?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study was conducted to find the constitutive antifungal phenolic lipids, phenolics contents and antioxidant activities in mango fruit peel extracts of anthracnose (Colletotrichum gloeosporioides Penz.) resistant (‘Kensington pride’) and susceptible (‘Badami’ and ‘Raspuri’) mango cultivars grown in India, during fruit development, maturity and ripening. Phytochemical tests were performed to determine 5-n-pentadecyl resorcinol content and total phenolics. Moreover, the mango peel methanolic extracts were subjected to evaluate their antioxidant potential using invitro DPPH assay. Results show that 5-n- pentadecyl resorcinol content in the mango fruit peel extract was at its peak during the early stage of fruit development (at 30DFS) in ‘Kensington pride’ as compared to ‘Badami’ and ‘Raspuri’. The upsurge in total phenolics content and DPPH activity at 30 DFS, in all three mango cultivars, indicated their higher radical scavenging activity during fruit development than the fruit maturity and fruit ripening. Endogenous production and retention of high levels (P ≤ .05) of constitutive antifungal 5-n-pentadecyl resorcinol in anthracnose resistant mango cultivar (‘Kensington pride’) during early stage of fruit development that imparts disease resistance during development, maturity, and fruit ripening process, could be one of the bases of plant defense mechanism against anthracnose disease. In conclusion, this study exhibited the important role played by the mango cultivars background on the constitutive antifungal 5-n-pentadecyl resorcinol content and antioxidant profiles.
Introduction
Mango (Mangifera indica L.) is an important fruit crop of the tropical and subtropical countries of the World. Both production and productivity of mangoes are severely affected and drastically reduced by latent infection of anthracnose disease caused by Colletotrichum gloeosporioides Penz through pre and postharvest losses that occur in humid tropics of the World (Dodd et al., Citation1997). Developing fruit buds are highly sensitive, susceptible and dropped prematurely when infected with the pathogen. The pathogen often infects immature, unripe fruits and remain in the quiescent phase of disease development until the onset of fruit ripening adds a reference. Fruit losses due to anthracnose reduced if fungal infections restricted in their quiescent phase of development during fruit development and maturity. Among the various bioactive compounds, phenolic lipids endogenously produced specific plant secondary metabolites in cereals, legumes, vegetables, and fruits characterized for their potential antioxidant (Prior and Cao, Citation2000), antimicrobial (Tapiero et al., Citation2002) and anti-obesity properties (Hsu et al., Citation2006). These phenolic lipids comprise alkyl phenols, alkylresorcinols, anacardic acids, and alkyl catechols. Alkylresorcinols (ARs) play a significant role in imparting resistance to plant and other living systems against abiotic and biotic stresses in the range of hosts and mitigates stress conditions. AR’s identified in a variety of plant species such as rye (Deszez and Kozubek, Citation2000), wheat (Gohil et al., Citation1988), rice (Suzuki et al., Citation1996), and mango (Cojocaru et al., Citation1986), fungi, bacteria and in few animal species (Ross et al., Citation2003) (Landberg et al., Citation2008). In vitro assays, the antimicrobial activity of ARs and its derivatives showed their defensive role in various plants grown under biotic stress conditions (Kozubek and Tyman, Citation2005; Stasiuk and Kozubek, Citation2010). Further, the highly localized pattern of deposition of ARs within regions surrounding plant structures against biotic and abiotic stresses is evidenced by the accumulation of significant levels of ARs within grains of wheat, rye, triticale and barley and within a thin cuticle layer external to the seed coat (Landberg et al., Citation2008; Ross et al., Citation2003).
In vitro studies, direct inhibitory effect of 5-n-alk(en)yl- resorcinols on phytopathogenic fungi was also reported (Suzuki et al., Citation1996). Similarly, a mixture of the 5-n-alkylresorcinol homologs isolated from rye grain inhibited the mycelial growth of Fusarium culmorum, Rhizoctonia solani, and Rhizoctonia cereals (Zarnowski et al., Citation1999). ARs are known to retain quiescence or latency state of infection in fungi causing postharvest diseases in mango highest levels of 5-n-heptadecenylresorcinol and 5-n-pentadecylresorcinol in the fruit peel of unripe mangoes, and their levels gradually decreased with the initiation of fruit ripening and senescence (Cojocaru et al., Citation1986; Droby et al., Citation1986, Citation1987). Studies on profiling and characterization of ARs into major and minor alk(en)ylresorcinols from mature Peruvian mango peels were also reported (Knodler et al., Citation2007). Significant correlations between the size of lesions following inoculation with Colletotrichum gloeosporioides Penz and pools of 5-n-pentadecylresorcinol and 5-n-heptadecenylresorcinol in mango fruit peel were reported (Hassan et al., Citation2007) and also a direct relationship between the percentage of non-aqueous sap and the concentrations of alk(en)ylresorcinols in different mango cultivars were reported (Hassan et al., Citation2009).
With this background, considering the latent infection nature of anthracnose disease in mango, heavy pre and postharvest losses of mango fruits due to this disease, present study was planned to assess the endogenous production levels of the constitutive antifungal phenolic lipids, phenolics contents and antioxidant activities in mango fruit peel extracts of both anthracnose resistant (‘Kensington pride’) and susceptible (‘Badami’ and ‘Raspuri’) mango cultivars grown in India, during fruit development, maturity and ripening.
Materials and Methods
Chemicals
All reagents used in the experiments were of analytical grade and purchased mostly from SD Fine Chem, Mumbai and Sigma chemical Co., Bangalore, India. Solvents used for extraction of fruit samples purchased from Fisher Scientific., Bangalore, India. The phenolic standards and alkyl resorcinol standard obtained from Sigma chemical Co., Bangalore, India. Water used was of Millipore quality.
Samples
Uniform size fruits were harvested at five different stages of fruit development and maturity from well-maintained healthy mango orchards of three mango cultivars ‘Badami’, ‘Raspuri’, and ‘Kensington Pride’ from Indian Institute of Horticultural Research, Hessarghatta, Bengaluru, Karnataka, India, during Mango season (November 2015 to June 2016). The samples were packed in corrugated fiber boxes and immediately transported to the laboratory for analysis and stored in cold room (12 ± 2°C) for 10–12 hr to remove field heat and then, samples were tap water washed. Mango peel samples from above mango cultivars were collected at five stages of development and maturity of fruit (Stage 1: 30 DFS (Days after Fruit Set), Stage 2: 60 DFS, Stage 3: 90 DFS, Stage 4: 110 DFS, Stage 5:120 DFS, Stage 6: Completely ripened fruits (with TSS, >20° Brix) and the mango fruit peels were packed in LDPE bags and finally stored at −20°C for further analysis of alkyl resorcinol content, total phenolics content and antioxidant activity.
Determination of 5-n-pentadecylresorcinol Content in Fruit Peels of Mango Cultivars
The peel slices from each fruit in replicates bulked, cut into approximately 4–9 mm2 pieces and a 25 g of sample was homogenized using a laboratory mixer. A hundred milliliters of acetone was added to the peel tissue and kept overnight under continuous stirring as per the method (Jose da, et al. Citation2005).
Then the slurry was filtered, and the sediment was additionally rinsed twice with 50 ml of fresh acetone. The filtrates were collected and centrifuged for 10 min (at 8385 g). The supernatant separated from the precipitate and the solvent gets entirely evaporated. The extracts weighed and stored at −20°C and then filtered through a 0.45 µm pore size membrane before analysis by HPLC. All samples analyzed with an HPLC (Shimadzu, Japan) equipped with a UV detector. A C18 column (250 mm× 4.6 mm) with an isocratic mobile phase of 90% (v/v) methanol with a flow rate of 1.0 mL/min used. A 10 µL sample injected into the HPLC and detection of the compounds was achieved at a wavelength of 280 nm. A unit pack of 10 mg, 5-n-pentadecylresorcinol, obtained from Sigma Aldrich was used to quantify resorcinol in mango peel samples.
Determination of Total Phenolic Content in Fruit Peels of Mango Cultivars
Total phenolic content in mango peel sample determined by using Folin-Ciocalteau assay (Singleton and Rossi, Citation1965), with slight modifications. Briefly, 100 µl of sample extract mixed with 2.9 ml of distilled water in a tube, followed by addition of 500 µl of Folin-Ciocalteau reagent. After 3 min, 2 ml of Na2CO3 (20%) solution was added in each container and kept in the dark for 30 min, at 28 ± 2°C. The absorbance measured at 765 nm against the reagent blank with UV-VIS spectrophotometer (UV-1700 Pharma Spec by Shimadzu). Total phenolic content reported as mg of Gallic acid equivalent (GAE)/g of peel extract.
Determination of Free Radical Scavenging Activity (DPPH) in Fruit Peels of Mango Cultivars
The DPPH free radical scavenging activity of mango peel extract determined as per the method (Wang et al., Citation2008), with slight modification. Briefly, an aliquot of 0.25 ml of diluted peel extract with methanol taken and 1 ml of 0.2 mM of DPPH added. It was then subjected to vortex for 30 s and kept for incubation in the dark for 30 min. The absorbance was measured using spectrophotometer at 517 nm. The scavenging activity calculated as per the following equation:
Statistical Analysis
All the experimental determinations were carried out in triplicate (n = 3), and the data reported as mean±standard deviation (SD) for all triplicate determinations. The design of all experiments has been made explicit and clear and verified and checked.
Results and Discussion
5-n-pentadecyl Resorcinol Content In Fruit Peels of Mango Cultivars
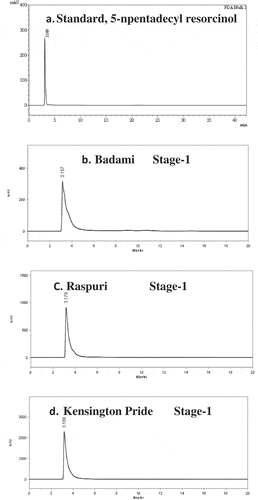
The present study attempted to investigate constitutive alkylresorcinol compounds in mango fruits of cultivars grown commercially. Results ( and ) indicated that the fruit peels of all the mango varieties initially at 30 DFS were recorded (P ≤ 0.05) higher pools of 5-n- pentadecyl resorcinol and it decreased as fruit growth advanced toward maturity and then declined to low levels at full ripe stage. At 30 DFS (Stage-1: 30DFS), anthracnose less susceptible mango variety ‘Kensington Pride’ had the (P ≤ 0.05) highest level of 5-n-pentadecylresorcinol (6.52 mg/g FW) as compared to anthracnose highly susceptible mango varieties ‘Raspuri’ (4.71 mg/g FW) and ‘Badami’ (4.47 mg/g FW). During developmental stages of mangoes. There was a slight decrease in the concentration of 5-n-pentadecylresorcinol content as the growth of these developing fruits advanced toward the physiological stage of maturity for harvest, followed by the complete stage of maturity (Stage-5: 120DFS). The stage of complete ripeness of mango peels (Stage-6) showed significant effects on the levels of 5-n-pentadecylresorcinol in peel component in both anthracnose less susceptible and anthracnose highly susceptible mango varieties, and their response differed. Both less susceptible (‘Kensington’) and highly susceptible (‘Raspuri’) mango varieties had statistically identical levels of 5-n-pentadecylresorcinol (1.96 to 2.01 mg/g of sample extract), whereas, ‘Badami’ as anthracnose highly susceptible mango variety had the (P ≤ 0.05) lowest levels of 5-n-pentadecylresorcinol content (0.94 mg/g of sample extract).
Table 1. Changes in 5-Pentadecyl resorcinol content (mg/g FW) in mango peel extract of mango cultivars during different stages of fruit development, maturity and ripening.
Total Phenolics Content in Fruit Peels of Mango Cultivars
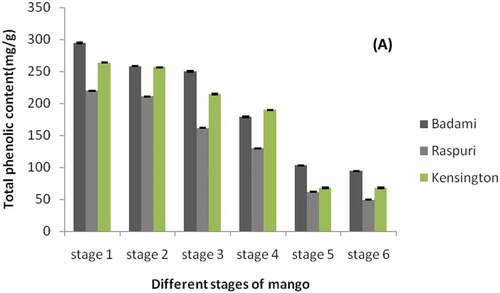
Total phenolics are secondary metabolites which have antioxidant potential and provide health benefits to human. Genotype, as well as environmental factors, control production of phenolics. So, the content of total phenolics may vary among the different varieties and geographical locations and time of harvest (Lee and Talcott, Citation2004). Results on the total polyphenol content () indicated that total Polyphenol content in acetone extracts of raw and ripe fruits (P ≤ 0.05) varied from 294 to 50 mg GAE/g peels of all the three mango varieties. Concentration of the total polyphenol content was (P ≤ 0.05) higher (between 294.46 ± 0.40 to 211.26 ± 0.75 mg of GAE/g of sample) in raw mango peels of all three mango varieties recorded during early stage of fruit development (Stage 1:30 DFS) as compared to rest stages of fruit development, maturity and complete ripe mango peels (Stage 6).
Figure 2. Total phenolic content in the peels of ‘Badami’, ‘Raspuri’, and ‘Kensingtonpride’mangoes harvested at different stages of fruit maurity and at complete ripe stage. Stage 1: 30 DFS, stage 2: 60 DFS, stage 3: 90 DFS, stage 4: 110 DFS, stage 5:120 DFS, stage 6: completely ripened fruits (with TSS, >20° Brix).
Scavenging Activity on DPPH˙
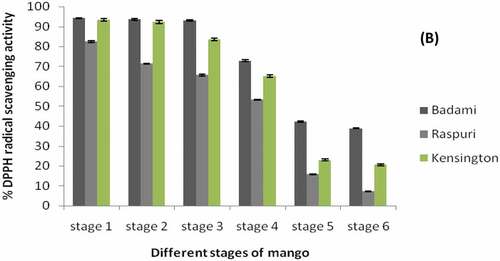
The peel extracts from the three different mango varieties () showed (P ≤ 0.05) different values of radical scavenging activities ranging from 94% to 84% during the initial stage of fruit set (stage-1) and decreased to 38.8–7.29% during ripening (stage-6). Presence of comparatively higher concentration of DPPH activity (between 94.13 ± 0.26 to 82.53 ± 0.45%), during an early stage of fruit development (at 30 DFS) than rest of the stage of fruit development and maturity and ripening, indicating higher radical scavenging activity during an early stage of fruit development in all three mango varieties. However, the concentration of DPPH activity decreased gradually to least levels (38.81 ± 0.16 to 7.29 ± 0.20%) (P ≤ .05) toward full maturity (at 120 DFS).
Figure 3. DPPH radical scavenging activity of mango peel extract of ‘Badami’, ‘Raspuri’ and ‘Kensingtonpride’mangoes harvested at different stages of fruit maurity and at complete ripestage. Stage 1: 30 DFS, stage 2: 60 DFS, stage 3: 90 DFS, stage 4: 110 DFS, stage 5:120 DFS, stage 6: completely ripened fruits (with TSS, >20° Brix).
Constitutive antifungal phenolic lipid compounds in fruit peel tissues have been reported to be a potential chemical barrier against anthracnose resistant mangoes (Hassan et al., Citation2007, Citation2009). The present study attempted to investigate constitutive alk(en)ylresorcinol compounds (5-n-pentadecyl resorcinol) in mango fruits of a range of commercially important anthracnose less susceptible and susceptible mango varieties, during fruit development, maturity, and fruit ripening. Among these mango varieties, anthracnose less susceptible variety (Kensington pride) initially during an early stage of fruit development (at 30 DFS) had higher levels of 5-n- pentadecyl resorcinol then the later stages of fruit development, maturity, and fruit ripening. However, 5-n- pentadecyl resorcinol content in the peel component of all mango varieties proportionately declined as fruit growth advanced toward complete fruit development and further down surged on complete fruit ripening, irrespective of their degree of susceptibility to anthracnose. Results ( and ) also revealed that there was consistent and positive correlation ship in the concentration of 5-n-penta decylresorcinol in the peel component of both anthracnose less susceptible and anthracnose highly susceptible mango varieties and the physiological stages of fruit development, maturity, and fruit ripening.
Results on endogenous production and retention of comparatively higher levels of 5-n- pentadecyl resorcinol in anthracnose less susceptible mango cultivar ‘Kensington Pride’ during the early stage of fruit development (at Stage-1:30DFS). This indicated fungi toxic levels of these antifungal bioactive compound (5-n- pentadecyl resorcinols) in the peel component of ‘Kensington Pride’ that imparts resistance to the developing fruits to latent infections by C. gloeosporioides Penz during later stages of fruit development, maturity till fruit attains complete ripe stage and this could be one of the reasons and the basis of defense mechanism against anthracnose disease. Similarly, the presence of fungi toxic levels of such antifungal resorcinol bioactive compounds in both mango peel and fruit latex in immature, developing fruits to infection by C. gloeosporioides Penz was reported (Hassan et al., Citation2007). Presence of antifungal compounds such as 5-n-pentadecyl resorcinol and 7-n-heptadecenyl resorcinol in the peel of mango fruits was found to be chemical barriers for Colletotrichum gloeosporioides Penz pathogens against anthracnose disease in mango were reported (Hassan et al., Citation2007). The resistant varieties had higher levels of both types of resorcinols (5-n-pentadecyl resorcinol and 7-n-heptadecenyl resorcinol) in their peel, suggesting the crucial role of the constitutive alk(en)yl resorcinol in anthracnose disease resistance. Alk(en)yl resorcinols present in high concentrations in the sap of Australian grown mangoes (Hassan et al., Citation2009). Recent studies have also shown that the defense responses generated in unripe mango fruits earlier and greater magnitude in the resistant cultivars against C.gloeosporiodes Penz (Sinniah et al., Citation2013).
Results on the total polyphenol content () indicated that endogenous production and accumulation of (P ≤ 0.05) higher concentration of total phenolics during early stage of fruit development (at 30 DFS) than rest of the stage of fruit development and maturity and ripening, indicating higher radical scavenging activity during early stage of fruit development in all three mango varieties. Similarly, it was also reported (Ueda et al., Citation2000) that the total phenolic content was higher in the peel component than the pulp component at any stage of mango fruit development. However, the concentration of total phenolics gradually decreased to (P ≤ 0.05) least levels (94.48 ± 0.55 to 49.46 ± 0.70 mg of gallic acid/g of sample extract) toward full maturity (at 120 DFS) in all three mango varieties. At the fully ripe stage, the total polyphenol contents in acetone extracts of ripe peels declined (P ≤ 0.05) to least levels in the peel component of all the three mango varieties. Earlier, it was also reported (Ajila et al., Citation2007) that the total polyphenols content in 80% acetone extract of ripe peel component of Raspuri and Badami mango varieties ranged from 100 to 55 mg/g.
Results revealed that presence of comparatively higher levels of total phenolics () and DPPH activity () respectively, during early stage of fruit development (at 30 DFS) than rest of the stage of fruit development and maturity and ripening, indicating higher radical scavenging activity during early stage of fruit development in all three mango varieties, irrespective of their degree of susceptibility to anthracnose disease. However, the concentration of total phenolics and DPPH activity decreased gradually to least levels toward full maturity (at 120 DFS) in all Mango varieties. Similarly, studies (Ribeiro et al., Citation2008) showed that the peel extracts from the Uba mango variety from Brazil had higher radical scavenging activity than kernel extract which is mainly due to the presence of a higher concentration of the antioxidant compounds in the peel.
Present studies on assessment of physiological changes in the pools of highly valuable bioactive phenolic lipids, total phenolics and DPPH radical scavenging activity in anthracnose susceptible three mango varieties during fruit development, maturity and ripening indicated that presence of higher levels of 5-n- pentadecyl resorcinol during early stage of fruit development (at 30 DFS) in anthracnose less susceptible mango cultivar ‘Kensington pride’ than the anthracnose highly susceptible ‘Badami’ and ‘Raspuri’. Presence of comparatively high levels of both total phenolics and DPPH activity in all three mango varieties, during early stage of fruit development (at 30 DFS) than rest of the stage of fruit development and maturity and ripening, indicating higher radical scavenging activity during early stage of fruit development Endogenous production and retention of comparatively higher levels of phenolic lipids particularly 5-n-pentadecyl resorcinol in anthracnose less susceptible mango variety during early stage of fruit development that imparts disease resistance during fruit development, maturity, and ripening, could be one of the basis of defense mechanism against biotic stress conditions such as anthracnose disease.
Conclusion
The objective of the present study was to investigate the constitutive antifungal 5-n-pentadecyl resorcinol content and phenolics contents and the antioxidant properties of commonly consumed three mango cultivars in mango fruit peel extracts of anthracnose (Colletotrichum gloeosporioides Penz.) resistant (‘Kensington pride’) and susceptible (‘Badami’ and ‘Raspuri’) mango cultivars grown in India, during fruit development, maturity and ripening. The results exhibited that mango fruit peel extracts contained the important quantity of 5-n-pentadecyl resorcinol content, phenolics, and antioxidant properties. These research findings may afford a useful basis to affirm mango cultivar property endogenous production and retention of high levels of constitutive antifungal 5-n-pentadecyl resorcinol in anthracnose resistant mango cultivar (‘Kensington pride’) during early fruit development that imparts disease resistance to developing fruits attains complete maturity and full ripe stage, could be one of the bases of plant defense mechanism against anthracnose disease. In conclusion, this study exhibited the important role played by the mango cultivars background on the constitutive antifungal 5-n-pentadecyl resorcinol content and antioxidant profiles.
Declaration of interest statement
There is no potential conflict of interest among the authors.
Acknowledgments
This work as part of the CSIR-Network project funded by the Council of Scientific and Industrial Research (CSIR), New Delhi, India. The authors acknowledge the CSIR, New Delhi, India for funding this Project. The authors are grateful to Director, CSIR-CFTRI, Mysore, for his keen interest, constant encouragement, and kind support during this investigation. Authors also extend their sincere thanks to the Head and the Staff of CIFS, CSIR-CFTRI for their technical assistance in instrumental analysis of the samples. Authors also thank Dr Giridhar, P. Senior Principal Scientist, Department of plant cell biotechnology, CFTRI, Mysore, for providing comments which improved the manuscript.
Additional information
Funding
References
- Ajila, C.M., S.G. Bhat, and U.J.S. Prasad Rao. 2007. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 102:1006–1011. doi: 10.1016/j.foodchem.2006.06.036.
- Cojocaru, M., S. Droby, and E. Glotter. 1986. 5-(12-heptadecenyl)-resorcinol, the major component of the antifungal agent from the peel of mango fruit. Phyto Chem. 25:1093–1095. doi: 10.1016/S0031-9422(00)81560-5.
- Jose da, C.F., B. Danielsson, and A. Kozubek. 2005. Application of supercritical carbon dioxide for the extraction of alkylresorcinols from rye bran. J. Supercrit. Fluids 35:220–226. doi: 10.1016/j.supflu.2005.01.010.
- Deszez, L., and A. Kozubek. 2000. Higher cardol homologs (5-alkylresorcinols) in rye seedlings. Biochim. Biophys. Acta 1483:241–250. doi: 10.1016/S1388-1981(99)00187-0.
- Dodd, J.C., D. Prusky, and P. Jeffries. 1997. Fruit diseases, p. 257–280. In: R.E. Litz (ed.). The mango: Botany, production, and uses. CAB International, Wallingford, UK.
- Droby, S., D. Prusky, and B. Jacoby. 1986. The presence of the antifungal compound and its relation to the latency of Alternaria alternate in unripe peels of mango fruits. Physiol. Mol. Plant Pathol. 29:173–183. doi: 10.1016/S0048-4059(86)80019-4.
- Droby, S., D. Prusky, and B. Jacoby. 1987. Induction of antifungal resorcinols in the flesh of unripe mango fruits and its relation to latent infection by Alternaria alternata. Physiol. Mol. Plant Pathol. 30:285–292. doi: 10.1016/0885-5765(87)90041-5.
- Gohil, S., D. Pettersson, and A.C. Salomonsson. 1988. Analysis of alkyl-and alkenylresorcinols in triticale, wheat, and rye. J. Sci. Food Agric. 45:43–52. doi: 10.1002/jsfa.2740450106.
- Hassan, M.K., E.K. Dann, and D.E. Irving. 2007. Concentrations of constitutive alk(en)ylresorcinols in the peel of commercial mango varieties and resistance to postharvest anthracnose. Physiol. Mol. Plant Pathol. 71:158–165. doi: 10.1016/j.pmpp.2007.12.005.
- Hassan, M.K., D.E. Irving, and E.K. Dann. 2009. Sap properties and alk(en)ylresorcinol concentrations in Australian grown mangoes. Ann. Appl. Biol. 154:419–427. doi: 10.1111/j.1744-7348.2008.00313.x.
- Hsu, C., S. Huang, and G. Yen. 2006. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 pre-adipocytes about their antioxidant activity. J. Agric. Food Chem. 54:4191–4197. doi: 10.1021/jf0609882.
- Knodler, M., N. Bernardini, and D.R. Kammerer. 2007. Characterization of major and minor alk-(en)ylresorcinols from mango (Mangifera indica L.) peel by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrosc. 21:945–951. doi: 10.1002/rcm.2919.
- Kozubek, A., and J.H. Tyman. 2005. Bioactive phenolic lipids, p. 119–190. In: A. Rahman (ed.). Studies in natural products chemistry. Elsevier BV Publishers, Amsterdam, The Netherlands.
- Landberg, R., A. Kamal-Eldin, and M. Salmenkallio-Marttila. 2008. Localization of alkylresorcinols in wheat, rye and barley kernels. J. Cereal Sci. 48:401–406. doi: 10.1016/j.jcs.2007.09.013.
- Lee, J.H., and S.T. Talcott. 2004. Fruit maturity and juice extraction influence ellagic acid derivatives and other antioxidant polyphenolics in muscadine grapes. J. Agric. Food Chem. 52(2):361–366. doi: 10.1021/jf034971k.
- Prior, R.L., and G. Cao. 2000. Antioxidants and phytochemicals in fruits and vegetables: Dietary and health implications. Hortic. Sci. 35:588–592.
- Ribeiro, S.M.R., L.C.A. Barbosa, and J.H. Queiroz. 2008. Phenolic compounds and antioxidant capacity of Brazilian mango varieties. Food Chem 110:620–626. doi: 10.1016/j.foodchem.2008.02.067.
- Ross, A.B., M.J. Shepherd, and M. Schüpphaus. 2003. Alkylresorcinols in cereals and cereal products. J. Agric. Food Chem. 51:4111–4118. doi: 10.1021/jf0340456.
- Singleton, V.L., and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144–158.
- Sinniah, G.D., N.K.B. Adikaram, and C.L. Abayasekara. 2013. Differential defense responses expressed in mango (Mangifera indica L.)cultivars resistant and susceptible to Colletotrichum gloeosporioides Penz. Indian Phytopathol. 66(1):34–40.
- Stasiuk, M., and A. Kozubek. 2010. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 67:841–860. doi: 10.1007/s00018-009-0193-1.
- Suzuki, Y., Y. Esumi, and H. Hyakutake. 1996. Isolation of 5-(8 Z-heptadecenyl)-resorcinol from etiolated rice seedlings as an antifungal agent. PhytoChemistry 41:1485–1489. doi: 10.1016/0031-9422(95)00809-8.
- Tapiero, H., K.D. Tew, and G.N. Ba. 2002. Polyphenols do they play a role in the prevention of human pathologies. Biomed. Pharmacother. 56:200–207.
- Ueda, M., K.S. Sasaki, and N. Utsunimiya. 2000. Variation of total polyphenol and polyphenol oxidase activity during maturation of mango fruit (Mangifera Indica L. Irwin) cultured in a green plastic house. Food Sci. Technol. Res. 6:299–305. doi: 10.3136/fstr.6.299.
- Wang, X., T.H. Beckham, and J.C. Morris. 2008. Bioactivities of gossypol, 6-methoxygossypol, and 6, 6ʹ-dimethoxygossypol. J. Agric. Food Chem. 56(12):4393–4398. doi: 10.1021/jf073297u.
- Zarnowski, R., A. Kozubek, and S.J. Pietr. 1999. Effect of rye 5-n-alkylresorcinols on in vitro growth of phytopathogenic Fusarium and Rhizoctonia fungi. Bull. Pol. Acad. Sci. Biol. Sci. 47:231–235. doi: 10.1021/jf5054518.