescine in three concentration levels (0, 1, and 2 mM), rootstocks in two species levels (Carrizo Citrange and Volkameriana), and stress in two type levels (control at 0.8 of field capacity, and flood conditions).
Before transferring to the new pots, roots of the seedlings were uniformly pruned and treated with a solution of 2/1000 fungicides of Rural TSA (Iprodione + Carbendazim) for five minutes, then they moved to a new pot and rose outside of the greenhouse. Immediately after planting in the new soil environment, plants were irrigated with 200 ml of 1/1000 of fungicides. In addition, to stimulate lateral branching, the plants head was pruned. The seedlings were daily fed with 200 ml of the nutrient solution of 2 ml/l NPK (20–20–20) for 2 months (until to exposure to the drought and flooding conditions). The treatments included 2 periods of 9-day flooding, alternating with 6 days of field capacity for the plant regeneration, and finally 15-day flooding. Putrescine was sprayed 5 days after each flooding treatment at concentrations of 0 (control), 1, and 2 mM.
Dependent Parameters
The studied characteristics were as follows:
Growth Indicators
For this purpose, plant roots and aerobic organs were separated and growth indicators of root length and root weight were measured in three stages including 45 days after submergence, after plant harvesting, and during complete washing.
Relative Water Content (RWC) of the Leaf
The relative water content of the leaves was determined for some leaf pieces (from the middle part of the lamina without the main vein) (Kirnak et al., Citation2001).
Chlorophyll Content or SPAD Unit
A chlorophyll meter (SPAD-502, Minolta, Japan) was employed to measure the leaf chlorophyll index.
Pigment Concentration
To measure the pigments concentration, young and same age leaves were taken from the different replications of each treatment. After weighing, they were homogenized with 80% acetone in a Chinese mortar. The Lichtenthaler and Welburn (Citation1983) method was applied to determine chlorophyll a, chlorophyll b, and carotenoids in mg/L at wavelengths of 664 nm for chlorophyll a and 645 nm for chlorophyll b using a spectrophotometer (WPA-Biowave II, UK).
Chlorophyll Fluorescence
A fluorometer (PAM-2500, Heinz Walz GmbH, Germany) was utilized to measure the chlorophyll fluorescence. To this aim, the youngest and perfect leaves were chosen, placed in the darkness for 20 minutes by special clips (Genty et al., Citation1989), and the ratio of Fv/Fm (the maximum quantum function of П photosystem) was recorded.
Leaf Gas Exchange
Photosynthesis rate, stomatal conductance, transpiration, and intercellular carbon dioxide concentration were determined by a portable infrared gas analyzer device (LCI-SD, ADC Hudson, UK) using fully developed leaves in the middle section of the branches at 9:00 to 11:00 am (to prevent high temperature and low humidity of the air in the evening, which affect the gas exchange parameters). Firstly, the device was adjusted to the desired parameters, and then the middle portions of the adult leaves were placed inside the measuring chamber. Leaf gas exchange was then recorded and averaged based on the photosynthesis active radiation (PAR) of 1000 μmol/m2. s, proposed by Iglesias et al. (Citation2002) for citrus, for three leaves per the selected seedlings.
Antioxidant Enzymes
SOD enzyme activity was determined by the spectrophotometric method based on its inhibitory ability to the photochemical revival of nitro blue tetrazolium at a wavelength of 560 nm (Beauchamp and Fridovich, Citation1971). The activity of the CAT enzyme was also measured by the spectrophotometric method (Citation1984), reported by Aebi (984) with respect to the disappearance level of hydrogen peroxide at 240 nm.
Statistical Analysis
The results of this study were analyzed by one-way analysis of variance using the PROC GLM procedure in SAS software (Version 9.1; SAS Institute, 2003) and the variance of each individual parameter and their interactions were compared using Duncan‘s multiple range test.
Results and Discussion
Growth Indicators
The results of variance analysis revealed that the triple interaction effect of rootstock species, stress type, and putrescine concentration was found to be significant on the vegetation growth indicators of root length and weight at 5% and 1% probability level, respectively (). Accordingly, in both rootstocks in comparison with the field capacity condition, the flooding state caused more reduction in the root length (). Under flooding treatment, the reduction values of the root length in different putrescine concentration (0, 1, and 2 mM) were recorded 70, 70.7, and 60% and 36, 47, and 38% at Volkameriana and Carrizo Citrange rootstocks, respectively. Based on key results, root length reduction of Volkameriana was greater than that of Carrizo Citrange, which is indicative of its intense flood-susceptible characteristic in dealing with flooding harmful stress. Further, under flooding and field capacity conditions, root length increased with increasing putrescine concentration in both citrus rootstocks. These results are consistent with the findings reported by some researchers (Verma et al., Citation2014; Wu et al., Citation2011; Yao et al., Citation2010; Zhang et al., Citation2015).
Table 1. Analysis of variance of putrescine effect on some morphological and physiological characteristics of Carrizo Citrange and Volkameriana rootstocks under flooding stress.
Figure 1. Effect of putrescine and flooding stress on root length and root fresh weight in Volkameriana and Carrizo Citrange rootstocks.
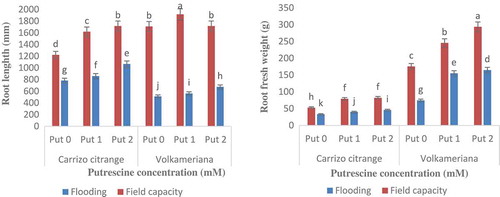
In both rootstocks, the root fresh weight of flooded samples was lower than that of field capacity, which was increased with the increase in the putrescine concentration (). So that the maximum and minimum root fresh weights were found in the field capacity condition with 2 mM putrescine concentration and flooding state without spraying, respectively (maximum and minimum root fresh weights in Volkameriana and Carrizo Citrange were recorded 293.5 and 74.4 g followed by 82 and 33.5 g, respectively). These conclusions are in a good compromise with the outcomes obtained by some researchers (Bendera et al., Citation2017; Partiya et al., Citation2018; Yin and Komatsu et al., Citation2017). Under flooding state, plant root respiration is completely restricted, and the ATP production capacity along with energy transfer through mitochondrial oxidative phosphorylation diminished due to the limited oxygen concentration. The flooding stress causes changes in the soil chemical properties including acidification and the oxidation potential and revival, which confine soil nutrients uptake and lead to the plant pesticides accumulation. It is revealed that plant growth under flooding state is limited by reducing food supply, energy, and accumulation of metabolic toxins (Yin and Komatsu, Citation2017).
With respect to the research statistics, with increasing putrescine concentration, the relative water content of the leaves in both rootstocks increased, as well under field capacity and flooding conditions. Other authors (Ebeed et al., Citation2017) reported similar results. Further, the relative water content of the leaves was found to be the highest in the field capacity condition while the lowest value belonged to the flooded samples (). Looking at , when seedlings submerged, the relative water content of the leaves in the Carrizo Citrange rootstocks was higher than Volkameriana in different levels of putrescine concentration arguing that Carrizo Citrange is more flood-tolerant. The higher relative water content in the citrus leaves may be attributed to the ability of osmotic adjustment or root potential in water absorption (Schonfeld et al., Citation1988). In a study, it was shown that during a 35-day flooding period, the relative water content of Carrizo Citrange leaves was not significantly reduced (Rodríguez-Gamir et al., Citation2011). In another investigation, two-year-old Verna lemon with Valencia, Castellano, and Orange rootstocks was exposed to the flooding treatment for 9 days during which leaf water potential, relative water content, and stomatal conductance decreased. Investigations showed that the water absorption ability of flooded Verna lemon seedlings was decreased because of the reduction in the root hydraulic conductivity (Gimeno et al., Citation2012).
Table 2. Analysis of variance of putrescine effect on some physiological and biochemical characteristics of Carrizo Citrange and Volkameriana rootstocks under flooding stress.
Table 3. Effect of putrescine concentrations on RWC (%), SPAD Value, chlorophyll a and b (mg g−1 FW) content in leaf of Carrizo Citrange and Volkameriana rootstocks.
Under flooding state, the chlorophyll content or SPAD unit fell down in both citrus rootstocks, but the reduction was found to be significantly higher in Carrizo Citrange than Volkameriana at different levels of putrescine concentration. The amount of reduction in the SPAD unit was recorded 19, 22, and 18% and 8, 9, and 3% in the Volkameriana and Carrizo Citrange rootstocks, respectively under flooding treatment at different putrescine concentrations (0, 1 and 2 mM) (). It expresses that Carrizo Citrange rootstock was more flood-tolerant compared to Volkameriana. Similar to the chlorophyll a and b concentrations, the flood-tolerance capability of Carrizo Citrange was also increased with an increase in the putrescine concentration. These results are consistent with the findings reported by some researchers (Yao et al., Citation2010).
The results concerning chlorophyll a and b concentrations reveal that an increase in the putrescine concentration caused a significant increase in both chlorophylls in the Carrizo Citrange and Volkameriana rootstocks under field capacity and flooding states. Chlorophyll a and b concentrations were the highest in the control samples and the lowest under flooding condition (). Based on , under flooding state at different levels of putrescine concentration, the chlorophyll a and b concentration in the Carrizo Citrange rootstock was greater than Volkameriana confirms the lower susceptibility of the Carrizo Citrange rootstock compared to the Volkameriana. Consistent findings were found in some investigations (Cardoso et al., Citation2017; Partiya et al., Citation2018). Since the most vulnerable part of the plant‘s optical system to the light induced injuries is the II photosystem, the damaged II photosystem is the first sign of stress induction in the leaf. Therefore, II photosystem plays an important role in the no optical responses to the environmental factors in plants (Bhardway et al., Citation1981). In addition, flooding stress reduces the maximum quantum function of the II photosystem (Fv/Fm) (Garcıa-Sanchez et al., Citation2007), which was also proved in this research for both citrus rootstocks, especially for Volkameriana one under flooding treatment. An increase in the putrescine concentration eventuated to more decrease in the maximum quantum function of the II photosystem in the Volkameriana rootstocks ().
Table 4. Effect of putrescine concentrations on maximum fluorescence efficiency (Fv/Fm), ACO2 (µmol CO2 m−2 s−1), E (mmol H2O m−2 s−1) and Ci (µmol mol-2), in leaf of Carrizo Citrange and Volkameriana rootstocks.
Based on research statistics, a significant difference was observed among the interaction effect of rootstock species, stress type, and putrescine concentration at 1% probability level (). The effect of flooding stress and putrescine foliar application were found to be significant on photosynthesis (Aco2), stomatal conductance (gs), transpiration (E), and carbon monoxide concentration (Ci) at 5% probability level (). So that under flooding conditions photosynthesis (Aco2), transpiration (E), and stomatal conductance (gs) rates decreased whereas intercellular carbon dioxide concentration (Ci) increased.
Higher concentration of intercellular carbon dioxide in plants under stress conditions (drought and flooding) emphasizes on the major role of the non-stomatal parameters including chlorophyll decomposition and chlorophyll fluorescence in reducing the photosynthesis rate rather than stomatal factors. (Garcıa-Sanchez et al., Citation2007). On the other hand, a reduction in the maximum quantum function of II photosystems (Fv/Fm) reinforces the idea that the reduction of the biochemical factors in mesophyll has been a factor in photosynthesis reduction (Perez-Perez et al., Citation2007). In the present research, the higher concentration of intercellular carbon dioxide at stress conditions argues that non-stomatal parameters are quite significant than stomatal factors in photosynthesis rate reduction. Based on key results, Volkameriana rootstock was more tolerant of the flooding-induced stress conditions.
Putrescine foliar application at 2 mM concentration improved the photosynthesis, transpiration, and stomatal conductance while diminished intercellular carbon dioxide concentration. Putrescine foliar application may also induce a reduction in the damaging non-stomatal factors and increase the flood-tolerance characteristics of the plant.
The results of the variance analysis of antioxidant enzymes (superoxide dismutase and catalase) showed a significant difference between the dual interaction effect of rootstock species and stress type at 1% probability levels (). Enzymes activity was significantly influenced by flooding stress and putrescine foliar spraying at 5% probability level (). Accordingly, the activity of both enzymes increased under flooding treatment compared to the field capacity condition, which is consistent with the results reported by other researchers (Partiya et al., Citation2018; Puyang et al., Citation2015; Tewari and Mishra, Citation2018). Considering the individual effect of putrescine, the activity of these enzymes increased with increasing putrescine concentration (). One of the first reactions of plants against pathogens, injuries, and drought and flooding stresses is the accumulation of reactive oxygen species (ROS). So that a strong correlation has been reported between the defense system of the antioxidant and flood-tolerance feature in many plants. One of the primary responses of the plants toward the flood stress is the stomatal closure to prevent water loss, which eventuates to a defection in the plant photosynthesis system (Garcıa-Sanchez et al., Citation2007; Yetisir et al., Citation2006). As a result, the electron transfer chain of photosynthesis forms superoxide radicals (O2−) and atomic oxygen (O2) in chloroplasts due to over-recovery.
Figure 2. Effect of flooding stress on antioxidant enzymes activity of superoxide dismutase (SOD) and catalase (CAT) in Volkameriana and Carrizo Citrange rootstocks.
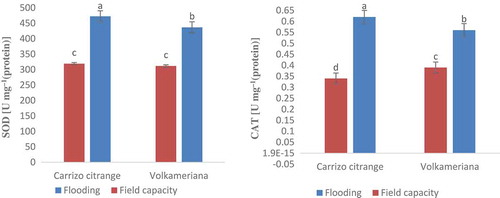
Figure 3. Effect of putrescine on antioxidant enzymes activity of superoxide dismutase (SOD) and catalase (CAT) in Volkameriana and Carrizo Citrange rootstocks.
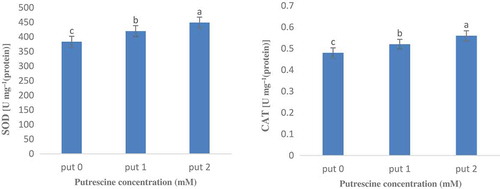
Reactive oxygen species that are abundantly produced because of submergence imbalance the cellular oxidation-reduction system and cause oxidative damages to proteins, nucleic acids, and lipids (Hossain et al., Citation2009). When plants are exposed to stress, the first active enzyme that eliminates ROS in the enzymatic mechanism is superoxide dismutase (SOD), which plays an important role in the cell defense against ROS and has high ability to eliminate active oxygen and superoxide radicals under flood stress. In addition, CAT enzyme activity is of great importance for plant salinity-tolerance, as it can decompose H2O2 into O2 and water (He et al., Citation2007). In this study, increasing the putrescine concentration from zero to 2 mM increased the activity of the two enzymes of superoxide dismutase and catalase and plant tolerance, as well and reduced the stress detrimental effects, which is similar to the reported given by other researchers (Yiu et al., Citation2009).
Conclusion
In general, the results of current research revealed that putrescine foliar spraying on plant aerobic organs could be a beneficial technique to increase the Carrizo Citrange and Volkameriana seedlings flood-tolerance characteristic. Putrescine foliar application improved some growth factors and increased the attributes associated with photosynthetic capacity and antioxidants enzymes activity. In addition, putrescine enabled to reduce the stress severe pressure, which ultimately led to increased tolerance of flooding stress in the citrus rootstocks. Therefore, putrescine foliar application is recommended to produce valuable rootstocks under flooding state.
Literature cited
- Aebi, H. 1984. Catalase in vitro. Meth. Enzymol. 105:121–126.
- Amri, E., and A.R. Shahsavar. 2010. Response of lime seedlings (Citrus aurantifolia L.) to exogenous spermidine treatments under drought stress. Aust. J. Basic Appl. Sci. 4:4483–4489.
- Arbona, V., M. Manzi, S.I. Zandalinas, V. Vives-Peris, R.M. Pérez-Clemente, and A. GَmezCadenas. 2017. Physiological, metabolic, and molecular responses of plants to abiotic stress, p. 1–35. In: M.Sarwat, A.Ahmad, M.Z.Abdin, M.M. Ibrahim, (Eds.). Stress signaling in plants: Genomics and proteomics perspective. Vol. 2. Springer, Cham
- Arbona, V., Z. Hossain, M.F. Lopez-Climent, M. Perez-Clemente, and A. Gomez-Cadenas. 2008. Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol. Plant. 132:452–466. doi: 10.1111/j.1399-3054.2007.01044.x.
- Ashraf, M. 2003. Relationships between leaf gas exchange characteristics and growth of differently adapted populations of blu panicgrass (Panicum antidotale Retz.) under salinity or waterlogging. Plant Sci. 165:69–75. doi: 10.1016/S0168-9452(03)00128-6.
- Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276–287.
- Bender, B., E.S. Capellesso, M.E. Lottici, J. Sentkovski, A.A. Mielniczki-Pereira, L.M.G. Rosa, and T.L. Sausen. 2016. Growth responses and accumulation of soluble sugars in Inga marginata Wild. (Fabaceae) subjected to flooding under contrasting light conditions. Braz. J. Biol. doi: 10.1590/1519–6984.11315.
- Bhardway, R., and G. Singhal. 1981. Effect of water stress on photochemical activity of chloroplasts during greening etiolated barley seedling. Plant Cell Physiol. 22(2):155–162.
- Blokhina, O., E. Virolainen, and K.V. Fagerstedt. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 91:179–194. doi: 10.1093/aob/mcf118.
- Cardoso, K.P., J.G. Palheta, J.D.C. Sousa, V.R. Nascimento, G.A.D. Nogueira, L.C. Machado, J.T. Martins, T.C. Costa, W.V.A. Júnior, C.F. Neto, et al. 2017. Physiological and biochemical metabolism in Jatoba plants (Hymenaea courbaril L.) affected by water stress and flooding. Aus. J. Cro Sci. 11:844–852. doi: 10.21475/ajcs.17.11.07.pne498.
- Colmer, T.D., and L.A.C.J. Voesenek. 2009. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 36:665–681. doi: 10.1071/FP09144.
- Domingo, R., A. Perez-Pastor, and M.C. RuızSanchez. 2002. Physiological responses of apricot plants grafted on two different rootstocks to flooding conditions. J. Plant Physiol. 159:725–732. doi: 10.1078/0176-1617-0670.
- Ebeed, H.T., N.M. Hassan, and A.M. Aljarani. 2017. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 118:438–448. doi:10.1016/j.plaphy.2017.07.014
- Flores, H.E., and A.W. Galston. 1982. Polyamines and plant stress: Activation of putrescine biosynthesis by osmotic shock. Science 217:1259–1261.
- Fu, X.Z., F. Xing, N.Q. Wang, L.Z. Peng, C.P. Chun, L. Cao, L.L. Ling, and C.L. Jiang. 2014. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotechnol. Biotechnol. Equip. 28:192–198. doi: 10.1080/13102818.2014.909152.
- Garcıa-Sanchez, F., J.P. Syvertsen, V. Gimeno, P. Botıa, and J.G. Perez-Perez. 2007. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 130:532–542. doi: 10.1111/j.1399-3054.2007.00925.x.
- Genty, B., J.M. Briantais, and N.R. Baker. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 990:87–92. doi: 10.1016/S0304-4165(89)80016-9.
- Gimeno, V., J.P. Syvertsen, I. Simon, V. Martinez, and F. Garcia-Sanchez. 2012. Interstock of ‘valencia’ orange affects the flooding tolerance in ‘verna’ lemon trees. HortScience. 47:403–409. doi: 10.21273/HORTSCI.47.3.403.
- He, Z.Q., C.X. He, Z.B. Zhang, Z.R. Zou, and H.S. Wang. 2007. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids Surf., B. 59:128–133. doi: 10.1016/j.colsurfb.2007.04.023.
- Hossain, Z., M.F. Lopez-Climent, V. Arbona, R.M. Perez-Clemente, and A. Gomez-Cadenas. 2009. Modulation of the antioxidant system in citrus under waterlogging and subsequent drainage. J. Plant Physiol. 166:1391–1404. doi: 10.1016/j.jplph.2009.02.012.
- Iglesias, D.J., I. Lliso, F.R. Tadeo, and M. Talon. 2002. gulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant. 116:563–572. doi: 10.1034/j.1399-3054.2002.1160416.x.
- Islam, M.A., M.E. MacDonald, and J.J. Zwiazek. 2003. Responses of black spruce (Picea mariana) and tamarack (Larix laricina) to flooding and ethylene. Tree Physiol. 23:545–552. doi: 10.1093/treephys/23.8.545.
- Kirnak, H., C. Kaya, I. Tas, and D. Higgs. 2001. The influences of water deficit on vegetative growth, physiology, fruit yield and quality in eggplants. Bulg. J. Plant Physiol. 27(3–4):34–46.
- Kozlowski, T.T. 1997. Responses of woody plants to flooding and salinity. Tree Physiol. Monograph No. 1. Heron Publishing, Victoria, Canada. doi: 10.1093/treephys/17.7.490.
- Kusano, T., K. Yamaguchi, T. Berberich, and Y. Takahashi. 2007. Advances in polyamine research in 2007. J. Plant Res. 120:345–350. doi: 10.1007/s10265-007-0074-3.
- Li, M., D. Yang, and W. Li. 2007. Leaf gas exchange characteristics and chlorophyll fluorescence of three wetland plants in response to long-term soil flooding. Photosynthetica. 45:222–228. doi: 10.1007/s11099-007-0036-y.
- Lichtenthaler, H.K., and A.R. Welburn. 1983. Determination of total carotenoids and chlorophylls A and B of leaf extracts in different solvents. Biochem. Soc. Trans. 11:591–592. doi: 10.1042/bst0110591.
- Lin, K.H., C.C. Tsou, S.Y. Hwang, L.F. Chen, and H.F. Lo. 2006. Paclobutrazol leads to enhanced antioxidative protection of sweetpotato under flooding stress. J. Plant Physiol. 163:750–760. doi: 10.1016/j.jplph.2005.07.008.
- Nayyar, H. and S. Chander. 2004. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J. Agron. Crop Sci. 190: 355–365. doi:10.1111/j.1439-037X.2004.00106.x
- Nayyar, H. 2005. Putrescine increases floral retention, pod set and seed yield in cold stressed chickpea. J. Agron. Crop. Sci 191:340–345. doi:10.1111/jac.2005.191.issue-5.
- Ndayiragije A. and S. Lutts. 2006. Do exogenous polyamines have an impact on the response of a salt-sensitive rice cultivar to NaCl? J. Plant Physiol. 163(5): 506–516. doi: 10.1016/j.jplph.2005.04.034.
- Partiya, R., R. Fotouhi Ghazvini, R. Fifaei, and M. Ghasemnezhad. 2018. Response of different citrus genotypes to continuous flooding conditions. Int. J. Hortic. Sci. Technol. 5:253–263.
- Perez-Perez, J.G., J.P. Syvertsen, P. Botia, and F. Garcia- Sanchez. 2007. Leaf water relations and net gas exchange responses of salinized carrizo citrange seedlings during drought stress and recovery. Ann. Bot. 100:335–345. doi: 10.1093/aob/mcm113.
- Puyang, X., M. An, L. Xu, L. Han, and X. Zhang. 2015. Antioxidant responses to waterlogging stress and subsequent recovery in two kentucky bluegrass (Poa pratensis L.) cultivars. Acta. Physiol. Plant. 37:197. doi: 10.1007/s11738-015-1955-z.
- Richards, F.J., and R.G. Coleman. 1952. Occurrence of putrescine in potassium-deficient barley. Nature. 170:395–401. doi: 10.1038/170460a0.
- Rodríguez-Gamir, J., G. Ancillo, M.C. González-Mas, and E. Primo-Millo. 2011. Root signalling and modulation of stomatal closure in flooded citrus seedlings. Plant Physiol. Biochem. 49:636–645. doi: 10.1016/j.plaphy.2011.03.003.
- Ruız-Sanchez, M.C., R. Domingo, D. Morales, and A. Torrecillas. 1996. Water relations of fino lemon plants on two rootstocks under flooded conditions. Plant Sci. 120:119–125. doi: 10.1016/S0168-9452(96)04494-9.
- Schonfeld, M.A., R.C. Johnson, B.F. Carver, and D.W. Mornhinweg. 1988. Water relations in winter wheat as drought resistance indicators. Crop Sci. 28:526–531. doi: 10.2135/cropsci1988.0011183X002800030021x.
- Sen, S., D. Ghosh, and S. Mohapatra. 2018. Modulation of polyamine biosynthesis in Arabidopsis thaliana by a drought mitigating pseudomonas putida strain. Plant Physiol. Biochem. 129:180–188. doi: 10.1016/j.plaphy.2018.05.034.
- Tewari, S., and A. Mishra. 2018. Flooding stress in plants and approaches to overcome, p.1–13. In: P. Ahmad, M.A. Ahanger, V.P. Singh, D.K. Tripathi, P. Alam and M.N. Alyemeni, (Eds). Plant metabolites and regulation under environmental stress. Academic Press
- Verma, K.K., M. Singh, R.K. Gupta, and C. Verma. 2014. Photosynthetic gas exchange, chlorophyll fluorescence, antioxidant enzymes, and growth responses of Jatropha curcas during soil flooding. Turk. J. Bot. 38:130–140. doi: 10.3906/bot-1212-32.
- Verma S. and S.N. Mishra. 2005. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J Plant Physiol. 162(6): 669–77. doi: 10.1016/j.jplph.2004.08.008.
- Watson M.B. and R.L. Malmberg. 1996. Regulation of Arabidopsis thaliana (L.) Heynh Arginine decarboxylase by potassium deficiency stress. Plant Physiol. 111(4): 1077–1083.
- Weinstein, L.H., R. Kaur-Sawnhey, M.V. Rajam, S. Wettlaufer, and A.W. Galston. 1986. Cadmium-induced accumulation of putrescine in oat and bean leaves. Plant Physiol. 82:641–645.
- Wu, Q.S., Y.N. Zou., Y.H. Peng, and C.Y. Liu. 2011. Root morphological modification of mycorrhyzal citrus (Citrus tangerine) seedlings after application with exogenous polyamines. J. Anim. Plant Sci. 21:20–25.
- Yao, Q., L.R. Wang, Q.X. Xing, J.Z. Chen, and H.H. Zhu. 2010. Exogenous polyamines influence root morphogenesis and arbuscular mycorrhizal development of Citrus limonia seedlings. Plant Growth Regul. 60:27–33. doi: 10.1007/s10725-009-9415-7.
- Yelenoski, G., J.C.V. Vu, and H.K. Wutscher. 1995. Influence of paclobutrazol in the soil on growth, nutrient elements in the leaves, and flood/freeze tolerance of citrus rootstock seedlings. J. Plant Growth Regul. 14:129–134. doi: 10.1007/BF00210914.
- Yetisir, H., M.E. Caliskan, S. Soylu, and M. Sakar. 2006. Some physiological and growth responses of watermelon (Citrullus lanatus Thunb) Matsum and Nakai, grafted onto Lagenaria siceraria to flooding. Environ. Exp. Bot. 58:1–8. doi: 10.1016/j.envexpbot.2005.06.010.
- Yin, X., and S. Komatsu. 2017. Comprehensive analysis of response and tolerant mechanisms in early-stage soybean at initial-flooding stress. J. Proteomics. 169:225–232. doi: 10.1016/j.jprot.2017.01.014.
- Yiu, J.C., L.D. Juang, D.Y.T. Fang, C.W. Liu, and S.J. Wu. 2009. Exogenous putrescine reduces flooding-induced oxidative damage by increasing the antioxidant properties of Welsh onion. Sci. Hortic. 120:306–314. doi: 10.1016/j.scienta.2008.11.020.
- Young, N.D., and A.W. Galston. 1983. Putrescine and acid stress. Plant Physiol. 71:767–771. doi: 10.1104/pp.71.4.767.
- Zhang, Y., X. Song, G. Yang, Z. Li, H. Lu, X. Kong, A.E. Eneji, and H. Dong. 2015. Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crops Res. 179:164–172. doi: 10.1016/j.fcr.2015.05.001.
