?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The use of drought-tolerant rootstocks is one of the available solutions for the cultivation of pear in semi-arid areas. In order to achieve drought-tolerant rootstocks, seeds of Pyrus syriaca, and Pyrus salicifolia species as well as Pyrus communis cv. Spadona, Khoj no. 1 and Khoj no. 2 were cultivated in the field conditions. This research was carried out as a factorial experiment with two factors of pear species (in five levels) and water stress (in two levels of control and drought stress) based on a randomized complete block design. In drought treatment, the irrigation time was considered based on 80% of allowed water depletion. In control blocks, normal irrigation was performed. The experiment began in July and continued to late September. In stress conditions, P. communis cv. Spadona and Khoj no. 1 had the highest seedling height and stem diameter and P. salicifolia and P. communis cv. Khoj no. 2 had the lowest of the rate of these traits. The lowest increase in electrolyte leakage (EL) and the highest proline content were in P. communis cv. Khoj no. 2 and P. salicifolia and the highest EL and the lowest proline were observed in P. communis cv. Spadonain under drought stress conditions. The most and the least relative water content (RWC) were belonged to P. communis cv. Khoj no. 2 and Spadona, respectively, in stress conditions. According to the studied traits, P. salicifolia and P. communis cv. Khoj no. 2 populations were more tolerant to drought stress.
Introduction
Pyrus species have been growing under Mediterranean climate for thousands of years (Reales et al., Citation2010). All wild species of pears are scattered from the east to the west in the northern hemisphere. About half of these species are native to Europe and North Africa and Asia Minor and the Mediterranean coast, and others are native to Asia. There are 12 wild pear species in Iran from 22 identified pear species in the world (Sabeti, Citation1995). In some regions of the world, these species are used as the rootstock for pear commercial cultivars. For example, a large number of pear cultivars in Turkey are grafted on P. elaeagrifolia, in Syria and Lebanon on P. syriaca, in ancient Yugoslavia, Turkey and Greece on P. amygdaliformis, in the south of Russia on P. Salicifolia and in Algeria and Morocco are grafted on P. longipes (Henareh, Citation2015).
Water deficit causes drought stress across plant tissues as root water uptake is lower than leaf transpiration (Aroca et al., Citation2012), resulting in a decline in plant growth and productivity. Trees have developed many sophisticated mechanisms to survive under drought stress. These mechanisms involve both physical and biological processes operating at the cellular and whole-organism level (Nardini et al., Citation2011). Drought stress effects on the vegetative characteristics of trees, including height, fresh and dry weight of the organs, the number and surface area of the leaves (Reddy et al., Citation2004). The relative water content of the leaves can be used to select genotypes that maintain cell turgor under water stress environment (Meher et al., Citation2018). The higher RWC means the ability of the leaf to retain more water or the ability of the root to absorb more water under stress conditions. Environmental stresses, such as drought, change cell membranes that often accompanied by an increase in their permeability. In these conditions, the sulfidryl bands of the membrane oxidize to the S-S bands (Blokhina et al., Citation2003). The ability of the cell membrane to control the amount of ions that transfer in and out of the cell is used as a criterion of damage to the tissues in stress condition (Blokhina et al., Citation2003). Plants tend to overcome drought stress with a process known as osmotic regulation, and reduce their osmotic potential by the accumulation of soluble materials. Proline is one of the most important compatible solutions that play an important role in osmotic regulation and cellular protection (Pinhero et al., Citation2001). Plant accrues certain osmolytes (proline) and sugars (raffinose and sorbitol) to prevent membrane disintegration and enzyme inactivation to diminish the turgor potential along with detoxification of reactive oxygen species by reestablishing the cellular redox level (Haider et al., Citation2018).
Climate change and declining rainfall in recent years have led to drought and its destructive effects on horticulture. The selection of drought-tolerant species with low water requirements is one of the solutions to overcome the drought problem in arid and semi-arid regions (Cheruth et al., Citation2009). Pear is one of the crops that has affected by drought stress in recent years. The pear and quince are used as rootstocks for pear trees. The quince rootstocks are very dwarf (EM-C) and semi-dwarf (BA29) that are used for different pear cultivars, but these rootstocks could not overcome the problem of graft incompatibility with some pear cultivars. These rootstocks are not also tolerant of winter frosts and chlorosis caused by iron deficiency in the calcareous soils (Harotko, Citation2007). Various breeding methods are used to produce new cultivars and rootstocks of pear and other species of fruit trees. Seed cultivation after cross or open pollination is one of the breeding methods that frequently used in breeding programs. In this way, it is possible to achieve a wide variation for choice of new rootstocks and cultivars. For example, the Manon cultivar was obtained from the open pollination of Beurre Bosc cultivar. The Pib-BU3 pear dwarf rootstock was also obtained from open pollination of P. longipesas, and Pi-BU4 and Pi-BU7 rootstocks were obtained from open pollination of the P. pyrifolia species (Mohan Jain and Priyadarshan, Citation2009). Wild germplasm has evolved in natural dry ecosystems for the tolerance of stress conditions such as high temperatures, drought and salinity. The identification of wild pear germplasm is very important to use as a rootstock in semi-arid regions (Zarafshar et al., Citation2014). It has been reported that wild pear genotypes grow in dry or low moisture soils. Some of them have a low growth vigor in the shrub forms, so they can be used as drought tolerant and slow growth rootstocks for commercial pear cultivars (Henareh, Citation2015).
In a research, three pear genotypes from P. syriaca species were exposed to four irrigation treatments. The results showed that the Coile genotype was more tolerant to drought conditions compared with other genotypes due to its high RWC during drought stress and non-decreasing dry weight (Javadi and Bahramnejad, Citation2011). In another study, drought tolerance was evaluated in three populations of wild pear germplasm (P. boisseriana) in greenhouse conditions. According to the results, the collected population of semi-arid regions showed higher drought tolerance than the other two populations that were collected from semi-humid areas. These seedlings were introduced as promising sources for use as rootstock for commercial pear cultivars in drought conditions (Zarafshar et al., Citation2014).
P. syriaca are distributed from west Azarbaijan to Fars in the Zagros Mountains and northwest of Iran (Abdollahi, Citation2011). This species showed grafting compatibility with Spadona and Kochia pear cultivars and improved the growth of the scion in calcareous soils. It is currently used as a rootstock in some countries (Fallouh et al., Citation2008). P. communis is scattered in the forests of the north, West Azarbaijan, Sardasht and Baneh in Kurdistan province. The fruit of this species is very diverse. Two types of native Khoj including large fruit (Khoj no. 1) and small fruit (Khoj no. 2) that are from this species, were studied in this research. Spadona cultivar is high yielding and has high resistance to chlorosis due to iron deficiency. It is also relatively tolerant of psylla and fire blight. P. salicifolia species spread in the northwest of Iran, including west and east Azerbaijan provinces (Abdollahi, Citation2011).
In recent years, the cultivation of fruit trees, such as pear, affected by climate change and decrease in rainfall, especially in the arid and semi-arid regions of the world. There are some ways to solve the drought problem in these regions, such as management of irrigation water and the use of drought-tolerant rootstocks with a low water requirement. There are pear species such as P. syriaca, P. glabra and P. salicifolia wild species that some of them can be used as drought-tolerant and also low growth vigor rootstocks for commercial pear cultivars (Henareh, Citation2015). The aim of this research was the evaluation of some wild species of pear for the screening of drought-tolerant populations. The compatibility of drought-tolerant populations as rootstock with pear commercial cultivars will be evaluated in a follow-up study.
Material and Methods
Plant Materials
In this study, seedlings from open pollination of Pyrus syriaca Boiss., and Pyrus salicifolia Pall. species along with P. communis L. cv. Spadona and two native pears from P. communis cv. Khoj including large fruit (Khoj no. 1) and small fruit (Khoj no. 2) were evaluated.
Fruits of pear species and cultivars were collected in August and September of 2016 from different regions of West Azarbaijan and Isfahan provinces as well as northern regions of Iran and transferred to the laboratory after ripening. The seeds were separated from the flesh and kept in a cool and dry place in paper bags after washing and drying. In order to satisfy the chilling requirement, seeds were sown in separate rows in the nursery soil with a sandy loam texture at Isfahan Agricultural and Natural Resources Research Center, in December. Seed cultivation was carried out in separate plots. From each population, six plots and in each plot, 500 seeds were cultivated at intervals of 2–3 cm in the row and 1 m between the rows.
During the chilling period, surveillance was carried out to provide adequate moisture and prevent drying of the culture bed.
The seedlings were irrigated for three months in order to establish in the nursery soil. Determination of irrigation time based on the allowed water depletion of pear trees is after a 50% decrease in humidity, but because the seedling roots had not yet been sufficiently developed, and needed enough moisture for better growth, so allowed water depletion was considered 35%. In the irrigation intervals, soil moisture at various depths of the soil was measured up to a depth of 100 cm by the Time Domain Reflectometry device (TDR, Trase6050X1) (Alizadeh, Citation2006; Doorenbos and Pruitt, Citation1977).
To obtain the amount of irrigation (irrigation volume), first, net irrigation depth was calculated according to formula 1.
In this formula, In is the net irrigation depth (mm), θFCi is the moisture content of the field capacity for each layer, θBLi is the soil moisture before irrigation for each layer, Di is the root development depth (mm), and i is the number of each soil layer. The number of soil layers in this study was three layers, which included depths of 0 to 20, 20 to 40 and 40 to 60 cm. According to formula 2, gross irrigation depth was calculated.
In this formula, Ig is the gross irrigation depth (mm), In is the net irrigation depth (mm), (1-Lr) is the amount of leaching and Ei is the irrigation efficiency (usually 80–90% for drip irrigation).
The amount of irrigation (irrigation volume) was obtained according to formula 3.
This volume was controlled by the installed counter on the pipe before irrigation plot. For irrigation of seedlings were used T-tape drip. The outlet flow of each drippler was measured in one liter per hour at an appropriate pressure. In this study, the efficiency of the drip system and the leaching requirement was considered 90% and 10%, respectively.
Drought Stress
Drought stress began in July, which coincided with the beginning of the stress period in the Isfahan region. In order to apply drought stress, irrigation frequency was changed, and irrigation time was considered based on 80% of allowed water depletion. shows the net and gross volume of irrigation water and the number of irrigation per month.
Table 1. Number of irrigation, net and gross volume of irrigation water in drought stress and control conditions.
Evaluated Traits
The number of seedlings was counted in stress-affected blocks before and after the drought stress, and the viability of each species was calculated as a percentage of initial numbers, according to formula 4.
Morphological and physiological traits of seedlings were separately recorded in control and drought treatments at the end of the experimental period (late September). These traits included the height of seedlings, diameter of main stem, number and length of internode, canopy width, number of sucker and branch, chlorophyll index, leaf dimensions, relative water content (RWC), electrolyte leakage (EL) and proline content. Developed young terminal leaves were used for the analysis of chlorophyll, leaf dimensions, EL, RWC and proline.
For measuring leaf length and width, the average of 10 leaves was considered. The diameter of stem and chlorophyll index was measured by caliper and chlorophyll meter (Spad), respectively.
Cell membrane stability was determined as EL. For EL analysis, whole fully expanded leaves (0.1 g from each pot) were incubated in 15 ml of distilled water in a shaker for 24 h. The conductance of the incubation solution was measured as an initial level of EL (Ci) using a conductance meter (YSI-3100, Guangzhou, China). Leaf tissue in the incubation solution was killed in an autoclave at 120°C for 30 min. The conductance of the incubation solution with killing tissues (Cmax) was determined following 24 h incubation on a shaker. Relative EL was calculated as (Ci/Cmax) ×100 (Blum and Ebercon, Citation1981).
Leaf relative water content was calculated according to Barrs and Weatherley (Citation1962). Leaf samples were detached from the plants and immediately weighed to determine fresh weight (FW). Samples were placed in covered petri dishes filled with water for leaves to reach full hydration. After approximately 24 h at 4°C, leaf samples were blotted dry with paper towels and weighted to determine turgid weight (TW). Leaf tissue was dried in an oven at 80°C for 48 h to determine dry weight (DW). Leaf RWC was calculated as (FW-DW)/(TW-DW)×100.
The proline content was determined by the method given by Bates et al. (Citation1973). For this, 10 ml of 3% (w/v) sulphosalicylic acid was added to 0.4 g leaf materials. The homogenate was filtered through filter paper. Two ml acetic acid and 2 ml acidic ninhydrin regent were added to a 2 ml filtered extract. The mixture was thoroughly stirred and incubated in a boiling water bath for 1 h and then the reaction was stopped by using an ice bath and warmed to room temperature. The mixture was extracted with toluene and the absorbance of the fraction was read at 518nm in a UV/visible spectrophotometer (model PG Instrument+80, Leicester, UK). The concentration of proline in the sample was computed from a standard curve of proline and was expressed asµmol−1g/FW.
Data Analysis
This research was carried out as a factorial experiment based on a randomized complete block design with two factors, including two levels of irrigation (normal and drought stress) and five pear species. Ten seedlings were considered in each plot to evaluate traits. Data analysis was calculated with SAS software (version 9.1) and ANOVA procedure was used. Mean comparison was performed in LSD method.
Results
Viability Percent
Viability percent of the pear seedlings in blocks affected by stress is shown in . According to the results, P. salicifolia showed the highest survival in drought stress conditions. After that, P. communis cv. Khoj no. 1 and Khoj no. 2 was placed in the next rank. The lowest percentage of seedling viability during drought stress was also belonged to P. communis cv. Spadona.
Table 2. Viability percent of the pear seedlings after three months of drought stress.
Morphological Traits
Analysis of variance showed that the interaction of pear species and drought stress on some morphological traits, including seedling height, stem diameter, internode number and canopy width were significant at p < .01 level.
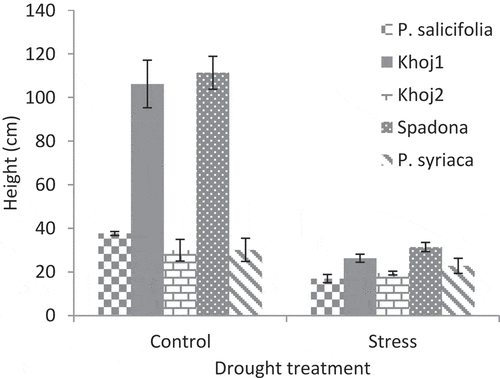
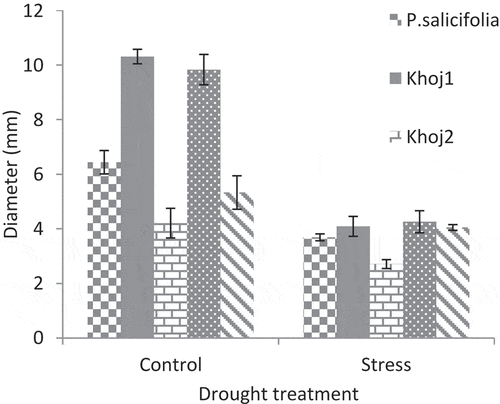
P. communis cv. Spadona and Khoj no. 1 showed the highest seedling height under both drought stress and normal conditions. The height of P. syriaca, P. salicifolia and P. communis cv. Khoj no. 2 seedlings in the control treatment was equal to the height of P. communis cv. Spadona and Khoj no. 1 seedlings in drought stress treatment.P. salicifolia showed the lowest seedling height in stress conditions (). In control treatment, P. communis cv. Spadona and Khoj no. 1 had the highest stem diameter and P. communis cv. Khoj no. 2 had the lowest rate of it. In stress conditions, P. communis cv. Khoj no. 2 also had the lowest stem diameter. In these conditions, the stem diameter of P. communis cv. Spadona, Khoj no. 1, P. salicifolia and P. syriaca did not have significant differences ().
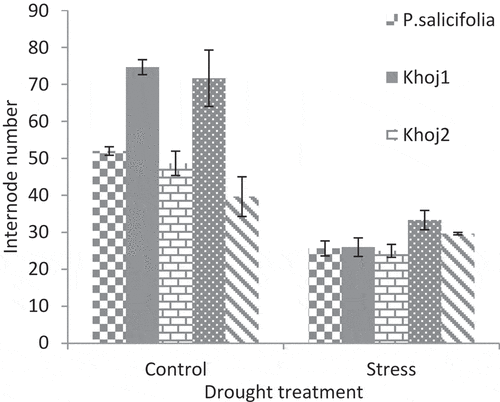
In the control treatment, P. communis cv. Spadona and Khoj no. 2 produced the highest internode number. The lowest rate of this trait was belonged to P. syriaca species. In drought stress conditions, P. communis cv. Spadona produced the most internode number and P. salicifolia, P. communis cv. Khoj no. 1 as well as Khoj no. 2 had the least internode number ().
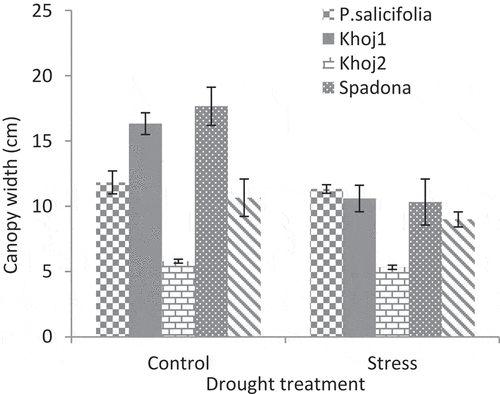
The highest canopy width belonged to P. communis cv. Spadona and Khoj no. 1 in the control treatment. P. communis cv. Khoj no. 2 had the lowest canopy width and there was no significant difference between control and stress treatments in this population. No significant difference was observed between the control and stress treatments in other species ().
According to , the most leaf length, leaf width and internode length was belonged to P. communis cv. Spadona population. P. communis cv. Khoj no. 2 produced the least leaf length. The lowest leaf width and internode length were also observed in P. communis cv. Khoj no. 2, P. salicifolia and P. syriaca. P. salicifolia showed the highest chlorophyll index. This species, along with P. syriaca species, had the highest leaf length-to-width ratio.
Table 3. Effect of species on some measured traits±SD.
The measured traits in the control treatment showed more rates than drought stress ().
Physiological Traits
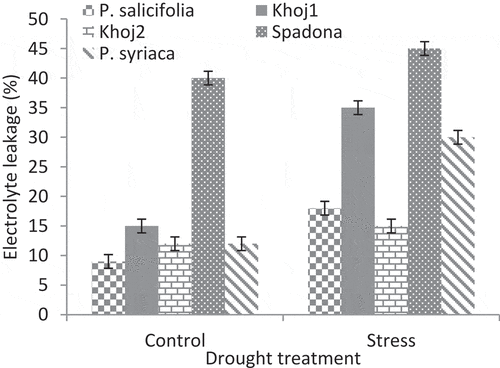
Analysis of variance showed that the interaction of pear species and drought stress on EL, RWC and proline content was significant at p < .01 level. EL in populations under stress was more than the control treatment (). The highest and the lowest EL was observed in Spadona under stress and P. salicifolia in control treatment, respectively. In drought stress conditions, the least EL was observed in P. communis cv. Khoj no. 2, P. salicifolia, P. syriaca, P. communis cv. Khoj no. 1 and Spadona, respectively ().In P. communis cv. Khoj no. 2 population, there was no significant difference in EL between control and drought treatments.
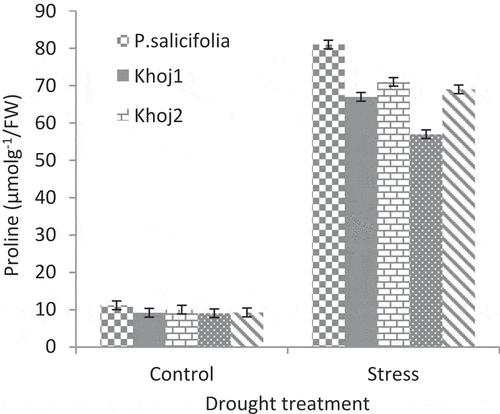
Applying drought stress in all populations led to the increased proline content of leaves (). The most increase in proline content was belonged to P. salicifolia, and then P. communis cv. Khoj no. 2.P. communis cv. Spadona showed the lowest increase in proline content. In non-stress conditions, there was no significant difference among populations in proline content.
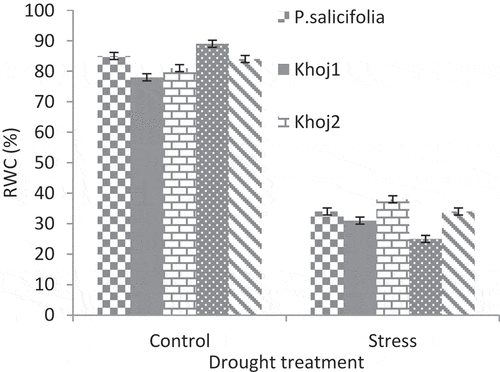
Drought stress reduced leaf RWC in pear populations. The highest and the least amount of RWC in stress conditions was observed in P. communis cv. Khoj no. 2 and Spadona, respectively. However, Spadona showed the highest RWC in the control treatment, but under the stress conditions, it reached the lowest level ().
Discussion
Viability Percent
After the end of the stress period, P. salicifolia showed the highest viability percentage in blocks under stress. From the old times, wild pear genotypes have been considered in the Iranian plateau because of tolerance to biotic and abiotic stresses (Javadi et al., Citation2005). The adaptation of wild pears to rocky and dry areas leads to more tolerance to drought stress compared to other commercial and native rootstocks (Henareh, Citation2015). In the present study, P. communis cv. Spadona that was a commercial cultivar and belonging to P. communis, had the lowest viability percentage in blocks under stress (). Wild species of pear showed higher viability percentage and tolerance to drought stress.
Morphological Traits
The studied pear species were significantly different from each other, which was due to the diversity among populations, so it is possible to select cultivars and species for a specific purpose.
In drought stress, initially, there is a reduction of growth of expanding tissues related to a decrease of cell turgor and cell division rates (Tardieu Citation2013). Drought stress led to reduce in the growth characteristics of pear seedlings. Similarly, the increasing water stress decreased the relative shoot length and diameter in M9 apple and MA quince rootstocks (Bolat et al., Citation2014). P. communis cv. Spadona and Khoj no. 1 had the most seedling height under stress and control conditions, but their height difference with other species in normal conditions was more than stress conditions (). P. salicifolia showed the lowest seedling height in stress conditions (). It seems that species with less susceptible to drought stress, when exposed to this stress show less decrease in growth. Morphological adaptations in plants can be one of the adaptive mechanisms under drought stress conditions (Pire et al., Citation2007). Similarly, the effects of drought stress on morphological factors have been reported in many experiments on crops such as sour cherry (Sivritepe et al., Citation2008) and apple (Nemeskeri et al., Citation2015). These morphological changes can help breeders for early selection of tolerant seedlings. P. communis cv. Spadona and Khoj no. 1 that had higher levels of growth in the control treatment showed a faster response in blocks under drought stress, but in the other three populations, the difference in seedling height between the blocks under stress and blocks with normal irrigation was relatively low.
Similarly, P. communis cv. Spadona and Khoj no. 1 had the highest stem diameter in the control treatment that was approximately equal to P. salicifolia and P. syriaca in blocks under stress. P. communis cv. Khoj no. 2 had the least stem diameter in stress conditions (). It has been reported that the negative effect of drought stress on stem diameter is less than its effect on seedling height (Haghighatian et al., Citation2013). In the present research, the stem diameter and seedling height were equally affected by drought stress, but this effect was different among populations.
In the control treatment, P. communis cv. Spadona and Khoj no. 2 produced the highest internode number and canopy width ( and ). In these two populations, the most difference was observed between control and stress treatments. P. communis cv. Khoj no. 2 had the lowest internode number and canopy width. Drought reduces organ size, namely internode and leaf, and whole-shoot growth through growth cessation (Lauri et al., Citation2014).
The highest leaf length and width, as well as internode length, were belonged to the P. communis cv. Spadona population (). Internode length is an appropriate index for determining the effect of drought on plants. So that, in the stress conditions, the internode length changes before the change in the water potential of the leaves (Grimplet et al., Citation2007). The lowest leaf length was belonged to P. communis cv. Khoj no. 2. This population along with P. salicifolia and P. syriaca had the lowest leaf width and internode length. Wild species had less leaf dimensions. The variation in leaf dimensions and area, as well as the effects of drought on these variables, has also been reported in two apple cultivars (Lauri et al., Citation2014).
P. salicifolia showed the highest leaf chlorophyll index. The chlorophyll meter indicates the relative chlorophyll concentration, based on the difference between the light transmittance in two red and infrared wavelengths, which correlates with the chlorophyll content of the leaves (Hoel and Solhaug, Citation1998). Preservation and not the decomposition of chlorophyll in one species during drought stress indicates the tolerance of that species to stress (Tarahomi et al., Citation2010). The P. salicifolia along with P. syriaca showed the highest leaf length-to-width ratio (). These two species also had the higher chlorophyll index. In general, the values of chlorophyll index, leaf dimensions and internode length were decreased in blocks under drought stress compared to normal irrigation blocks (). Drought stress is one of the limiting factors in the early stages of plant growth that strongly affects cell division and elongation (Yordanov et al., Citation2003).
Physiological traits
The increase in ion leakage is associated with increased cell permeability and leads to electrolyte leakage from the cell, therefore maintaining cell membrane is important in increasing drought tolerance (Bolat et al., Citation2014). In this study, El in populations under stress was higher than control populations (). Increase EL in drought stress treatment has also been reported in apple (Bolat et al., Citation2014) and fig (Gholami et al., Citation2012). EL increases under drought stress, but this increase in the tolerant plants is less than sensitive plants (Taiz and Zeiger, Citation1998). Increase EL in P. communis cv. Khoj no. 2 and P. salicifolia populations were less than other populations and these two populations were more tolerant in drought conditions. Jinrong et al. (Citation2008) also stated that tolerant Blackberry cultivars had more cell membrane stability than sensitive cultivars which was determined by less EL. Abraham et al. (Citation2004) introduced the EL as the most sensitive indicator of drought stress because of its rapid increase in response to stress, which is consistent with the current research.
Proline is one of the compounds commonly affected by drought in leaves under stress (Lee et al., Citation2013; Rejeb et al., Citation2014). In addition, proline can act as an antioxidant (Ramachandra Reddy et al., Citation2004). According to Pinhero et al. (Citation2001), the synthesis and accumulation of osmolites among plant species or different cultivars of a species are different. In this study, the highest proline content belonged to P. salicifolia and P. communis cv. Khoj no. 2. In P. salicifolia and P. communis cv. Khoj no. 2, proline content in stress treatment compared to control treatment was increased 24.7 and 7.7 times, respectively (). Similarly, Morot Guadry et al. (Citation2001) reported that with decreasing leaf water potential, proline concentration increased several times. According to Jia et al. (Citation2002), proline accumulation had no positive correlation with tolerance to stress and it was only a response to drought damage. In another study, apple seedlings did not show a positive correlation between drought stress and proline content (Sircelj et al., Citation2005), but in the present study, applying stress led to increased proline content in all populations, but this increase was different. In fact, there is genetic variation between species in osmotic adjustment (Dacosta, Citation2006).
Taiz and Zeiger (Citation1998) stated that for most species when water absorption by roots is equal to the transpiration of the leaf, the optimal percentage of RWC is between 85% and 95%. They showed that the appropriate amount of RWC is at least about 60%, which varies depending on the type of tissue and plant species. In the present study, leaf RWC varied among populations from 78% to 89% in the control treatment that was optimal. These values reached 25–38% in stress conditions. Drought stress reduced RWC in pear populations (). Similarly, there was no much difference among RWC of irrigated apple cultivars, but RWC of apple leaves decreased by applying drought stress (Hamann et al., Citation2018). The most and the least leaf RWC in stress conditions were observed in P. communis cv. Khoj no. 2 and Spadona, respectively. Almond genotypes also showed different responses in their RWC under drought stress conditions (Yadollahi et al., Citation2011). The leaf RWC is calculated based on the amount of water in the leaves relative to the amount of water that can be in the leaves, thus reducing leaf RWC is accompanied by the increase in water stress levels (Bolat et al., Citation2014).
Conclusions
Genetic diversity helps plants to overcome environmental changes and also provides more chance to select cultivars or new rootstocks (Liu, Citation2006). In P. communis species, Spadona and Khoj no. 2 populations showed less tolerance to drought stress than other populations (with the lowest RWC and proline content, and the highest EL), but in this species, P. communis cv. Khoj no. 2 along with some wild species showed high tolerance to drought stress, so it seems that the behavior of three populations of P. communis species had high variance in drought exposure. As regards, P. salicifolia and P. communis cv. Khoj no. 2 populations in the drought stress blocks had the highest RWC and proline content, and the lowest EL; therefore, these species can be introduced as more tolerant species to drought stress. Although P. syriaca and P. communis cv. Khoj no. 2 had less EL and more proline and RWC than P. communis cv. Khoj no. 1, they showed a lower viability percentage than this population. It seems that other physiological factors, such as the activity of antioxidant enzymes, are involved.
References
- Abdollahi, H. 2011. Pear, botany, cultivars and rootstocks. Publication of Agricultural Education, Iranian Ministry of Agriculture, Tehran, Iran, p. 196.
- Abraham, E.M., B. Huang, S.A. Bonos, and W.A. Meyer. 2004. Evaluation of drought resistance for Texas bluegrass, Kentucky bluegrass and their hybrids. Crop Sci. 44:1746–1753. doi: 10.2135/cropsci2004.1746.
- Alizadeh, A. 2006. Principles of irrigation systems design, p. 452. Mashhad, Iran: Imam Reza University Press.
- Aroca, R., R. Porcel, and J.M. Ruiz-Lozano. 2012. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 63:43–57. doi: 10.1093/jxb/err266.
- Barrs, H.D., and P.E. Weatherley. 1962. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 15:413–428. doi: 10.1071/BI9620413.
- Bates, L.S., R.P. Waldren, and I.D. Teare. 1973. Rapid determination of free proline for water stress studies. Plant Soil. 39:205–207. doi: 10.1007/BF00018060.
- Blokhina, O., E. Violainen,and, and K.V. Fagerstedt. 2003. Antioxidants, oxidative damage and oxygen deprivation stress. Ann. Bot. 91:179–194. doi: 10.1093/aob/mcf118.
- Blum, A., and A. Ebercon. 1981. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 21:43–47. doi: 10.2135/cropsci1981.0011183X002100010013x.
- Bolat, I., M. Dikilitas, S. Ercisli, A. Ikinci, and T. Tonkaz. 2014. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 8. doi: 10.1155/2014/769732.
- Cheruth, A.J., P. Manivannan, A. Wahid, M. Farooq, H. Al-Juburi Somasundaram, and R. Panneerselvam. 2009. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 11:1560–8530.
- Dacosta, M. 2006. Physiological and morphological characteristics associated with drought resistance mechanisms in bent grass species. Rutgers the State University of New Jersey, New Brunswick, p. 222.
- Doorenbos, J., and W.H. Pruitt. 1977. Crop water requirements. FAO Irrigation and Drainage, paper No. 24, Romme, Italy.
- Fallouh, I., K. Al-Maarri, and S. Haddad. 2008. Study of the grafting compatibility between some clones of Syrian wild pear Pyrus Syriaca Boiss. with four pear commercial cultivars. Damascus J. Agron. Sci. 24:237–250.
- Gholami, M., M. Rahemi, B. Kholdebarin, and S. Rastegar. 2012. Biochemical responses in leaves of four fig cultivars subjected to water stress and recovery. Sci. Hort. 148:109–117. doi: 10.1016/j.scienta.2012.09.005.
- Grimplet, J., L.G. Deluc, G.R. Gramer, and J.C. Cushman. 2007. Integrating functional genomics with salinity and water deficit stress respones in wine grape-Vitis vinifera, p. 643–668. In: M.A. Jencks ed. Advances in molecular breeding towards drought and salt tolerant crops. Reno, USA: University ofNevada.
- Haghighatian, H., H. Nadian, F. Rejali, and A.R. Tavakoli. 2013. The effect of two arbuscular mycorrhizal fungi on vegetative growth and phosphorus absorption on citrus aurantifolia under drought stress conditions. Seed Plant Prod. J. 28:403–417.
- Haider, M.S., M.M. Kurjogi, M. Khalil-ur-Rehman, T. Pervez, J. Songtao, M. Fiaz, S. Jogaiah, C. Wang, and J. Fang. 2018. Drought stress revealed physiological, biochemical and gene-expressional variations in ‘Yoshihime’ peach (Prunus Persica L) cultivar. J. Plant Interact. 13:83–90. doi: 10.1080/17429145.2018.1432772.
- Hamann, F.A., S. Czaja, M. Hunsche, G. Noga, and A. Fiebig. 2018. Monitoring physiological and biochemical responses of two apple cultivars to water supply regimes with non-destructive fluorescences sensors. Sci. Hort. 242:51–61. doi: 10.1016/j.scienta.2018.07.008.
- Harotko, K. 2007. Advances and challenges in fruit rootstock research. Acta. Hortic. 732:33–42. doi: 10.17660/ActaHortic.2007.732.1.
- Henareh, M. 2015. Investigation of Iranian pear species capability in order to achieve a dwarfing and fire blight tolerant rootstocks. Final Research Report, Agricultural and Natural Resources Research Center of Azarbaijan- Gharbi 95pp.
- Hoel, B.O., and K.A. Solhaug. 1998. Effect of irradiance on chlorophyll estimation with the minolta SPAD-502 leaf chlorophyll meter. Ann. Bot. 82:389–392. doi: 10.1006/anbo.1998.0683.
- Javadi, T., and B. Bahramnejad. 2011. Relative water content and gas exchanges of three wild pear genotypes under water stress conditions. J. Hortic. Sci. 24:223–233.
- Javadi, T., K. Arzani, and H. Ebrahim Zadeh. 2005. Evaluation of soluble carbohydrates and proline in nine Asian pear cultivars (Pyrus seratonia) under drought stress. Iran. J. Biol. 17:12–24.
- Jia, W.S., Y.Q. Wang, S.Q. Zhang, and J.H. Zhang. 2002. Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J. Exp. Bot. 53:2201–2206. doi: 10.1093/jxb/erf079.
- Jinrong, L., X. Xiaorong, D. Jianxiong, S. Jixiong, and B. Xiaomin. 2008. Effects of simultaneous drought and heat stress on Kentucky bluegrass. Sci. Hort. 115:190–195. doi: 10.1016/j.scienta.2007.08.003.
- Lauri, P.E., A. Marceron, F. Normand, A.D. Ambreville, and J.L. Regnard. 2014. Soil water deficit decreases xylem conductance efficiency relative to leaf area and mass in the apple. J. Plant Hydraul. 1(1):e0003. doi: 10.20870/jph.2014.e003.
- Lee, B.R., S. Muneer, S.H. Park, Q. Zhang, and T.H. Kim. 2013. Ammonium-induced proline and sucrose accumulation, and their significance in antioxidative activity and osmotic adjustment. Act. Physiol. Planta. 35:2655–2664. doi: 10.1007/s11738-013-1297-7.
- Liu, M.J. 2006. Chinese jujube: Botany and horticulture. Hortic. Rev. 32:229–299.
- Meher, M.P., R.K. Ashok, and R.D. Manohar. 2018. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 25:285–289. doi: 10.1016/j.sjbs.2017.04.008.
- Mohan Jain, S., and P.M. Priyadarshan. 2009. Breeding plantation tree crops. Temperate species, Springer Science Business Media, LLC, 233 Spring Street, New York NY 10013, USA: Springer Science + Business Publishing, p.290.
- Morot Guadry, J.F., D. Job, and P.J. Lea. 2001. Amino acid metabolism, p. 167–211. In: P.J. Lea and J.F. Morot-Guadry (eds.). Plant nitrogen. Springer, Berlin.
- Nardini, A., M.A.L. Gullo, and S. Salleo. 2011. Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Sci. 180:604–611. doi: 10.1016/j.plantsci.2010.12.011.
- Nemeskeri, E., E. Kovacs- Nagy, J. Nyeki, and E. Sardi. 2015. Responses of apple tree cultivars to drought: Carbohydrate composition in the leaves. Turk. J. Agric. For. 39:949–957. doi: 10.3906/tar-1409-154.
- Pinhero, R.G., M.V. Rao, G. Palyath, D.P. Murr, and R.A. Fletcher. 2001. Changes in the activities of antioxidant enzymes and their relationship to genetic and Paclobutrazol-induced chilling tolerance of maize seedling. Plant Physiol. 114:695–704. doi: 10.1104/pp.114.2.695.
- Pire, R., A. Pereira, J. Diez, and E. Fereres. 2007. Drought tolerance assessment of a Venezuelan grape rootstock and possible conditions mechanism. Agrociencia 41:435–446.
- Reales, A., D.J. Sargent, K.R. Tobutt, and D. Rivera. 2010. Phylogenetics of Eurasian plums, Prunus L. section Prunus (Rosaceae), according to coding and non-coding chloroplast DNA sequences. Tree Genet. Genomes. 6:37–45. doi: 10.1007/s11295-009-0226-9.
- Reddy, R.A., K.V. Chaitanya, and M. Viveka. 2004. Drought induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 161:1189–1202. doi: 10.1016/j.jplph.2004.01.013.
- Rejeb, K.B., C. Abdelly, and A. Savoure. 2014. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80:278–284. doi: 10.1016/j.plaphy.2014.04.007.
- Sabeti, H. 1995. Forests, trees and shrubs in Iran. Publication of Yazd University, Yazd, Iran, p. 81.
- Sircelj, H., M. Tausz, D. Grill, and F. Batic. 2005. Biochemical responses in leaves of two apple tree cultivars subjected to progressing drought. J. Plant Physiol. 162:1308–1318. doi: 10.1016/j.jplph.2005.01.018.
- Sivritepe, N., U. Erturk, C. Yarlikaya, I. Turkan, M. Bor, and F. Ozdemir. 2008. Response of the cherry rootstock to water stress induced in vitro. Biol. Plantarum. 52:573–576. doi: 10.1007/s10535-008-0114-4.
- Taiz, L., and E. Zeiger. 1998. Plant physiology. Sinauer Association, Sunderland, MA, p. 792.
- Tarahomi, G., M. Lahoti, and F. Abasi. 2010. Effect of drought stress on variations of soluble sugar chlorophyll and potassium in Salvia leriifolia benth. Q.J. Biol. Sci. 3:1–7.
- Tardieu, F. 2013. Plant response to environmental conditions: Assessing potential production, water demand, and negative effects of water deficit. Front. Plant Physiol. 4:1–11.
- Yadollahi, A., K. Arzani, A. Ebadi, M. Wirthensohn, and S. Karimi. 2011. The response of different almond genotypes to moderate and severe water stress in order to screen for drought tolerance. Sci. Hort. 129:403–413. doi: 10.1016/j.scienta.2011.04.007.
- Yordanov, I., V. Velikova, and T. Tsonev. 2003. Plant responses to drought and stress tolerance. Bulg. J. Plant Physiol. Special Issue:187–206.
- Zarafshar, M., M. Akbarinia, H. Askari, S.M. Hosseini, M. Rahaie, D. Struve, and G.G. Striker. 2014. Morphological, physiological and biochemical responses to soil water deficit in seedlings of three populations of wild pear tree (Pyrus boisseriana). Biotechnol. Agron. Soc. Environ. 18:353–366.
- Zeinolabedini, M., K. Majourhat, M. Khayam Nekoui, V. Grigorian, M. Torchi, F. Dicenta, and G. Martinez. 2008. Comparison of the use of morphological, protein and DNA markers in the genetic characterization of Iranian wild Prunus species. Sci. Hort. 116:80–88. doi: 10.1016/j.scienta.2007.10.022.