ABSTRACT
Recalls of frozen foods contaminated with Listeria monocytogenes have highlighted a need to optimize postharvest processing practices. Peroxyacetic acid (PAA), electrolyzed oxidizing water (EO), and chlorine were applied to wild blueberries inoculated with Listeria innocua. Following treatment, product was blast frozen and analyzed after 2 weeks of storage at −20°C. L. innocua was reduced by 2.2 log CFU/g after immersion and spraying with PAA. Yeast was not significantly reduced by any treatment. L. innocua survival was largely unaffected by freezing. Application of maximum sanitizer concentration by immersion followed by spraying is recommended to maximize the safety of wild blueberries.
Introduction
In recent years, outbreaks linked to Listeria monocytogenes (Order Bacillales, Family Listeriaceae) have caused concern regarding survival of this pathogen in frozen foods. In 2015, Blue Bell Creamery products contaminated with L. monocytogenes caused 10 illnesses and three deaths (CDC, Citation2015). Additionally, in 2016, a multistate outbreak occurred in frozen vegetables involving more than 350 products sold under 42 brands produced by CRF frozen foods. Product contaminated with L. monocytogenes associated with nine illnesses and three deaths (CDC, Citation2016).
Wild blueberries (Vaccinium angustifolium), native to Maine and Eastern Canada, offer many potential health benefits (USDA-ARS, Citation2016). Also called lowbush blueberries, this crop is distinct from the more common cultivated blueberry and certain aspects of its growth and harvest affect the likelihood for microbial contamination (Kniel and Shearer, Citation2014; Tefera et al., Citation2018). Wild blueberries are grown close to the ground, with average plant heights between 4 and 15 inches, as compared to 4–6 feet for highbush plants (Yarborough, Citation2015). This proximity to soil increases the likelihood of contamination by soilborne organisms (Tefera et al., Citation2018; Yarborough, Citation2015). Wild blueberries are harvested in late summer through a process of mechanical or hand raking. The majority of the crop (approximately 99%) is processed for frozen storage and sale. This process commonly includes a winnowing step followed by sanitization through a process of immersion or spraying in a wash tank/conveyer system. Wash tanks can serve as a reservoir for contamination if a contaminated product is brought through the facility (Luo et al., Citation2010, Citation2012). Further, locations within the processing environment may serve as harborage points for pathogens (particularly L. monocytogenes), as demonstrated in the 2016 CRF outbreak.
Due to cooler temperatures and the presence of moisture, processing facilities provide a suitable environment for survival of Listeria spp. (Hill et al., Citation2001). Listeria monocytogenes is a pathogen of concern in frozen products due to this pathogen’s ability to survive frozen storage (Flessa et al., Citation2005; Palumbo and Williams, Citation1991). The lack of a “kill step” in the processing of wild blueberries and other frozen fruits is problematic due to the fact that they may not be cooked before consumption. Current industry standards include the use of chlorine (up to 200 ppm) as the main source of decontamination of fresh produce (FDA-CFSAN, Citation1998). The use of alternative sanitizers has been of interest to producers and consumers due to the potential human health risks associated with the use of chlorine (Ruiz-Cruz et al., Citation2007).
While alternative sanitizing treatments may be effective at the bench scale, it is imperative to consider the constraints of existing equipment configuration at the commercial scale. Economic feasibility of treatment application, heterogeneity of product, inoculum distribution, and sanitizer application at a larger scale should also be taken into account. This study aimed to evaluate the use of alternative sanitization methods on wild blueberries and the survival of L. innocua, a nonpathogenic surrogate for L. monocytogenes, after 2 weeks of frozen storage. Specific attention was given to scale by comparing bench-scale results with those from a pilot-scale version of the common wild blueberry processing line configuration.
Materials and Methods
Bacterial Strains and Inoculum Preparation
Listeria innocua ATCC 33090 (American Type Culture Collection, Manassas, VA) was grown in tryptic soy broth (TSB, Alpha Biosciences, Baltimore, MD) at 30°C for 24 h. Overnight cultures were spread onto tryptic soy agar (TSA, Alpha Biosciences, Baltimore, MD) and incubated for 24 h at 30°C to promote increased resistance to treatment (Gandhi and Chikindas, Citation2007). Listeria innocua lawns were harvested by scraping with sterile 0.1% peptone (Difco, Sparks, MD) and diluted to 5 mL of 0.1% peptone. The resulting cell suspension was used to inoculate fresh wild blueberries to achieve a final concentration of ~4 log CFU/g. In this study, L. innocua was used as a surrogate for L. monocytogenes due to the high potential for environmental contamination during pilot-scale work. This species is known to be genetically similar to the pathogenic members of the genus while lacking a common virulence locus (Buchrieser et al., Citation2003; Chakraborty et al., Citation2000).
Fresh Wild Blueberries
Fresh wild blueberries were obtained from Allen’s Freezer Incorporated (Ellsworth, ME) for 4 consecutive weeks during August 2018. Blueberries were transported in perforated plastic produce bins and stored at 4°C for up to 3 days before treatment.
Inoculation of Wild Blueberries
Fresh wild blueberries were rinsed with cold tap water and laid out on sterile plastic trays for manual removal of large debris. Blueberries were stored at ambient temperature in a biological safety cabinet (SterilGARD Hood, Sanford, ME) to dry for 24 h to dry before inoculation. Rinsed wild blueberries were weighed into sterilized, smooth-bottomed aluminum tins for inoculation. Each tin of blueberries was inoculated with prepared L. innocua cell suspension (as described above) and shaken for 45 s to ensure even distribution of the inoculum. Inoculated wild blueberries were left to dry at ambient temperature for 24 h in a biological safety cabinet.
Microbiological Analysis-Enumeration of Native Microflora on Unwashed Fresh Wild Blueberries
Duplicate samples of fresh, unwashed and washed wild blueberries were combined with 0.1% peptone agitated for 2 min. After agitation, the samples were serially diluted in 0.1% peptone and spread plated onto TSA and acidified potato dextrose agar (APDA, Alpha Biosciences, Baltimore, MD). Plates were incubated at 37°C for 48 h (TSA), and room temperature for 5 days (APDA). Microbial populations were determined (log CFU/g) for aerobic mesophiles (AMC) and yeast.
Microbiological Analysis-Enumeration of L. innocua on Inoculated Wild Blueberries
After inoculation, triplicate samples were taken to determine L. innocua levels on the wild blueberries. Samples were homogenized and plated as described above with the addition of modified Oxford agar (MOX, Alpha Biosciences, Baltimore, MD), incubated at 30°C for 48 h.
Sanitizer Treatment Preparation
Individual sanitizer solutions were prepared using sterilized tap water. The concentration of electrolyzed water (stock solution concentration of 500 ppm, pH 6.5; JDP-Clarentis®, Palm Beach Gardens, FL) was determined using a HACH digital titrator kit (HACH, Model 16900, Loveland, CO) and diluted to achieve a final concentration of 200 ppm active chlorine constituents. A 5% Sodium Hypochlorite solution (Lab Chem, Zelienople, PA) was diluted to yield a final concentration of 200 ppm and verified using commercial test strips (Micro Essential Lab, Brooklyn, NY). Concentrated peroxyacetic acid (15% vol/vol) was diluted to achieve a final concentration of 80 ppm and was verified using a commercial peracetic acid test kit (Alpha Chemical, Stoughton, MA). Diluted solutions were stored for no longer than 24 h before use.
Benchtop Study
Sanitizer Treatment of Inoculated Wild Blueberries
Inoculated wild blueberries were subjected to a variety of sanitizer treatments to determine the efficacy of the application method (immersion, spraying or sequential combination), and sequence of sanitizer application on L. innocua and native microflora. Inoculated blueberries were distributed into sterile steam pans for treatment. For sanitizer treatments, the blueberries were subjected to spraying or immersion (contact time varied between 30 s and 5 min with duplicate samples taken at predetermined time points), or combination treatments consisting of multiple sequential sprays or immersion followed by spraying (3-min total contact time with duplicate samples taken at 1:30 s and 3 min). Post-treatment microbiological analysis was carried out as previously described.
Pilot Scale Study
Microbiological Analysis – Enumeration of L. innocua and Native Microflora on Unwashed, Washed, Inoculated, and Treated Fresh Wild Blueberries
Microbiological analysis was carried out as described above with the addition of coliform enumeration. To determine the coliform population, samples were plated on 3M Petrifilm™ (3M, Maplewood, MN) and incubated at 35°C for up to 48 h.
Sanitizer Treatment Preparation
Sanitizers were prepared as described above. Tap water was used in place of sterile water.
Pilot Scale Setup
Pilot-scale work was completed on a conveyer consisting of three spray bars and a mechanized, perforated belt (). The system was controlled with a DirectSoft5 (Automation Direct, Cumming, GA, USA) programmable logic controller (PLC) interface. Three peristaltic pumps were run at 30 Hz with a flow rate of 0.5 GPM. Two nozzles discharging a flat cone spray pattern were mounted to each spray bar. Parameters for the conveyer were chosen based on equipment capabilities as well as current industry processing practices (personal communication).
Figure 1. Custom-built pilot-scale processing line and setup for treatment of inoculated wild blueberries

Sanitizer Treatment and Freezing of Inoculated Wild Blueberries
Wild blueberries were immersed in then sprayed with chosen sanitizer combinations. Prior to treatment, the conveyer lines were purged for 1 min with the corresponding sanitizer for each line. Wild blueberries were immersed in sanitizer for 1.5 min followed by spraying for 1.5 min. Samples were taken in duplicate after each step to determine the efficacy of individual steps. After treatment, blueberries were blast frozen in a single layer for 4 h, packaged in sterile bags and stored at −20°C for up to 2 weeks. Upon completion of sanitization, the lines were purged with water for 30 s to remove residual sanitizer.
Microbiological Analysis – Enumeration of L. Innocua and Native Microflora Treated Inoculated Wild Blueberries after 24 h of Freezing
At 24 h after freezing, L. innocua and native microflora populations were determined as described previously.
Microbiological Analysis – Enumeration of L. Innocua and Native Microflora on Frozen Treated Inoculated Wild Blueberries after 2 Weeks of Frozen Storage
After 2 weeks of frozen storage, samples were taken to assess the survival of L. innocua and native microflora on wild blueberries. For enrichment, 10 g of blueberries was diluted 1:10 in Listeria Enrichment Broth (LEB, Alpha Biosciences, Baltimore, MD) which was incubated at 30°C for 24 h. Incubated enrichment was spread onto MOX and incubated at 30°C for 48 h, at which time plates were examined for colonies with morphology consistent with Listeria spp.
Statistical Analysis
Data were analyzed to determine statistical significance (p < .05) between sanitizer, application method and contact time using a multiway ANOVA and Tukey HSD post hoc test for multiple mean comparisons in R studio.
Results
Benchtop Study
Efficacy of Sanitizer Treatments and Method of Application on Inoculated Wild Blueberries
Immersion
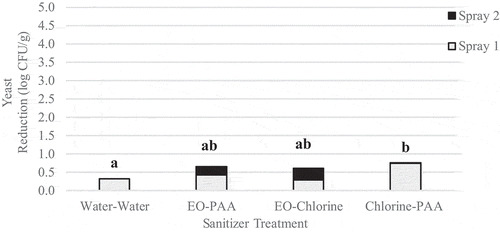
Pre-treatment L. innocua populations on berries ranged from 4.2 to 4.5 log CFU/g with an average of 4.3 log CFU/g. Pre-treatment levels of native yeast ranged from 6.9 to 7.1 log CFU/g with an average of 7.0 log CFU/g. For immersion treatments, L. innocua population reductions ranged from 0.3 to 1.1 log CFU/g (). No significant effect of contact time was observed. Immersion in EO was the most effective at reducing L. innocua populations with a reduction of 1.1 log CFU/g. However, due to high variability, none of the treatments investigated significantly (p > .05) reduced L. innocua populations compared to the untreated control. Initial yeast populations were high and reductions were relatively low, ranging from 0.1 to 0.6 log CFU/g (). Disregarding contact time, immersion in chlorine (p = .01) or PAA (p = .01) was significantly more effective against yeast than immersion in water alone. The application of chlorine and PAA reduced yeast populations by 0.6 log CFU/g compared to water which reduced yeast populations by 0.2 log CFU/g. The application of EO for 5 min and PAA for 3 min reduced yeast populations by 0.7 log CFU/g and significantly (p < .05) reduced yeast populations compared to the untreated control.
Figure 2. Reduction of L. innocua on inoculated wild blueberries immersed in sanitizer for increasing time intervals
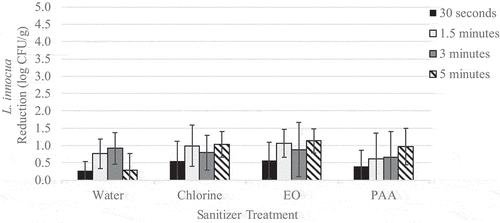
Figure 3. Reduction of native yeast on wild blueberries immersed in sanitizer for increasing time intervals
Spray
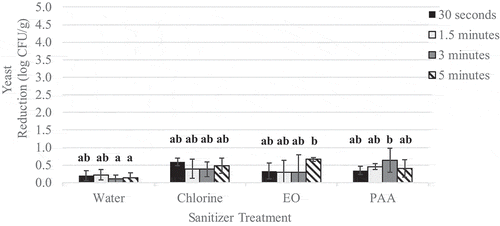
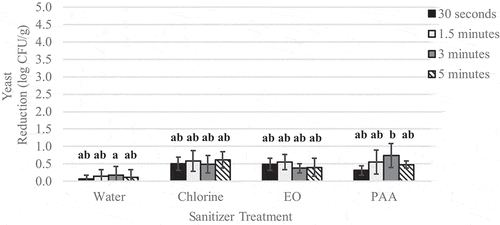
Spraying alone resulted in L. innocua reductions of 0.1–2.5 log CFU/g () and yeast population reductions of 0.1–1.0 log CFU/g (). Across all contact times, spraying with chlorine, PAA or EO resulted in yeast population reductions of 0.6–0.7 log CFU/g, and was significantly (p < .05) more effective than spraying with water alone (0.2 log CFU/g reduction).
Figure 4. Reduction of L. innocua on inoculated wild blueberries sprayed with sanitizer for increasing time intervals
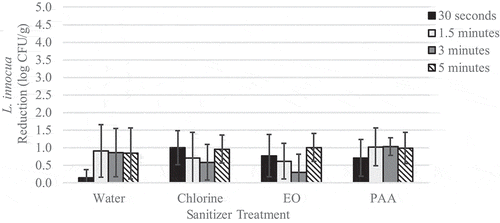
Figure 5. Reduction of native yeast on wild blueberry spray treated with sanitizer for increasing time intervals
Combination Spray
Sequential sprays of sanitizer reduced L. innocua populations by 0.5–2.1 log CFU/g (). Except for EO followed by chlorine, none of the treatments applied significantly (p < .05) reduced L. innocua populations compared to the untreated control or treatment with water alone. Yeast population reductions ranged from 0.0 to 0.8 log CFU/g () and with the exception of water followed by water, and EO followed by chlorine were significantly (p = .0) reduced when compared to the untreated control. The application of chlorine followed by PAA was the most effective treatment and resulted in a population reduction of 0.8 log CFU/g.
Figure 6. Reduction of L. innocua on inoculated wild blueberries sequentially sprayed with sanitizer
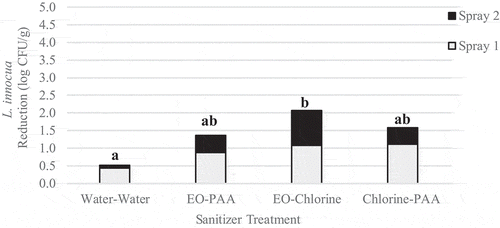
Figure 7. Reduction of native yeast on wild blueberries sequentially sprayed with sanitizer
Immersion Followed by Spray
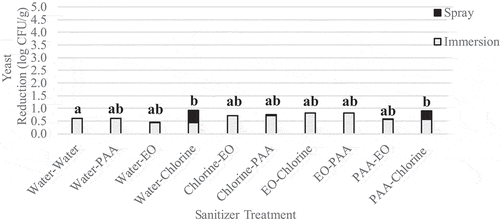
Immersing followed by spraying was the most effective method of application at reducing L. innocua populations. L. innocua population reductions ranged from 1.3 to 3.2 log CFU/g (). All treatments investigated, excluding water followed by water, significantly (p < .05) reduced Listeria populations compared to the untreated control. Immersion in EO followed by spraying with PAA was the most effective treatment against L. innocua, and significantly (p = .03) reduced L. innocua populations to a greater extent than immersion in water followed by spraying with water. Immersion in PAA followed by spraying with chlorine and water followed by chlorine was the most effective treatment against yeast, and significantly (p = .02) reduced yeast populations compared to the untreated control ().
Figure 8. Reduction of L. innocua on inoculated wild blueberries sequentially immersed then sprayed with sanitizer
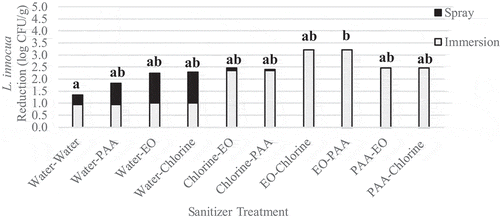
Figure 9. Reduction of native yeast on wild blueberries sequentially immersed then sprayed with sanitizer
Pilot-Scale Study
Pre-treatment L. innocua populations ranged from 4.0 to 5.2 log CFU/g with an average of 4.7 log CFU/g. Pre-treatment levels of native yeast ranged from 7.0 to 7.1 log CFU/g with an average of 7.0 log CFU/g.
Efficacy of Sanitizer Treatments and Frozen Storage on Inoculated Wild Blueberries
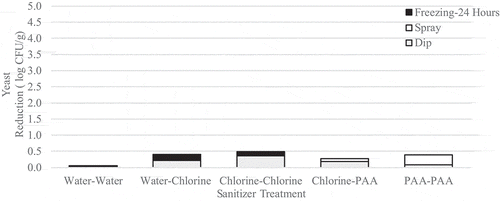
Immersion followed by spraying with sanitizer reduced L. innocua populations by up to 2.2 log CFU/g (). Statistically, none of the treatment combinations significantly (p > .05) reduced L. innocua populations compared to the untreated control, but immersion in PAA followed by spraying with PAA resulted in the largest reduction of L. innocua. Similar to the benchtop study, yeast population reductions remained low and reached a maximum reduction of only 0.5 log CFU/g (). While none of the treatments investigated significantly (p > .05) reduced yeast populations when compared to the untreated control, the application of chlorine followed by chlorine was the most effective combination against these organisms. Coliform presence appeared to be unaffected by treatment but was reduced by subsequent freezing (). Although we observed slight variability in coliform positive samples, there was a downward trend in presence post-freezing (4 out of 30 positive samples, or 13%). The same cannot be said for L. innocua, which was present in 11/15 samples tested following 2 weeks of frozen storage ().
Table 1. Positive coliforms detected in sanitizer-treated, frozen wild blueberries
Table 2. Survival of L. innocua in sanitizer-treated, inoculated wild blueberries after 2 weeks of frozen storage
Figure 10. Reduction of L. innocua on inoculated wild blueberries immersed then spray treated with sanitizer followed by freezing
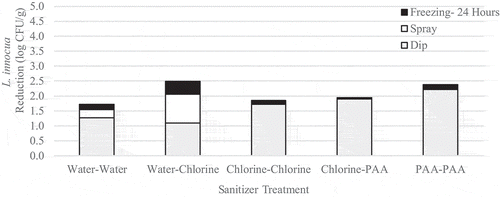
Figure 11. Reduction of native yeast on wild blueberries immersed then spray treated with sanitizer followed by freezing
Discussion
The goal of this study was to explore chlorine-alternative, aqueous sanitization methods for wild blueberries that could be integrated into current processing practices with a minimal capital expense. Treatment of inoculated wild blueberries at the pilot scale was conducted for evaluation of sanitizer efficacy more representative of a large-scale processing environment. Additionally, frozen storage for up to 2 weeks allowed for exploration of the synergy between sanitization and freezing against L. innocua. Our results can be used as a tool for producers to choose proper processing interventions to mitigate food safety risks and quality concerns associated with this product.
Due to wild blueberries being produced solely in the Northeastern United States, relatively little research on aqueous sanitization of this crop has been published. Those studies that have been published have not taken into account equipment limitations of existing processing lines and/or have been conducted at bench scale. As demonstrated by the results of this study, the introduction of confounding factors unavoidably introduced as scale increases is expected to result in reduced treatment efficacy in commercial settings. A similar study on wild blueberries previously conducted at the University of Maine investigated the efficacy of chlorine, 0.5% citric acid, and 0.5% hydrogen peroxide against native yeast, mold, coliforms, and total aerobic bacteria with the addition of frozen storage for up to 5 weeks (Crowe and Bushway, Citation2002). Investigators found that chlorine was most effective at reducing populations of interest followed by hydrogen peroxide, and citric acid (Crowe and Bushway, Citation2002). Authors reported that none of the treatments investigated significantly (p > .05) reduced L. innocua, yeast, coliforms, total aerobic bacteria, or mold populations which may be attributed to inherent variability in the product and present microflora on incoming fruit. These results agree with those in our study, which showed that despite L. innocua of up to 2.2 log CFU/g variability was too great to observe any significant differences between treatments.
In our study, wild blueberries were inoculated via swirling in the inoculum solution to achieve purposeful heterogenous distribution of bacterial cells. Additionally, propagation of bacterial cells on solid, as opposed to the liquid medium, and an extended post-inoculation drying step were both employed to increase the resistance of inoculum to treatment. The purpose of this type of inoculation was to mimic the uneven distribution and desiccation of microbial populations typically encountered in the field. Uneven distribution of bacterial cells in addition to the location of bacterial cells can affect how the sanitizer interacts with the product and ultimately how effective the sanitizer is against microbial populations (Allende et al., Citation2008). In addition to the uneven distribution of bacterial cells, yeast populations on incoming fruit were approximately 7.0 log CFU/g. The presence of competing microorganisms, such as yeast, has been shown to interfere with the efficacy of sanitizer treatments due to an increase in the organic load (Allende et al., Citation2008). Yeast is found in the environment and populations change year-to-year due to the current conditions in the field. Weather, soil amendments, harvesting practices, and geographic location can contribute to native microflora on produce and may vary significantly across harvest seasons (Beuchat, Citation2002). Processors should be aware of the potential for fluctuations in native microflora to affect the success of post-harvest mitigation strategies against pathogenic bacteria.
Similar to previous research on L. monocytogenes survival in frozen products, we found viable L. innocua cells in a high percentage of samples after 2 weeks of frozen storage. Other studies have assessed the survival of L. monocytogenes at freezer temperatures and have recorded survival for up to 12 weeks of frozen storage (Flessa et al., Citation2005; Oyarzábal et al., Citation2003). This is a concern for processers due to outbreaks that have been associated with frozen products (CDC Citation2015, Citation2016). Sanitizer treatments investigated reduced L. innocua by up to 2.2 log CFU/g at the pilot scale. To be considered a “kill step”, post-harvest treatments must reduce pathogenic bacteria by 5 log CFU/g (FDA Preventive Controls, Citation2018). Our results indicate that sanitization cannot be used as a kill step in this product due to insufficient reduction as well as demonstration of considerable variability. Wild blueberries are considered a low-risk food product and there are no historical records of outbreaks linked to this fruit. Our results suggest that if contamination was to occur, it is likely that a sanitization step would reduce the risk, but not eliminate it. Additionally, finding viable L. innocua cells after frozen storage further illustrates that freezing is not a reliable strategy for the inactivation of L. monocytogenes, even when used in combination with sanitizer application. The combination of sanitization and freezing was, however, more effective against coliforms and can likely be used as an effective strategy to reduce coliform populations below the detectable limit.
The parameters used in this study were chosen to mimic current industry processing lines and experiments were conducted at two levels of scale to explore the effect of. Wild blueberries were inoculated to mimic field contamination and treated in crowded conditions they would encounter in the processing environment. Aqueous sanitizers were applied through a dip tank followed by an overhead spray, which is the method of application most commercial-scale processers currently use. Treatment of wild blueberries at the pilot scale with the addition of frozen storage mimics the interactions between microbial communities, the product, and the effect of sanitizers. The results of this study, which illustrate minimal efficacy and significant variability in regard to Listeria inactivation, demonstrate the need for additional investigation of pre-harvest interventions to maximize product safety and quality.
Conclusion
This study aimed to assess the efficacy of alternative sanitization treatments and freezing against L. innocua and native microflora on wild blueberries. Sanitizer treatment, regardless of contact time, method or order of application, was largely unsuccessful in reducing L. innocua populations when compared to the untreated control. Application of sanitizer by immersion followed by spraying and use of maximum sanitizer concentration is recommended to achieve the largest possible population reductions on wild blueberries. A focus on reducing yeast populations as well as further investigation into cell adherence and sanitizer interference will be crucial for the production of a high-quality product. Additionally, freezing may be an effective strategy to reduce native coliform populations. Producers interested in alternatives to chlorine should not see significant differences in microbial quality if adopting PAA-based sanitizers.
Data availability
Data are available by request to the corresponding author.
Acknowledgments
Maine Agricultural and Forest Experiment Station Publication Number 3685. Funding for this project was provided by the Maine Department of Agriculture, Conservation and Forestry’s Agricultural Development Grant Program, the Maine Wild Blueberry Commission and the USDA National Institute of Food and Agriculture, Hatch project number ME0-31916 through the Maine Agricultural & Forest Experiment Station.
Disclosure statement
Authors have no financial interest in and have received no financial benefit from direct applications of this research.
Additional information
Funding
References
- Allende, A., M.V. Selma, F. Lopez-Galvez, R. Villaescusa, and M.L. Gil. 2008. Role of commercial sanitizers and washing systems on epiphytic microorganisms and sensory quality of fresh-cut escarole and lettuce. Postharvest Biol. Technol. 49(1):155–163. doi: 10.1016/j.postharvbio.2007.12.010.
- Beuchat, L.R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4(4):413–423. doi: 10.1016/S1286-4579(02)01555-1.
- Buchrieser, C., C. Rusniok, F. Kunst, P. Cossart, and P. Glaser. 2003. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: Clues for evolution and pathogenicity. FEMS Immunol. Med. Microbiol. 35(3):207–213. doi: 10.1016/S0928-8244(02)00448-0.
- Chakraborty, T., T. Hain, and E. Domann. 2000. Genome organization and the evolution of the virulence gene locus in Listeria species. Int. J. Med Microbiol. 290:167–174. doi: 10.1016/S1438-4221(00)80086-7.
- Crowe, K.M., and A.A. Bushway, (2002). Effects of post-harvest treatments on the microbiological quality and pesticide residues of lowbush blueberries. Electronic Thesis and Dissertations. 1–92. https://digitalcommons.library.umaine.edu/etd/92
- Flessa, S., D.M. Lusk, and L.J. Harris. 2005. Survival of Listeria monocytogenes on fresh and frozen strawberries. Int. J. Food Microbiol. 101(3):255–262. doi: 10.1016/j.ijfoodmicro.2004.11.010.
- Food and Drug Administration (FDA), (2018). Hazard analysis and risk-based preventive controls for human food: Draft guidance for industry. 1–306. 8 Mar. 2019 https://www.fda.gov/media/100002/download
- Gandhi, M., and M.L. Chikindas. 2007. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 113(1):1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008.
- Hill, C., P.D. Cottera, R.D. Sleatora, and C.G.M. Gahana. 2001. Bacterial stress response in Listeria monocytogenes. Int. Dairy J. 12(2–3):273–283. doi: 10.1016/S0958-6946(01)00125-X.
- Kniel, K.E., and A.E.H. Shearer. 2014. Berry Contamination. Academic Press, Cambridge, MA, 313–339.
- List of Selected Multistate Foodborne Outbreak Investigations | Foodborne Outbreaks | Food Safety | CDC [WWW Document], 2015. 22 Feb. 2019 https://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html.
- List of Selected Multistate Foodborne Outbreak Investigations | Foodborne Outbreaks | Food Safety | CDC [WWW Document], 2016. 22 Feb. 2019. https://www.cdc.gov/foodsafety/outbreaks/multistate-outbreaks/outbreaks-list.html.
- Luo, Y., W.N. Xiang, P. Millner, B. Zhou, C. Shen, Y. Yang, Y. Wu, Q. Wang, H. Feng, and D. Shelton. 2012. A Pilot plant scale evaluation of a new process aid for enhancing chlorine efficacy against pathogen survival and cross-contamination during produce wash. Int. J. Food Microbiol. 158(2):133–139. doi: 10.1016/j.ijfoodmicro.2012.07.008.
- Luo, Y., X.W. Nou, Y. Yang, I. Alegre, E. Turner, H. Feng, M. Abadias, and W. Conway. 2010. Determination of free chlorine concentrations needed to prevent escherichia coli O157. J. Food Prot. 74(3):258352. doi: 10.4315/0362-028X.JFP-10-429.
- Oyarzábal, O.A., M.C.L. Nogueira, and D.E. Gombas. 2003. Survival of Escherichia coli O157: H7,Listeria monocytogenes, and Salmonella in juice concentrates. J. Food Prot. 66(9):1595–1598. doi: 10.4315/0362-028X-66.9.1595.
- Palumbo, S.A., and A.C. Williams. 1991. Resistance of listeria monocytogenes to freezing in foods. Food Microbiol 8:63-68.
- Ruiz-Cruz, S., E. Acedo-Felix, M. Diaz-Cinco, M.A. Islas-Osuna, and G.A. Gonzalez-Aguilar. 2007. Efficacy of Sanitizers in reducing Escherichia coli O157. J. Food Control 18(11):1383–1389. doi: 10.1016/j.foodcont.2006.09.008.
- Tefera, T., K.R. Tysnes, K.S. Utaaker, and L.J. Robertson. 2018. Parasite contamination of berries. Food Waterborne Parasitol. 10:23–38. doi: 10.1016/j.fawpar.2018.04.002.
- United States Department of Agriculture (USDA), Food and Drug Administration (FDA), Center for Food Safety and Applied Nutrition (CFSAN), (1998) Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables. 1–41.
- United States Department of Agriculture Agricultural Research Service, (2016). Blueberries and Health. 1. 24 Feb. 2019 https://www.ars.usda.gov/plains-area/gfnd/gfhnrc/docs/news-2014/blueberries-and-health/.
- Yarborough, D., (2015). Production- 220- wild blueberry culture in maine. cooperative extension maine wild blueberries, 1. 24 Feb. 2019. https://extension.umaine.edu/blueberries/factsheets/production/wild-blueberry-culture-in-maine/.
