ABSTRACT
There is an increasing demand for fresh fruits such as strawberry enriched in antioxidants and vitamins. Strawberry fruit quality is highly affected by genotype and environmental conditions. In this study, six day-neutral strawberry cultivars, Albion, Monterey, Portola, San Andreas, Seascape, and Sweet Ann were grown under low tunnels covered with three UV selective films: Standard Clear (STD), TIII TES/TR (TES), and Temp cool (TEM). STD and TES blocked 5% and 10% UV transmittance, respectively, whereas TEM blocked 40% UV transmittance and 15% visible/infrared light. Strawberries were harvested and evaluated for fruit color, total anthocyanins, total phenolics, total soluble solids. After refrigerated storage, the strawberries were evaluated again for these traits plus decay incidence and weight loss. Genotype (cultivar) was the major factor affecting fruit quality, metabolite composition, and percentage decay in storage. Weight loss during storage was the only trait not significantly affected by cultivar. Cultivar × film type interaction effects were significant for all strawberry quality parameters.
Introduction
From 1980 to 2015, consumption of fresh fruit increased from 5 million short tons to almost 8 million in US (Basu et al., Citation2010; USDA, Citation2016a). In 2016, the consumer price index for fresh fruit reached an historic high of 18.4% (USDA, Citation2016b). Strawberry (Fragaria ×ananassa Duchesne ex Rozier) is the fifth-most-consumed fresh fruit by US consumers, based on the USDA-ERS’s food availability data. The US is the second-largest strawberry producing country in the world and contributes 28.58% to the world’s strawberry market. The value of strawberry production has also increased from 106 million dollars to 2.4 billion dollars from 1970 to 2012 in the US (USDA, Citation2013).
Strawberries are low in calories and fat, and high in Vitamin C and phenolic flavonoid compounds such as anthocyanins and ellagic acid (Battino et al., Citation2009). Strawberries are also credited with preventing human diseases like cardiovascular disease (Basu et al., Citation2010; Huntley, Citation2009; Sesso et al., Citation2007), certain types of cancers (Carlton et al., Citation2001; Xue et al., Citation2001), Type 2 diabetes (Pinto et al., Citation2010), and neurodegeneration (Vauzour et al., Citation2010). Strawberry antioxidant potential and phenolic content are largely affected by cultivar and environmental factors in the production environment, such as weather, production system, and management techniques (Scalzo et al., Citation2005). Strawberry fruit is non-climacteric with no significant change in respiratory activity or ethylene biosynthesis during ripening (Kader, Citation2002; Knee et al., Citation1977). Thus, they must be harvested at full maturity to obtain maximum fruit quality in terms of both visual appearance and nutritional value (Mitcham, Citation1996). This requirement results in significant fruit loss during harvesting, transportation, and storage.
Several different kinds of strawberry production systems have been studied to determine their effects on antioxidant capacity as well as fruit yield and size. Examples include field cultural systems (Wang and Millner, Citation2009; Wang et al., Citation2002) including “annual plasticulture” with plastic mulch on raised beds (Shiukhy et al., Citation2015), soilless systems (Hernanz et al., Citation2007), and protected environments such as high tunnels (Castilho et al., Citation2015) and controlled growth environments (Wang et al., Citation2003; Wang and Zheng, Citation2001). Protected environments such as greenhouses, high tunnels, and low tunnels can increase fruit quality by protecting the fruit and plants from pests and weather extremes (Freeman and Gnayem, Citation2004), but some types can lead to increases in certain pests (Demchak, Citation2009; Xiao et al., Citation2001). Low tunnels are less likely to increase pest problems and result in higher yield and better fruit quality than strawberries grown in open fields (Lewers et al., Citation2017). Light quantity was found to be an important factor in improved strawberry production under low tunnels (Condori et al., Citation2017).
A range of novel plastic films have been developed for protected environments with film-specific radiation wavelengths to help growers better control their crop growth and development (Fletcher et al., Citation2004). These different-colored light-quality selective plastic films have also exhibited distinct effects in total phenolics and anthocyanin content (Miao et al., Citation2017, Citation2016). For example, with UV-blocking films, an increased biomass of leaves, stems and roots were observed in cucumber (Krizek et al., Citation1997). A decreased content of phenolic acids was reported in tomato fruit (Bacci et al., Citation1999). In eggplant, UV-blocking film increased size (Kittas et al., Citation2006). In strawberry, higher fruit fresh weight, lighter surface color and softer fruit at harvest were observed under UV-blocking film (Ordidge et al., Citation2012). Differences in UV light during production provided inconsistent findings with regard to phenolics content of strawberry (Ordidge et al., Citation2010; Xu et al., Citation2017; Zhao et al., Citation2007). Yet, UV light treatment has improved postharvest shelf life of ‘Kent’ strawberries (Baka et al., Citation1999). Systematic studies of the effect of multiple UV-blocking films on fruit quality and shelf life of multiple strawberry cultivars is lacking.
In this study, we investigated the effects of three different plastic films with different UV-transmission properties on strawberry fruit quality and shelf life of six cultivars. In particular, we studied the differences in the chemical compositions, and nutrient variation during cold storage.
Material and Methods
Field Preparation
The experimental field was established in 2015 on the North Farm of the USDA-ARS Henry A. Wallace Beltsville Agricultural Research Center at Beltsville, MD (39°01ʹ48.42”N, 76°56ʹ07.99”W, 49.4 m elevation), on Rumford series, coarse-loamy, siliceous, thermic Typic Hapludults soils, supplemented each year with potassium, sulfur, boron, and calcitic lime to correct deficiencies reported by annual soil tests. Day lengths ranged from 9 h, 25 min to 14 h, 54 min. Field preparation was as described by Lewers et al. (Citation2017). Briefly, crop rotation minimized tillage and managed pest populations. Neither fumigation nor fungicides were used to control pathogens.
Experimental Design, Cultivar and Film Treatment Assignments
In March, raised beds with trickle irrigation were covered with white-on-black plastic mulch. Six beds were used for the experiment. Low tunnels were erected over the six beds after planting as described by Lewers et al. (Citation2017). The six beds were divided into two blocks of three beds each for replication of plastic film treatment. Within each block, three plastic films, 4 mm-thick × 366-cm-wide, with differing light-transmission properties were randomly assigned to the three beds: Clear Greenhouse TIII film (standard clear, STD), Tufflite IV™ TES/IR film (TES), and TempCool™ film (TEM) (Berry Plastic Corporation, Greenville, SC). Each bed was divided in two blocks for replication of cultivar treatment within bed. Six cultivars were randomly assigned to six-plant plots within each block. A six-plant plot was the experimental unit, and there was a total of 72 six-plant plots in the experiment (three films × two beds per film × six cultivars × two cultivar reps per bed). The six “day-neutral” strawberry (Fragaria × ananassa) cultivars used in this study were: Albion (PP16, 228), Monterey (PP19, 767), Portola (PP20, 552), San Andreas (PP19, 975), Seascape (PP7, 614), and Sweet Ann (PP22, 472). Dormant bare-root plants (Lassen Canyon Nursery, Redding, CA) were hand-planted in pre-moistened soil in March. Border beds and plots were used to eliminate border effects: two beds with low tunnels bordered the sides of the experimental beds, and a six-plant plot of ‘Albion’ was planted at each end of each bed.
Measuring Total Transmittance of Films
Directional-hemispherical transmittance of the plastic sheeting was measured with a 50-mm integrating sphere accessory (RSA-PE-20, Labsphere Inc. North Sutton, NH) for a spectrometer (PerkinElmer Lambda 40, Boston, MA) across the 190 to 1100 nm wavelength range at 1 nm intervals. A Spectralon standard (Labsphere Inc. North Sutton, NH) was used for calibration. Multiple locations on each sample were measured and mean transmittance factors for each sample were calculated (Daughtry et al., Citation1989).
Plant Growth, Harvesting and Postharvest Storage
To establish the plants, flowers and stolons were removed, and the planting holes were weeded. After establishment, about 6 weeks, flower removal ceased but removal of weeds and runners continued through the growing season. The field was fertigated weekly, alternating between calcium nitrate, to increase fruit firmness, and potassium nitrate, to increase fruit sweetness. During establishment, the rate of nitrogen was with 5.6 kg∙ha–1, and was reduced to 3.4 kg∙ha–1 after establishment. Fully ripe strawberries from six harvests were hand-harvested from each six-plant plot twice weekly. Twenty four fruits for each cultivar from each harvest were further selected based on the absence of physical damage, and on uniformity of size, shape, and color. These strawberries were placed with the calyx down (for consistency) in clear plastic egg cartons which were stacked in plastic egg boxes for immediate transport to a storage room set at 4°C with 85% relative humidity. The boxes were stacked two high and the stack was draped with a black unscented plastic garbage bag to help retain humidity.
Determination of External Color and Firmness
Strawberry external color was evaluated with a CR-400 colorimeter (Konica Minolta Sensing, INC, Japan). The chromaticity coordinates are L* for lightness, a* for the red to green variation (-greenness to +redness) and b* for the yellow to blue variation (-blueness to +yellowness). Chroma (C) which indicates the overall freshness and intensity of the fruit color was calculated from fruit surface redness (a*) and blueness (b*) by the equation C = (a*2 + b*2)1/2. Hue angle is a basic unit of color and was calculated by the equation h = tan−1(b*/a*) and can be interpreted, for example, as 0° = red and 90° = yellow. Readings were taken from both sides of each strawberry, using a total of 24 fruits for each sample.
Firmness was measured, using a TA-XTplus Texture Analyzer (Texture Technologies Corp, NY), as the maximum penetration force (N) reached during tissue breakage, and determined with a 10 mm diameter cylinder probe, penetration depth of 3 mm, and cross-head speed of 1 mm s−1. Strawberries were sliced into halves and each half was measured in the central zone. Fruit firmness values were averaged from measures of three fruits for each sample. After firmness analysis, strawberries were cut into small pieces, crushed with liquid nitrogen and freeze-dried for total phenolics and anthocyanin assays.
Determination of Total Soluble Solids (TSS), Total Phenolic Content (TPC) and Total Anthocyanin Content (TAC)
Total phenolic content (TPC). TPC was determined using a method described by Lester et al. (Citation2012) with small modifications as follows. Briefly, 100 mg of lyophilized strawberry fruit powder was extracted with 5 mL of 80% methanol. The samples were then centrifuged at 6,650 g for 10 min. Then 25 μL of 0.1% Fast Blue BB solution was added to 250 μL aliquots of the supernatants, followed by 25 μL of 5% NaOH. The absorbance of the mixtures was read at 420 nm using EonTM High-performance microplate spectrophotometer (Bioteck Instruments, Inc). Results were expressed as micrograms of gallic acid equivalent per gram of dry weight. Reactions were done in triplicate.
Total anthocyanin content (TAC). The same extraction was used for TAC by the method of Lee et al. (Citation2005). Total anthocyanin content was estimated by a pH differential method. Absorbance was measured at 510 nm and at 700 nm in buffer at pH 1.00 and pH 4.5, using A = (A510 − A700)pH1.0 − (A510 − A700)pH4.5 with a molar extinction coefficient for pelargonidin-3-glucoside of 15,600. Results were expressed as micrograms of pelargonidin-3-glucoside equivalent per gram of dry weight using a calibration curve for pelargonidin-3-glucoside. The calibration curve (y = 0.0278x + 0.0445, where y is absorbance and x is sample concentration) ranged from 10 to 1000 μg/mL (R2 = 0.9989).
Total soluble solids (TSS). The TSS of strawberry puree was measured with an Atago PR-101 digital refractometer (Atago Co. Ltd., Tokyo, Japan) at room temperature and expressed as Brix%. Measurements were done in triplicate.
Determination of Fruit Decay and Water Loss during Storage
The strawberries were weighed individually on the harvest date, and thereafter every week during the storage period. Water loss was expressed as the percentage loss of the initial fruit weight. The results from four out of six harvests were presented due to decay loss of two harvests during storage. In addition, strawberries were visually inspected daily during the storage period for the presence of molds. Strawberries showing surface mycelial development were considered decayed and were discarded to prevent fruit-to-fruit contamination during storage. Decay incidence was expressed as the percentage of decayed fruits on the measuring days over fruits on day 0.
Statistical Analysis
SPSS statistics software (SPSS for Windows, SPSS Inc., Chicago, IL) and Prism graphing illustration software (Graphpad Prism for windows, Graphpad software, Inc. La Jolla, CA) was used for statistical analysis in order to determine the contributions of genotypes, films and interactions between genotypes and films to each quality trait. Factorial design analysis of variance (ANOVA) was performed using a general linear model, with genotype (cultivar) and plastic films as factors. There were six cultivars and three film types, with four replicates for each fruit sample. Where cultivar × film interaction effects were significant, Tukey’s HSD post hoc test was performed to compare differences between means. Correlation analyses were performed using a two-tailed Pearson’s correlation test. Statistical significance was declared at P < .05.
Results and Discussion
Difference of UV Transmittance among Films
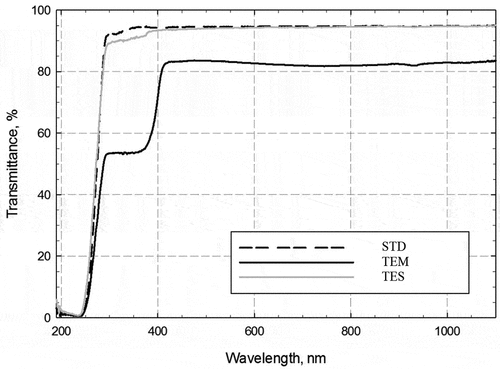
In this study, UV-blocking film and UV-attenuated film were compared with UV transmitting film and the total percentages of light transmittance at 200–1100 nm wavelength was measured (). STD blocked about 5% UV light between ~250–400 nm, TES blocked almost 10% UV light and TEM blocked about 40% UV light. STD and TES blocked about the same amount of visible light and infrared light from 400–1100nm, including photosynthetically active light. TEM blocked 15% visible and infrared light at 400–1100nm. Thus light transmission was somewhat similar for STD and TES, but significantly reduced for TEM.
Effect of Films and Genotypes on Strawberry Fruit Quality on Harvest Day
Strawberry Fruit Firmness
Although film type contributed significantly to the overall variation in strawberry firmness on harvest day (N), the cultivar × film interaction contributed more, and the most variation was from cultivar differences (, ). Fruit produced under TEM, which blocked more UV light than the other films, were generally softer (7.73N-average of all six genotypes) than fruit grown under STD (8.19N) and TES (8.10N), appearing to support previous findings that used just one cultivar (Ordidge et al., Citation2012). But fruit firmness differences between films were not significant for any of the cultivars (), as the significant cultivar × film interaction effects () were contradictory. TES film resulted in firmer fruit for ‘Albion’, ‘Monterey’, and ‘Portola’, but the differences were not significant (), and STD film resulted in firmer fruit for ‘San Andreas’, ‘Seascape’, ‘Sweet Ann’, but these differences also were not significant (). Cultivar differences contributed most to total variation (), and differences in fruit firmness on harvest day were observed between ‘Seascape’ and ‘San Andreas’ under all three films (). ‘San Andreas’ produced the firmest fruit, but differences were significant only under STD film. ‘Seascape’ was significantly softer than all the other cultivars under all three films.
Table 1. Fruit surface color, texture, total soluble solids, total phenolic contents and total anthocyanin contents of on harvest day.
Table 2. Genotype vs Film types contribution among fruit quality attributes.
Strawberry Fruit Color
Film type made no difference in fruit-color lightness, as measured by L value (). There were no differences in average L value among the films across cultivars, and none of the cultivars were significantly lighter under one film vs. another. However, cultivar differences were significant. The average L value for ‘Sweet Ann’ was significantly higher than for the other three cultivars under all three films (). The visual appearance of ‘Sweet Ann’ strawberries was noticeably different from the others; they appeared cleaner and fresher. Perhaps part of the reason was the lightness of the fruit as was measured by L value.
Strawberries produced under TES film were generally redder (38.95) than those produced under TEM film (38.43), as reported by a* value where higher values are more red (). But these differences were not consistent across cultivars. For any individual cultivar, none of the films made a significant difference in a* value, even though general trends were observed that appeared like interaction effects. Strawberries produced under TES film were generally redder for ‘Albion’, ‘Portola’, ‘San Andreas’, and ‘Seascape’, but ‘Monterey’, though ‘Sweet Ann’ strawberries were reddest when grown under STD film. Strawberries produced under TEM film were generally less red for ‘Portola’, ‘San Andreas’, ‘Seascape’, and ‘Sweet Ann’, but STD film resulted in the least red strawberries from ‘Albion’, and TES film resulted in the least red fruit from ‘Monterey’. Cultivar differences were more evident and were statistically significant. ‘San Andreas’ strawberries were reddest, and the difference was significant under STD and TES films (). ‘Albion’ strawberries were least red, and the difference was significant under STD film.
Chroma (C) was calculated from fruit surface redness (a*) and blueness (b*) by the equation C = (a*2 + b*2)1/2. Although film type contributed significantly to the overall variation in chroma, the cultivar × film interaction contributed more, and the most variation was from cultivar differences (). Strawberries produced under TEM film, with the lowest UV transmission, had generally lower C values for most of the cultivars; exceptions were ‘Monterey’ and ‘Sweet Ann’, which produced strawberries with slightly lower C values under TES film. ‘Albion’ strawberries had the lowest C value, and ‘Sweet Ann’ strawberries had the highest C value under all three films, and these differences were significant for both the STD and TEM films (). It’s possible that the visually observed clean, fresh appearance of ‘Sweet Ann’ strawberries was partly due to its higher chroma in addition to its lightness.
Fruit Chemical Content
Strawberry total soluble solids (TSS or %Brix) in this study ranged from 6.07% to 9.88% (). Film type in production made little difference in strawberry average TSS, compared with the effect of genotype (cultivar) differences (). The dependence of TSS on genotypes (cultivars) was also reported for strawberries grown in Italy (Sabatino et al., Citation2017). ‘Sweet Ann’ strawberries contained highest TSS although the color of the berries was the least red, and this was significant when grown under STD film. ‘Portola’ strawberries showed significantly least TSS when grown under all three films even though the berries were redder than ‘Sweet Ann’ (). Hence, the fruit maturity (redness) is not necessary correlated to fruit TSS as compared between different genotypes. However, the STD film type-maximized separation of TSS means for the group of cultivars studied. TSS is the most important factor in the strawberry eating experience (Colquhoun et al., Citation2012). Perhaps, all other factors aside, use of STD film by strawberry breeding programs may result in a greater ability to more easily identify the high TSS potential strawberry cultivars for mid-summer production in the Mid-Atlantic.
Although none of the individual cultivars produced fruit with significantly higher total phenolics content (TPC) under any of the films compared with any other film (), strawberry fruit was generally higher when produced under TEM film (), the film with reduced UV transmission. In contrast, tomato fruit under ambient UV light displayed higher TPC than those under UV-blocking films (Ordidge et al., Citation2010). The UV-blocking film used by Ordidge et al. (Citation2010) did not block photosynthetically active radiation (PAR, 400nm – 700nm), while the UV-blocking film used in the current study (TEM) blocked PAR by 15%. This difference may contribute to the condradiction in results from this study and Ordidge et al. (Citation2010), and indicate that PAR and UV light may both affect accumulation of phenolics accumulation in fruit. Cultivar differences were the greatest source of variation for TPC. ‘Monterey’, ‘Seascape’, and ‘San Andreas’ all had significantly higher TPC than ‘Portola under all three films ().
The positive correlation between TAC and TPC (0.716*) was significant. Film type did not have any effect of total anthocyanin content (TAC) (). Josuttis et al. (Citation2010) also found TAC levels to be unresponsive to UV. The greatest source of variance for anthocyanin content was cultivar differences, as even the cultivar × film variation for TAC was not significant (). ‘Sweet Ann’ strawberry fruit anthocyanin content was significantly lower than that of strawberries from all the other cultivars. ‘Sweet Ann’ strawberries also had the highest chroma (C) levels and lightness (L) values (). A significant negative correlation observed in this study between chroma (C) and total anthocyanin content (TAC) (−0.640**) (). A major anthocyanin contributing to strawberry fruit color is pelargonidin 3-glucoside (Tonutare et al., Citation2014).
Table 3. Pearson correlation coefficients of TPC, TSS, Color (C), Texture, TAC, Decay ratio and weight loss as fruit quality attributes.
Effect of Film and Genotype on Fruit Quality during Cold Storage
Fruit Decay in Storage
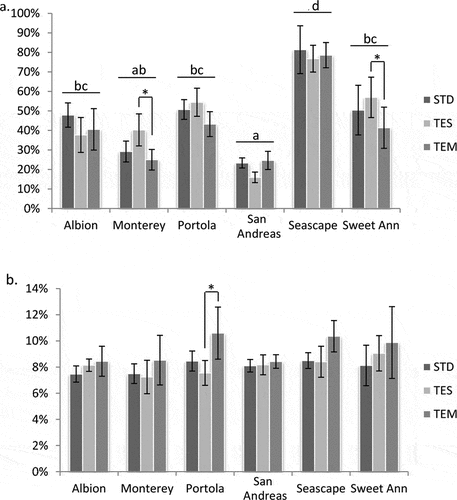
Fruit decay is a very important aspect of shelf life. Decay incidence in storage was affected significantly by film type, and significant cultivar × film interaction effects were observed (). Both ‘Monterey’ and ‘Sweet Ann’ strawberries had significantly lower percentage decay when produced under TEM film compared with TES film, with the strawberries produced under STD film having an intermediate percentage decay for these two cultivars (). The greatest source of total variation in decay of strawberries after 2 weeks in storage was the differences between cultivars (). The average percentage of decayed berries across film types for ‘San Andreas’, ‘Monterey’, ‘Albion’, ‘Sweet Ann’, ‘Portola’ and ‘Seascape’ were 21.4%, 32.1%, 40.3%, 49.4%, 49.9% and 78.7%, respectively. ‘San Andreas’ strawberries had the lowest percentage of decayed fruit, significantly lower than all but ‘Monterey’ when averaged across film types (). ‘Seascape’ had a significantly higher percentage of decayed strawberries than all the other cultivars. The percentage of decayed strawberries after 2 weeks in storage was significantly correlated with the firmness (N) of the strawberries at harvest (r2 = −0.789**) but not with any other trait (). ‘San Andreas’ strawberries were significantly firmer than ‘Seascape’ strawberries, and ‘Seascape’ strawberries were significantly softer than those of all other cultivars grown under all three films ().
Figure 2. Fruit shelf life changes of different genotypes under three plastic films. a. decay ratio of strawberry fruit with mycelia development after 2-weeks storage at 4°C. b. fruit weight loss after 2-weeks storage at 4°C. The data are based on six biological replicates. Letters represent the statistical differences between cultivars. * represent significant difference between film types (P < .05). STD, standard clear; TEM, Temp Cool.
Fruit Weight Loss in Storage
Another very important aspect of shelf life is water loss in storage. The dehydration, shrinkage, and wrinkling of fruit during storage is important to the appearance of strawberries on store shelves. Strawberry fruit is highly susceptible to rapid water loss due to respiration rate and the evaporation of moisture from the strawberry to the surrounding air through its extremely thin “skin” (Bartz and Jeffrey, Citation2002; Petriccione et al., Citation2015). Weight loss in storage was the only trait measured in this study for which the greatest source of variation was film type and not cultivar differences, and differences between cultivars were not significant (). Weight loss of strawberries in storage ranged from 7% to 10% and was generally greater for strawberries grown under TEM film (). The treatment difference in weight loss was significant only for ‘Portola’ strawberries grown under TEM film compared with ‘Portola’ strawberries grown under TES film (), which also explains the significant cultivar × film interaction effect ().
Fruit Firmness in Storage
An increase in strawberry firmness during cold storage was reported previously for the cultivars Diamante and Selva (Pelayo et al., Citation2003). The cultivar-specific changes in firmness observed during cold storage were explained as being due to different patterns of pectin and cellulose matrix disassembly during storage (Jimenez-Bermudez et al., Citation2002; Knee et al., Citation1977; Santiago-Domenech et al., Citation2008) which depended highly on genotype. In the current study, when the strawberries were measured for firmness on the day of harvest, then one and two weeks later, there were no significant changes in firmness over time for strawberries produced from any of the cultivars, when averaged across film type (Figures S1d, S2a, S2d).
Fruit Color in Storage
When averaged across film types, color lightness (L) did not change for any of the cultivars in 2 weeks of cold storage (, ). However, ‘Albion’ strawberries produced under STD film darkened significantly more than when produced under TES film, and ‘San Andreas’ strawberries produced under STD film darkened significantly more than when produced under TEM film (, ).
Figure 3. Changes on fruit surface color of different genotypes under three plastic films. a-d. Chroma (a), Lightness (b) and Redness (c) of fruits on harvest day. e-h. Chroma (d), Lightness (e) and Redness (f) of fruits after 2-weeks storage at 4°C. The data are based on six biological replicates. Letters represent statistical differences between cultivars (above line) or between film types (underneath line). STD, standard clear; TEM, Temp Cool.
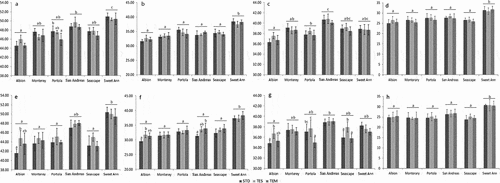
When averaged across film types, changes in redness (a*) from higher to lower values were significant only for ‘San Andreas’ strawberries. At harvest, ‘San Andreas’ strawberries were reddest, and the difference was significant under STD and TES films (). After storage, ‘San Andreas’ strawberries lost some redness (, , S3) and were significantly redder than only the ‘Albion’ berries, which were the least red at harvest (). Generally strawberries grown under TES film stayed redder in cold storage, but production-film differences were not always significant, and cultivar × film type interaction effects were significant (, ). ‘Seascape’ strawberries grown under TES film stayed significantly redder in storage than when grown under either STD or TEM. ‘Albion’ strawberries stayed significantly redder in storage when grown under TES film instead of STD film, and ‘Portola’ berries stayed significantly redder in storage when grown under TES film instead of TEM film.
Chroma (C) was calculated from fruit surface redness (a*) and blueness (b*) at harvest and after cold storage by the equation C = (a*2 + b*2)1/2. When averaged across film types, the drop in chroma value (C) was significant only for ‘Sweet Ann’ strawberries (Figures S1a, 3a, 3d), which had the highest chroma value at harvest (). Cultivar × film interaction effects were significant (, ), but there no general trend like that observed for redness (a*). Chroma for ‘Albion’ strawberries dropped more when produced under STD film instead of TES film (, ).
Fruit Chemical Content
Previously, changes in sweetness during cold storage were reported to be dependent on cultivar (Cordenunsi et al., Citation2005; Shin et al., Citation2007). In the current study, when averaged across film types, any apparent changes in sweetness (%Brix or TSS) during storage were not significant (Figure S1e). ‘Sweet Ann’ strawberries maintained their superior TSS in cold storage. When averaged across film types, total phenolic content of ‘Albion’ and ‘Seascape’ strawberries dropped significantly in 2-weeks cold storage, while TPC reductions of strawberries from the other cultivars were not significant (Figures S2c, S2f). Slightly reductions of TPC during cold storage was also observed for established cultivars but not in new-bred cultivars (Sabatino et al., Citation2017). Reductions in TPC were not dependent on film type, as there were no significant cultivar × film interaction effects. Any apparent changes in TPC were not lasting (Figure S1e). Any apparent changes in total anthocyanin content were not significant or lasting for any of the cultivars, film types, or their interaction effects (Figures S1g, S2b, S2e).
Altogether, our results confirm that all the fruit quality parameters measured were mainly determined by genotypes. Based on the molecular markers, Sabatino et al. (Citation2017) were able to identify the sources of better agronomy and quality traits in new breeding lines. Further analysis of genetic relationship among the cultivars used in this study will help identify the source of better quality traits breed the new cultivars with better quality.
Conclusion
In this study, we investigated the effect of UV-selecting films on quality and shelf life of six strawberry cultivars. Cultivar/genotype differences were the primary source of variation observed, ranging from 49% to 81% of total variation, for all quality parameters monitored, except for weight loss in cold storage. For weight loss, film type was the primary contributor of variation at over 17%. Yet film type during production significantly affected weight loss in storage for fruit of only one cultivar. Cultivar × film type interaction effects were significant for all strawberry quality parameters. When considering growing low-tunnel strawberries under the three films tested in this study, choice of cultivar should be considered over film type. For the cultivar selected, whether any of the films had a significant effect on key traits affecting profit should be considered. If not, then the availability and cost of the films can be the main consideration.
Acknowledgments
This project was funded by USDA-ARS Projects 8042-43000-015-00D and 8042. The authors wish to thank Jonathan Alcazar, John Enns, Philip Edmonds, Jessica Lahocki, and the BARC Research Farm Services, for their contributions to this research. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the US Dept. of Agriculture or any of the other agencies involved in this research, and does not imply its approval to the exclusion of other products or vendors that also may be suitable.
Additional information
Funding
Literature cited
- Bacci, L., D. Grifoni, F. Sabatini, and G. Zipoli. 1999. UV-B radiation causes early ripening and reduction in size of fruits in two lines of tomato (Lycopersicon esculentum Mill.). Glob. Chang. Biol. 5(6):635–646. doi: 10.1046/j.1365-2486.1999.00258.x.
- Baka, M., J. Mercier, R. Corcuff, F. Castaigne, and J. Arul. 1999. Photochemical treatment to improve storability of fresh strawberries. J. Food Sci. 64(6):1068–1072. doi: 10.1111/jfds.1999.64.issue-6.
- Bartz, J.A., and K.B. Jeffrey. 2002. Postharvest physiology and pathology of vegetables. 2nd ed. Boca Raton, USA: CRC Press.
- Basu, A., M. Rhone, and T.J. Lyons. 2010. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 68(3):168–177. doi: 10.1111/j.1753-4887.2010.00273.x.
- Battino, M., J. Beekwilder, B. Denoyes-Rothan, M. Laimer, G.J. McDougall, and B. Mezzetti. 2009. Bioactive compounds in berries relevant to human health. Nutr. Rev. 67:S145–S150. doi: 10.1111/j.1753-4887.2009.00178.x.
- Carlton, P.S., L.A. Kresty, J.C. Siglin, M.A. Morse, J. Lu, C. Morgan, and G.D. Stoner. 2001. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis 22(3):441–446. doi: 10.1093/carcin/22.3.441.
- Castilho, R.C., V.S. Duarte, G.J. de Moraes, K. Westrum, N. Trandem, L.C.D. Rocha, I. Delalibera, and I. Klingen. 2015. Two-spotted spider mite and its natural enemies on strawberry grown as protected and unprotected crops in Norway and Brazil. Exp. Appl. Acarol. 66(4):509–528. doi: 10.1007/s10493-015-9913-4.
- Colquhoun, T.A., L.A. Levin, H.R. Moskowitz, V.M. Whitaker, D.G. Clark, and K.M. Folta. 2012. Framing the perfect strawberry: An exercise in consumer-assisted selection of fruit crops. J. Berry Res. 2(1):45–61.
- Condori, B., D.H. Fleisher, and K.S. Lewers. 2017. Relationship of strawberry yield with microclimate factors in open and covered raised-bed production. Trans. ASABE 60(5):1511–1525. doi: 10.13031/trans.12371.
- Cordenunsi, B.R., M.I. Genovese, J.R.O. Do Nascimento, N.M.A. Hassimotto, R.J. Dos Santos, and F.M. Lajolo. 2005. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 91(1):113–121. doi: 10.1016/j.foodchem.2004.05.054.
- Daughtry, C.S.T., K.J. Ranson, and L.L. Biehl. 1989. A new technique to measure the spectral properties of conifer needles. Remote Sens. Environ. 27(1):81–91. doi: 10.1016/0034-4257(89)90039-4.
- Demchak, K. 2009. Small fruit production in high tunnels. HortTechnology 19(1):44–49. doi: 10.21273/HORTSCI.19.1.44.
- Fletcher, J.M., A. Tatsiopoulou, P. Hadley, F.J. Davis, and R.G.C. Henbest. 2004. GROWTH, YIELD AND DEVELOPMENT OF STRAWBERRY CV. ‘ELSANTA’ UNDER NOVEL PHOTOSELECTIVE FILM CLAD GREENHOUSES. 633 ed. International Society for Horticultural Science (ISHS), Leuven, Belgium. p. 99–106.
- Freeman, S., and N. Gnayem. 2004. Use of plasticulture for strawberry plant production. Small Fruits Rev. 4:21–32.
- Hernanz, D., A.F. Recamales, A.J. Melendez-Martinez, M.L. Gonzalez-Miret, and F.J. Heredia. 2007. Assessment of the differences in the phenolic composition of five strawberry cultivars (Fragaria x ananassa Duch.) grown in two different soilless systems. J. Agric. Food Chem. 55(5):1846–1852. doi: 10.1021/jf063189s.
- Huntley, A.L. 2009. The health benefits of berry flavonoids for menopausal women: Cardiovascular disease, cancer and cognition. Maturitas 63(4):297–301. doi: 10.1016/j.maturitas.2009.05.005.
- Jimenez-Bermudez, S., J. Redondo-Nevado, J. Munoz-Blanco, J.L. Caballero, J.M. Lopez-Aranda, V. Valpuesta, F. Pliego-Alfaro, M.A. Quesada, and J.A. Mercado. 2002. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiol. 128(2):751–759. doi: 10.1104/pp.010671.
- Josuttis, M., H. Dietrich, D. Treutter, F. Will, L. Linnemannstons, and E. Kruger. 2010. Solar UVB response of bioactives in strawberry (Fragaria x ananassa Duch. L.): a comparison of protected and open-field cultivation. J. Agric. Food Chem. 58(24):12692–12702. doi: 10.1021/jf102937e.
- Kader, A.A. 2002. Postharvest technology of horticultural crops. 3rd ed. University of California, Oakland.
- Kittas, C., A. Tchamitchian, N. Katsoulas, P. Karaiskou, and C. Papaioannou. 2006. Effect of two UV-absorbing greenhouse-covering films on growth and yield of an eggplant soilless crop. Sci. Hortic. 110(1):30–37. doi: 10.1016/j.scienta.2006.06.018.
- Knee, M., J.A. Sargent, and D.J. Osborne. 1977. Cell-wall metabolism in developing strawberry fruits. J. Exp. Bot. 28(103):377–396. doi: 10.1093/jxb/28.2.377.
- Krizek, D.T., R.M. Mirecki, and S.J. Britz. 1997. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cucumber. Physiol. Plant. 100(4):886–893. doi: 10.1111/ppl.1997.100.issue-4.
- Lee, J., R.W. Durst, and R.E. Wrolstad. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 88(5):1269–1278.
- Lester, G.E., K.S. Lewers, M.B. Medina, and R.A. Saftner. 2012. Comparative analysis of strawberry total phenolics via fast blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Compos. Anal. 27(1):102–107. doi: 10.1016/j.jfca.2012.05.003.
- Lewers, K.S., D.H. Fleisher, and C.S.T. Daughtry. 2017. Low tunnels as a strawberry breeding tool and season-extending production system. Int. J. Fruit Sci. 17:1–26. doi: 10.1080/15538362.2017.1305941.
- Miao, L.X., Y.C. Zhang, X.F. Yang, J.P. Xiao, H.Q. Zhang, G.H. Jiang, Z. Zhang, Y. Wang, and G. Jiang. 2017. Fruit quality, antioxidant capacity, related genes, and enzyme activities in strawberry (Fragaria ×ananassa) grown under colored plastic films. HotScience 52(9):1241–1250. doi: 10.21273/HORTSCI12062-17.
- Miao, L.X., Y.C. Zhang, X.F. Yang, J.P. Xiao, H.Q. Zhang, Z.F. Zhang, Y.Z. Wang, and G.H. Jiang. 2016. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria x ananassa) fruit. Food Chem. 207:93–100. doi: 10.1016/j.foodchem.2016.02.077.
- Mitcham, B. 1996. Quality assurance for strawberries: A case study. Perishables Handling Newslett. (85):3.
- Ordidge, M., P. Garcia-Macias, N.H. Battey, M.H. Gordon, P. Hadley, P. John, J.A. Lovegrove, E. Vysini, and A. Wagstaffe. 2010. Phenolic contents of lettuce, strawberry, raspberry, and blueberry crops cultivated under plastic films varying in ultraviolet transparency. Food Chem. 119(3):1224–1227. doi: 10.1016/j.foodchem.2009.08.039.
- Ordidge, M., P. Garcia-Macias, N.H. Battey, M.H. Gordon, P. John, J.A. Lovegrove, E. Vysini, A. Wagstaffe, and P. Hadley. 2012. Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 92(8):1597–1604. doi: 10.1002/jsfa.4744.
- Pelayo, C., S.E. Ebeler, and A.A. Kader. 2003. Postharvest life and flavor quality of three strawberry cultivars kept at 5 degrees C in air or air+20 kPa CO2. Postharvest Biol. Technol. 27(2):171–183. doi: 10.1016/S0925-5214(02)00059-5.
- Petriccione, M., F. Mastrobuoni, M.S. Pasquariello, L. Zampella, E. Nobis, G. Capriolo, and M. Scortichini. 2015. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 4(4):501–523. doi: 10.3390/foods4040501.
- Pinto, M.D., J.E. de Carvalho, F.M. Lajolo, M.I. Genovese, and K. Shetty. 2010. Evaluation of antiproliferative, anti-type 2 diabetes, and antihypertension potentials of ellagitannins from strawberries (Fragaria x ananassa Duch.) using in vitro models. J. Med. Food 13(5):1027–1035. doi: 10.1089/jmf.2009.0257.
- Sabatino, L., C. De Pasquale, F. Aboud, F. Martinelli, M. Busconi, E. D’Anna, S. Panno, G. Iapichino, and F. D’Anna. 2017. Properties of new strawberry lines compared with well-know cultivars in winter planting system conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 45(1):9–16. doi: 10.15835/nbha45110477.
- Santiago-Domenech, N., S. Jimenez-Bemudez, A.J. Matas, J.K.C. Rose, J. Munoz-Blanco, J.A. Mercado, and M.A. Quesada. 2008. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J. Exp. Bot. 59(10):2769–2779. doi: 10.1093/jxb/ern142.
- Scalzo, J., A. Politi, N. Pellegrini, B. Mezzetti, and M. Battino. 2005. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21(2):207–213. doi: 10.1016/j.nut.2004.03.025.
- Sesso, H.D., J.M. Gaziano, D.J.A. Jenkins, and J.E. Buring. 2007. Strawberry intake, lipids, C-reactive protein, and the risk of cardiovascular disease in women. J. Am. Coll. Nutr. 26(4):303–310. doi: 10.1080/07315724.2007.10719615.
- Shin, Y., R.H. Liu, J.F. Nock, D. Holliday, and C.B. Watkins. 2007. Temperature and relative humidity effects on quality, total ascorbic acid, phenolics and flavonoid concentrations, and antioxidant activity of strawberry. Postharvest Biol. Technol. 45(3):349–357. doi: 10.1016/j.postharvbio.2007.03.007.
- Shiukhy, S., M. Raeini-Sarjaz, and V. Chalavi. 2015. Colored plastic mulch microclimates affect strawberry fruit yield and quality. Int. J. Biometeorol. 59(8):1061–1066. doi: 10.1007/s00484-014-0919-0.
- Tonutare, T., U. Moor, and L. Szajdak. 2014. Strawberry anthocyanin determination by pH differential spectroscopic method - How to get true results. Acta Sci. Pol. Hortorum. Cultus. 13(3):35–47.
- USDA. 2013. Fruit and tree nuts outlook FTS-361. USDA Economic Research Service.
- USDA. 2016a. U.S strawberry industry 95003. USDA Economic Research Service.
- USDA. 2016b. USDA fruit year book_noncitrus fruit production and value, price, yield per acre, and bearing acreage. USDA Economic Research Service.
- Vauzour, D., K. Vafeiadou, C. Rendeiro, G. Corona, and J.P. Spencer. 2010. The inhibitory effects of berry-derived flavonoids against neurodegenerative processes. J. Berry Res. 1(1):45–52.
- Wang, S.Y., J.A. Bunce, and J.L. Maas. 2003. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 51(15):4315–4320. doi: 10.1021/jf021172d.
- Wang, S.Y., and P. Millner. 2009. Effect of different cultural systems on antioxidant capacity, phenolic content, and fruit quality of strawberries (Fragaria x aranassa Duch.). J. Agric. Food Chem. 57(20):9651–9657. doi: 10.1021/jf9020575.
- Wang, S.Y., and W. Zheng. 2001. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 49(10):4977–4982. doi: 10.1021/jf0106244.
- Wang, S.Y., W. Zheng, and G.J. Galletta. 2002. Cultural system affects fruit quality and antioxidant capacity in strawberries. J. Agric. Food Chem. 50(22):6534–6542. doi: 10.1021/jf020614i.
- Xiao, C.L., C.K. Chandler, J.F. Price, J.R. Duval, J.C. Mertely, and D.E. Legard. 2001. Comparison of epidemics of Botrytis fruit rot and powdery mildew of strawberry in large plastic tunnel and field production systems. Plant Dis. 85(8):901–909. doi: 10.1094/PDIS.2001.85.8.901.
- Xu, Y., M.T. Charles, Z. Luo, B. Mimee, P. Vernneau, D. Rolland, and D. Roussel. 2017. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 65(46):9970–9979.
- Xue, H.W., R.M. Aziz, N.J. Sun, J.M. Cassady, L.M. Kamendulis, Y. Xu, G.D. Stoner, and J.E. Klaunig. 2001. Inhibition of cellular transformation by berry extracts (vol 22, pg 351, 2001). Carcinogenesis 22(5):831–833. doi: 10.1093/carcin/22.5.831.
- Zhao, X., E.E. Carey, J.E. Young, W. Wang, and T. Iwamoto. 2007. Influences of organic fertilization, high tunnel environment, and postharvest storage on phenolic compounds in lettuce. HortScience 42(1):71–76. doi: 10.21273/HORTSCI.42.1.71.