 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Brazil is one of the world’s leading fruit producers and its processing must be exploited as a means of value aggregation. The new food habits of the population show a concern with health and the search for healthier foods, but the current population model of life requires that these products do not lose their convenience and practicality. The purpose of this work was to produce spray-dried mixed pulp of passion fruit and mango and to assess physical, chemical, microbiological and morphological stability during the shelf life. The bioactive compounds of microspheres were analyzed as to the content of carotenoids, phenolic compounds, vitamin C and antioxidant activity. Microspheres were also evaluated for the stability of color, water activity, solubility, and microbiology. The microstructure and morphology of the microspheres were obtained from the analysis of the distribution of particle size, x-ray diffraction, and electron microscopy scanning. No significant losses were observed (p < .05), for bioactive compounds. The carotenoids, phenolic compounds, Vitamin C and antioxidant activity remained stable throughout the 90 days of storage of the microspheres. The powder presented stable coloring, highly soluble, microbiologically safe for consumption and low water activity. The microstructure and morphology presented the typical nature of microencapsulated products obtained by spray drying. The microencapsulation was effective in protecting the bioactive compounds by keeping them stable throughout the study period. The powder obtained can be used as a functional ingredient in food products thereby enriching them with bioactive compounds.
Introduction
The food industry has investigated several methods to preserve the bioactive compounds of fruit that are lost over their short shelf life. Some factors such as temperature, oxygen, light, and pH tend to accelerate the process of deterioration of the fruit and the loss of their nutrients, in particular, the bioactive compounds (Dias et al., Citation2015). The microencapsulation is a technique that has been used as a form of food preservation since the use of encapsulating agents in microencapsulation tends to protect the bioactive compounds against the factors of degradation (Gouin, Citation2004)
Tropical fruits such as mango and passion fruit are rich in bioactive compounds like carotenoids, phenolic compounds, and vitamin C, besides possessing pleasant flavors and smells (Da Silva and Mercadante, Citation2002; Mercandante et al., Citation1998; Ribeiro et al., Citation2007; Tharanathan et al., Citation2006). The combination of fruit as mixed pulp could increase the potential of bioactive compounds and microencapsulation tends to stabilize these bioactive compounds during the shelf life.
The bioactive compounds in fruits have been strongly associated with the reduction of the risk of non-communicable diseases, such as cardiovascular diseases, cancer, diabetes, cataracts, Alzheimer’s and other diseases linked to aging (Liu, Citation2013). Mango is a potential source of phenolic compounds and carotenoids with high antioxidant activity that helps protect the body against damage linked to oxidative stress, aiding in the reduction of degenerative diseases such as cancer, atherosclerosis, diabetes, and obesity (Masibo and He, Citation2008). The passion fruit is a tropical fruit rich in various substances such as flavonoids, carotenoids, minerals and vitamin C, which can contribute to beneficial effects such as antioxidant activity, anti-hypertensive treatment, reduction of the contents of glucose and cholesterol from the blood (Zeraik et al., Citation2010).
The obtaining of microencapsulated pulp fruit is a great way to increase the keeping qualities of the fruit due to the low water activity of the microencapsulated pulp, hindering their physical-chemical and microbiological deterioration, and in addition to retain pigments and flavorings during the storage and also facilitate the transport, storage, and handling of the final product, be it for direct consumption or as an ingredient in the preparation of other foods (El-Abbassi et al., Citation2015; Thies and Thies, Citation2005).
The microencapsulation technique consists of entrapping of an active agent, which may be small solid particles, drops of liquid or gaseous compounds, in an encapsulating agent that will act as a protective enclosure (Gharsallaoui et al., Citation2007). The spray-drying technique is well established and the most used in the food industry due to the wide availability of equipment on the market, low operating costs, high production rates, reproducibility, wide variety of encapsulation materials, encapsulation efficiency of 10–90%, powders with low water activity, ease of transportation and storage and easy handling (Dordevic et al., Citation2014; Gharsallaoui et al., Citation2007; Gouin, Citation2004). The technique consists of nebulization of a solution, emulsion or suspension in a chamber with circulating hot air and particles in contact with the hot air will lose their moisture and become spherical, with the encapsulating agent involving the active material (De Azeredo, Citation2005).
Many encapsulating agents are used in microencapsulation, but the combination of materials already widely studied with substances with functional properties is a new area to be explored. Maltodextrin is mostly used because of its high solubility and low viscosity, which are important conditions for the process of spray drying (Tonon et al., Citation2010), in addition, the possibility to combine it with encapsulating agents with functional properties such as inulin (Rivas, Citation2012), which acts as a prebiotic in the body by promoting a selective stimulation of growth and activity of a limited number of endogenous beneficial colonic bacteria such as lactobacillus and bifidobacteria, leading to luminal and systemic beneficial effects within the host (Brownawell et al., Citation2012; Gibson et al., Citation1995). Several studies have reported other important functions of prebiotics in the maintenance of health such as a decrease in serum cholesterol (Letexier et al., Citation2003) and assistance in the modulation of the immune system (Seifert and Watzl, Citation2007). A small consumption of inulin per day, 8–10 g per day, contributes to the reduction of serum triglycerides, cholesterol, and LDL cholesterol while consumption of 15 − 20 g per day assists in relieving constipation (Gibson et al., Citation1995; Rao, Citation2001).
There is a gap of exploitation of combinations of mixtures of fruit in natura and its microencapsulation, aiming to increase the potential of bioactive compounds and exploiting its potential as a functional ingredient in a foodstuff product besides being able to replace flavorings and artificial colorings. The objective of this work was to produce a powder from mixed pulp of passion fruit and mango in a spray dryer and to assess the physical-chemical, microbiological and morphological stability together with its shelf life.
Material and Methods
Materials
Mango and passion fruit was acquired from the company BelaIschia in the city of Rio de Janeiro, Brazil and stored under freezing at −18°C. The carrying agents used were maltodextrin Mor-Rex 1910, Corn Products (Mogui-Guaçu, Brazil) and inulin Orafiti Beneo Raftline ST (Tienen, Belgium). All agents used were of analytical grade.
Statistical Design
The assays were performed according to a complete factorial design 22 with triplicate in central point. The independent variables were drying air temperature and encapsulating agent concentration. The responses evaluated were encapsulation efficiency and carotenoid content. The encapsulation efficiency was calculated according to Pessoa Lemos et al. (Citation2017), as the ratio between the total carotenoids content present in the final product and the content of total carotenoids present in the mixed pulp of mango and passion fruit before entering the dryer by the equation presented below:
shows the essays performed for the statistical design, respectively.
Table 1. Statistical design of drying in spray dryer
Total Carotenoid
The method of extraction of carotenoids was performed according to Rodriguez-Amaya (Citation2001), by macerating the sample with celite and acetone, until its total discoloration. The carotenoid solution obtained was transferred to a separatory funnel with petroleum ether and then the solution was washed in distilled water until all the acetone was removed. The carotenoid solution was funnel filtered over anhydrous sodium sulfate and collected in an amber volumetric flask swelled with petroleum ether. The absorbance was read at 453 nm in a Spectrophotometer (Shimadzu Model UV-1800) (Kyoto, Japan) for the calculation of total carotenoids.
Pilot Scale Spray-Drying Production of Microspheres for Analysis of Shelf Life
Initially, mango and passion fruit pulps were defrosted and mixed in the ratio 70% of mango pulp and 30% of passionfruit pulp. They were added to 10% encapsulation agents in the ratio 2:1 inulin and maltodextrin, according to the established statistical design.
The pilot scale spray-drying was performed in a spray dryer Niro Atomizer Copenhagen – Denmark, with injector nozzle of 1.0 mm in diameter and mass flow supply used was 14 L/h and input and output air temperatures were 160°C and 90°C, respectively.
Stability Study
The stability study was performed according to Bastos et al. (Citation2012) with some modifications. For the assessment of stability, the samples were placed in Tradpouch 60 M polyethylene packaging (Iperó, Brazil) and vacuum-sealed in a Selovac sealer (São Paulo, Brazil) and stored in the incubator B.O.D. model MA 415, Marconi (Piracicaba, Brazil) at a temperature of 25°C. The samples were analyzed at 0, 30, 60 and 90 days, with regards to the contents of total phenolic compounds, ascorbic acid, total carotenoids, antioxidant activity, color, microbiology, water activity, morphology and microstructure of microspheres.
Characterization of Bioactive Compounds
Characterization of bioactive compounds and antioxidant activity of microspheres were performed at 0, 30, 60 and 90 days. The quantification of ascorbic acid was performed by the titrimetric method using 2,6-dichlorophenol indophenol from Merck (Darmstadt, Germany) (Benassi and Antunes, Citation1988). The determination of total phenolic compounds was performed through the reaction with the Folin-Ciocalteau reagent from Sigma Chemical Co (Saint Louis, MO, USA) (Singleton and Rossi, Citation1965) (Georgé et al., Citation2005). The antioxidant activity was quantified by the spectrophotometric method based on the discoloration of the free radical ABTS•+ [2,2ʹ-acid-azino–bis-(3-ethylbenz-thiazoline-6–6-sulfonic) diammonium salt] from Sigma Chemical Co. (Saint Louis, MO, USA) (Rufino et al., Citation2007). The total carotenoids were quantified as described previously.
The Colorimetric Analysis
The colorimetric analysis, using the scale CIE L*, a*, b* and CIELCh, was performed on the microspheres at 0, 30, 60 and 90 days. A Minolta colorimeter, Color Quest XE (Northants, UK) with openness of 0.375 mm in diameter, with illuminant D65/15, recommended by the CIE (International Commission on Illumination), was used to represent the average light of the day.
The coordinated L* represents the brightness of the sample, 100 (white color) to 0 (black color). The coordinated a* matches the red when it assumes positive values and the green when it assumes negative values. The coordinated b* matches shades of blue for negative values and shades of yellow for positive values. C (Chroma) indicates the color saturation, ranging from 0 to 100. The value h (hue) indicates the tone in which 90° represents the yellow tone, 180°green, 270° the blue and 0/360° the red, tone.
The total difference of color was calculated by the following equation:
The difference between the microspheres on the first day and after 90 days of shelf life was expressed by the equation, where I is the initial value and f is the final value.
Microbiological Analyses and Water Activity
In accordance with Brazilian legislation fruit products must follow the microbiological standards of RDC n°12 of January 2001, which requires that the analyses of counts of coliforms at 35°C and coliforms at 45°C and detection of Salmonella spp. (American Public Health Association, Citation2001) should be performed. The counts of coliforms at 35°C and coliforms at 45°C were analyzed quantitatively, with the results expressed in most probable number per gram (MPN/g) and detection of Salmonella spp. was done qualitatively, with the results expressed as presence or absence of the microorganism in 25 g of food. Under current law, the presence of 25 g of this microorganism in food makes the product inadequate for human consumption.
Water activity was performed with a water activity meter AquaLab model series 3TE (Pullman, EUA). The analyses were performed at 0, 30, 60 and 90 days.
Solubility of Microspheres
The solubility of the microspheres in water was determined by placing 1 g of powder in 100 ml of distilled water and then agitated for 5 min (Wang et al., Citation2009). The powder was considered soluble when after the time of solubilization no deposit was observed. The tests were carried out in triplicate.
Morphological Characteristics
The sample was placed directly on carbon double-faced adhesive tape in a metal cylinder of 10.0 mm in diameter and 10.0 mm in height. They were then coated with platinum under high vacuum in pulverization equipment brand LEICA SPRAY IN ACE600 (Wetzlar, Germany). The morphology of the microcapsules was observed with a scanning electron microscope QUANTA FEG 250, and photographed with acceleration of voltage of 30 kV.
Distribution of Particles Size
The size distribution of microcapsules was determined through the laser equipment analyzer of particles size, Sald – 2201 (Shimadzu, Japan). The samples were dispersed in isopropyl alcohol. The spread of light intensity was measured and the data distribution in cumulative volumes of 25%, 50%, and 75% were supplied by the software of the equipment.
Statistics
All analyses were performed in triplicate, with the results being expressed as the average of the three determinations and standard deviation. The results were statistically analyzed using the Tukey test at 5% significance level (p < .05) using software XLStat Pro 7.5.
Results and Discussion
Statistical Design
The results of the statistical design are presented in , together with the input temperature of the product for each test and the concentrations of encapsulating agents used.
Table 2. Results of statistical design of drying mango and passion fruit mixed pulp in spray dryer
The content of carotenoids of powders obtained in the process ranged between 2484,43 and 6216,87 µg/100g. The coefficient of determination (R2) of the carotenoid content-dependent variable was 0.86653, and was not significant at 95% confidence. The concentration of the encapsulating agent had a negative effect, that is, larger amounts of encapsulating agent led to the production of powders with smaller carotenoid contents, the interaction concentration encapsulating agent ×carotenoid was significant at 95% confidence level, this variable was a value of p = .0009.
In guac fruit microencapsulation it was observed that the total carotenoids content in powder reduced from 1.95 mg/g to 0.61 mg/g of carotenoids when the concentration of maltodextrin increased from 10% to 30% (Kha et al., Citation2010).
The efficiency of the process of encapsulation ranged between 53% and 73%, the coefficient of determination (R2) was 0,58039. The variable concentrations of encapsulating agents were significant (p = 0,02), while the variable temperature was not significant (p = 1,00), at 95% of confidence.
The efficiency of encapsulation depends on several factors such as: type of equipment used, temperature of input and output of the air, pressure, mass flow, choice of wall material, type of active material to be encapsulated, proportion wall material/active material, total solid contents, etc. (Dordevic et al., Citation2014; Robert and Fredes, Citation2015)
The use of encapsulating agents combined to the encapsulation of fruit and fruit extracts has presented greater efficiency of encapsulation than when the encapsulating agent was used alone. In astaxanthin carotenoid microencapsulation using various wall materials, such as gum Arabic, whey protein, maltodextrin, and inulin, it was observed that the use of the whey protein combined with gum arabic presented the best efficiency of encapsulation, 70.1% (Bustos-Garza et al., Citation2013).
The input temperature used in microencapsulation has been associated with the oxidation reactions and/or degradation of bioactive compounds induced by heat (Robert and Fredes, Citation2015). The use of relatively high temperatures in microencapsulation entails considerable risks of thermal degradation of bioactive compounds. When the input temperature achieved is high, it tends to result in excessive evaporation and it may cause cracks and deformations in the wall material, causing premature release of its contents and degradation of the encapsulated ingredient (Ramírez et al., Citation2015).
The best condition for drying the mango and passion fruit mixed pulp was the one that had the largest carotenoid content using the lowest concentration of encapsulating agent, resulting in greater efficiency. As the variable temperature had no influence in the process it is possible to choose the use of lower inlet temperature of microencapsulation of the mixed pulp with the intention of less energy expenditure. Thus the inlet temperature of 160°C and 10% of encapsulating material was used for drying of the mango and passion fruit mixed pulp in pilot scale for assessment of stability of the microspheres.
Shelf Life of Microspheres
Bioactive Compounds Stability
The mango and passion fruit mixed pulp remained stable over time, with no significant losses during 90 days for contents of phenolic compounds, vitamin C, total carotenoids and antioxidant activity (). These results demonstrated that the conditions of microencapsulation studied such as: percentage of mixing inulin and maltodextrin used, percentage of encapsulating agents used in the encapsulation of the mixed pulp and the input and output temperatures in the spray dryer used were ideal for the protection of bioactive compound degradation.
Table 3. Bioactive compounds of microspheres over time
Products in which their bioactive compounds are stable over time have greater potential for application in the food industry, because, in addition to enriching with ingredients rich in antioxidants, it can also be used as a source of natural coloring since the carotenoids present in mango and passion fruit give very pleasant color to foods.
Stability studies of microspheres produced from fruit showed the loss of bioactive compounds and antioxidant activity over time due to instability of these compounds combined to encapsulating agents. In the organic passionfruit encapsulation with maltodextrin stored in vacuum packaging for 360 days at a temperature of 25°C a significant loss of vitamin C, from 16.75 to 15.86 mg/100g and β-carotene, from 12.75 to 6.50 mg/100g along the shelf life was observed (Costa et al., Citation2013). In another study with passion fruit juice encapsulated with starch n-ocetenylsuccinate stored for 77 days at 7°C and 25°C, retained 77,1% and 71,5%, of vitamin C, respectively (Borrmann et al., Citation2013).
In microencapsulation of pre-concentrated lycopene in watermelon juice by microfiltration using maltodextrin and gum arabic as encapsulating agent, it was observed that after 15 days of storage at room temperature there was a significant loss of antioxidant activity and at the end of the storing period only 8,8% of initial antioxidant activity remained (Gomes et al., Citation2014).
Color Stability
The results for the coloring of the microencapsulated mango and passion fruit mixed pulp showed values with significant differences over the 90 days of shelf life (). However, the result for the total difference of colors (ΔE*) was 2.70, values of ΔE* < 3 indicated that there was no visual difference in the colors.
Table 4. Microspheres coloring over time
The luminosity (L*) increased as time went by, which may be associated with the interaction between the encapsulating agents and the pigments. In a study with organic passion fruit microencapsulation with maltodextrin, an increase in luminosity occurred during the storage. This increase of brightness was attributed to the use of additives in the processing or because of the chemical reactions resulting in degradation of the pigments (Costa et al., Citation2013).
The coordinates a* and b* presented with coloring in the range of yellow and red and yellow hue (h*) (). There was no significant difference between time 0 and 90 days in the value of Chroma. The Hue angle (h*) showed a significant difference (p ≤ 0.05) during storage, with linear behavior. Meaning the reduction of this variable was a reflection of the oscillatory behavior of b* during storage, with the degradation of pigments occurring, such as carotenoids and thus defining the behavior of Hue angle. In the study of shelf life with cashew fruit microencapsulation significant differences in coordinates a* and b* were also observed (Bastos et al., Citation2012).
Microbiological Stability
All the analyzed variables were in accordance with Brazilian legislation for fruit products, as presented in . The coliforms at 35°C and 45°C presented lower counts than 3 MPN/g throughout the shelf life, a value that is lower than that established by Brazilian legislation (102 MPN/g). For the microorganism Salmonella sp., the Brazilian legislation advocates absence of salmonella in 25 g of food. The microbiological analyses showed the product is microbiologically suitable for human consumption according to Brazilian law.
Table 5. Microbiological stability, water activity and particle size of microspheres over time
Water activity of a product is a determining factor for microbial growth. Water activity below 0.60 is considered as a limiting value for the multiplication of any microorganism, but water activity, temperature, and availability of nutrients are interdependent; thus, the closer the temperature for multiplication, the wider the range of water activity is in which microbial growth is possible, the same happens with the highest availability of nutrients. Water activity for growth of certain intrinsic microorganisms depends on factors that may act both as the medium pH, potential for oxide reduction, the presence of antimicrobial substances, etc. (Franco and Landgraf, Citation2008).
When the water activity of the microencapsulated mixed pulp was observed over the 90 days (), very low values of aw were found, where microbial growth is difficult. This result demonstrates the strength of the spray-drying process by a spray dryer that obtained lower values of aw than those found by various studies of microencapsulation of fruits that presented water activity between 0,50 and 0,38 (Costa et al., Citation2013; Ramírez et al., Citation2015; Sofía et al., Citation2014).
Water activity also has an influence on degradation reactions. Products with higher water activities cause deterioration of bioactive compounds through oxidation. Enzymatic reactions are reduced from water activities in intervals of 0.90–0.35 and the speed of the oxidation of lipids tends to decrease (Damodaran et al., Citation2009). The results of the water activity showed that the low content reflected in the stability of bioactive compounds prevented the processes of deterioration and oxidation of these compounds.
Studies of microencapsulation with water activity superior to those found in the present study reported significant losses of bioactive compounds over time. On average, at values of water activity of 0.44 significant losses of polyphenols, β-carotene, and ascorbic acid over time were observed (Costa et al., Citation2013). In bayberry microencapsulation the high water activity has promoted increased degradation of bioactive compounds, therefor the author suggested that the microspheres must have a water activity of less than 0.33 for greater stability of bioactive compounds (Fang and Bhandari, Citation2011).
Particle Size
The microspheres obtained by the spray dryer showed Gaussian distributions and unimodal, with no significant difference in the distribution of particle size during the 90 days of storage (). Thus the formation of agglomerates was not observed showing that the product is favorable to be used in ingredients of powder formulations.
The particle size found in this study is much lower than that established for the microspheres obtained by spray drying that is 10–100 µm (El-Abbassi et al., Citation2015). Several studies performed with microencapsulation of fruit juices also reported larger particle sizes than those found in the study by obtaining particles between 12,21 and 17,25 µm (Borrmann et al., Citation2013; Santana et al., Citation2013; Tatar Turan et al., Citation2015). Differences in sizes of particles can occur due to the fact that some juices and fruit pulps do not pass through the stages of refinement and these may have higher contents of soluble fibers and soluble solids and thus an increase in viscosity occurs, contributing to the increase in the size of drops during the process of atomizing (Bastos et al., Citation2012).
The encapsulating agents chosen for microencapsulation may also have an influence on the viscosity and contribute to the size of the spill generating powder with smaller sizes. The results obtained in this study can be explained through the combined use of the inulin and maltodextrin as encapsulating agents, since the proportion used of encapsulating agents (10%) contained a greater quantity of inulin, thus contributing to the formation of a less viscous product.
Solubility Over Time
The microspheres were completely solubilized after 5 min of agitation and deposits were not observed after the solubilization time. The good solubility of the powder can be attributed to the small size of particles obtained in spheres that increases the contact surface of the material with water, making its dissolution easier.
Limitations in solubility may occur according to the type of material to be encapsulated and the encapsulating agent chosen. Fruits rich in soluble substances, with high levels of cellulose tend to have low solubility requiring steps of refinement of the pulp for better use of the fruit. In guac fruit microencapsulation, low solubility was observed due to the high content of fatty acids, carotenoids, tocopherol and cellulose present in fruit (Kha et al., Citation2010).
Morphological Characteristics
The morphological characteristics of the microspheres during the period of 90 days are presented in . The microspheres produced by spray drying showed the same characteristics over time, presenting uniformity between the sizes of the microspheres, with smooth surfaces and some small depressions. The external surfaces showed continuous walls without fissures or cracks. It is known that the presence of fissures and cracks can influence the gas permeability and the loss of volatile compounds responsible for the aroma of the product. The absence of rough surfaces is a good indicator for the flow properties, as the lower presence of depressions in the microspheres makes the flow easier. The presence of a large number of intact walls and uniformity of the microspheres resulted from a process of more perfect microencapsulation (Barros and Stringheta, Citation2006)
Figure 1. Scannning electronic microscopy (SEM) photographs of mixed pulp of mango and passion fruit microspheres in times of shelf life of 0, 30, 60, and 90 days
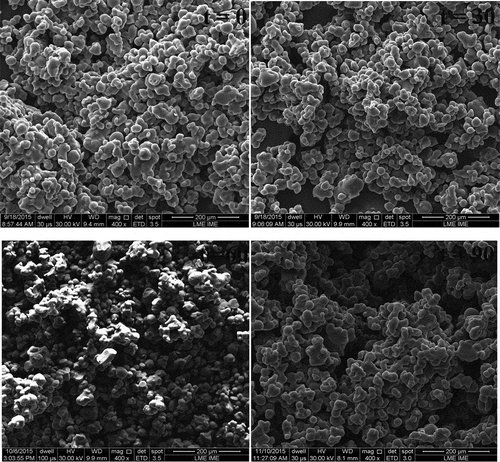
The morphology presented in the work is typical of microspheres obtained by spray drying (Borrmann et al., Citation2013; Tatar Turan et al., Citation2015). The amount of toothed surfaces or depressions and the uniformity of the microspheres are related according to the type of encapsulated material.
X-Ray Diffraction
In the inulin and maltodextrin used as encapsulating agent diffractograms are indicated, respectively. It is possible to observe the presence of a single large peak and many noises, indicating that these materials are in the amorphous phase. The diffractograms of not only the inulin but also the maltodextrin presented signs of diffraction 2Ɵ equal to 20°.
Figure 2. X-ray diffractogram of encapsulating agents inulin (A) maltodextrin (B) and microspheres during storage
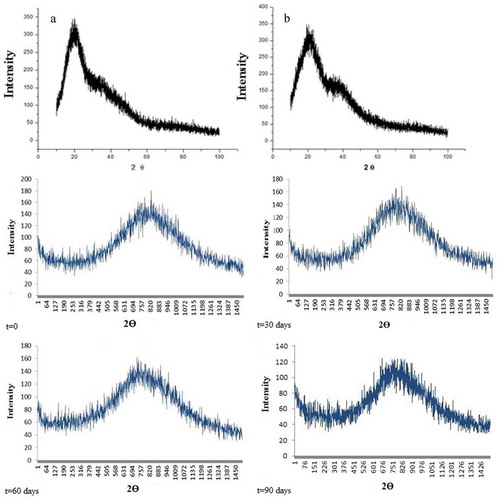
The X-ray diffractograms showed that the microspheres obtained from mango and passion fruit mixed pulp presented an amorphous state over the 90 days of storage (). It is possible to observe the absence of crystallinity of the matrix, which is presented in the vitreous condition. The diffractogram demonstrated the presence of many noises and the presence and a sign of diffraction 2Ɵ equal to 820°.
This result showed that during this period the sample did not suffer changes such as collapse, agglomeration, and viscosity. Typical changes occur when the change of amorphous state to “elastic state” and then to the crystal state. Thus the amorphous state of microspheres confirms the stability of powder in respect of bioactive compounds, particle size, water activity, microbiology, and solubility.
Some studies of microencapsulation of fruits have analyzed the behavior of the vitreous structure of microspheres over time and have observed that the change of the amorphous state to the crystalline state caused changes in the stability of the compounds and in the solubility of the microspheres. The change from the amorphous state to the crystalline state tends to impair the storage of the microencapsulated powders since this change implies water absorption, weight gain, potential collapse of the microstructure and microbiological instability (Borrmann et al., Citation2011, Citation2013).
Conclusion
The spray drying of a mixed formulation of 70% mango pulp and 30% of passion fruit pulp with maltodextrin and inulin resulted in stable microspheres storage for 90 days. The microspheres showed no significant decrease in the levels of phenolic compounds, carotenoids, vitamin C and the amount of antioxidant activity. There was no change in the total difference of color (ΔE) of microspheres over time, thus showing stability in coloration of the product. The powder was maintained with low water activity over the 90 days of storage, showing its ability for consumption, according to microbiological characteristics recommended by Brazilian law. The morphological characteristics of the powder showed that the microspheres remained physically stable, maintaining its medium size and its shape. Regarding its thermal properties (glassy state) microspheres are presented in the amorphous state. The powder obtained has the potential to be used as functional ingredient in food products as a source of bioactive compounds, natural aroma, and colorant.
References
- American Public Health Association. 2001. Compendium of methods for the microbiological examination of foods. American Public Health Association, Washington, DC.
- Brasil. (1997). Portaria No. 451 de 19 de setembro de 1997. Regulamentos técnicos.
- Barros, F.A.R.D., and P.C. Stringheta. 2006. Microencapsulamento de Antocianinas – Uma Alternativa Para o Aumento de Sua Aplicabilidade Como Ingrediente Alimentício. Biotecnol. Ciênc. E Desenvolvimento 36:18–24.
- Bastos, D.D.S., M.D.P. Gonçalves, C.T. De Andrade, K.G.D.L. Araújo, and M.H.M. Da Rocha Leão. 2012. Microencapsulation of cashew apple (Anacardium Occidentale, L.) juice using a new chitosan-commercial bovine whey protein isolate system in spray drying. Food Bioprod. Process. 90(4):683–692. doi: 10.1016/j.fbp.2012.04.005.
- Benassi, M.T., and A.J. Antunes. 1988. Comparison of metaphosphoric and oxalic acids as extractant solutions for the determination of vitamin C in selected vegetables. Braz. Arch. Biol. Technol. 31:507–513.
- Borrmann, D., S.G.F. Leite, and M.H.M. Rocha-Leão. 2011. Microencapsulation of passion fruit (Passiflora) juice in capsul®. Int. J. Fruit Sci. 11(4):376–385. doi: 10.1080/15538362.2011.630299.
- Borrmann, D., A.P.T.R. Pierucci, S.G.F. Leite, and M.H.M. Rocha-Leão. 2013. Microencapsulation of passion fruit (Passiflora) juice with n-octenylsuccinate-derivatised starch using spray-drying. Food Bioprod. Process. 91(1):23–27. doi: 10.1016/j.fbp.2012.08.001.
- Brasil. 2001. Resolução da Diretoria Colegiada, RDC no 12 de 02 de Janeiro de 2001. Aprova o “Regulamento técnico sobre padrões microbiológicos para alimentos“ ANVISA - Agencia Nacional de Vigilância Sanitária.
- Brownawell, A.M., W. Caers, G.R. Gibson, C.W.C. Kendall, K.D. Lewis, Y. Ringel, and J.L. Slavin. 2012. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J. Nutr. 124:962–974. doi: 10.3945/jn.112.158147.
- Bustos-Garza, C., J. Yáñez-Fernández, and B.E. Barragán-Huerta. 2013. Thermal and PH stability of spray-dried encapsulated astaxanthin oleoresin from haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 54(1):641–649. doi: 10.1016/j.foodres.2013.07.061.
- Costa, J.N., R.W. Da, P.H.M. Figueiredo, M.L.D.C. De Sousa, P.B.L. Gonzaga Constant, and D.J. Soares. 2013. Study of the stability of passion fruit powder (Passiflora Edullis f. Flavicarpa) from organic farming. Semina Cienc. Agrar. 34:705–716.
- Da Silva, S.R., and A.Z. Mercadante. 2002. Composição de Carotenóides de Maracujá-Amarelo (Passiflora Edulis Flavicarpa) in Natura. Ciênc. Tecnol. De Alimentos 22(3):254–258. doi: 10.1590/S0101-20612002000300010.
- Damodaran, S., K.L. Parkin, and O.R. Fennema. 2009. Química de Alimentos de Fennema. Artmed Editora.
- De Azeredo, H.M.C. 2005. Encapsulação: Aplicação À Tecnologia De Alimentos. Alimentos E Nutrição 16:89–97.
- Dias, M.I., I.C.F.R. Ferreira, and M.F. Barreiro. 2015. Microencapsulation of bioactives for food applications. Food Funct. 6(4):1035–1052. doi: 10.1039/C4FO01175A.
- Dordevic, V., B. Balanc, A. Belscak-Cvitanovic, S. Levié, K. Trifkovic, A. Kalusevic, I. Kostic, D. Komes, B. Bugarski, and V. Nedovic. 2014. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 7(4):452–490.
- El-Abbassi, A., S. El Fadeli, L. El-Bouzidii, M. Lahrouni, and K. Naumam. 2015. Recent advances in microencapsulation of bioactive compounds. Anal. Process. Tech. 41:129–146.
- Fang, Z., and B. Bhandari. 2011. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 129(3):1139–1147. doi: 10.1016/j.foodchem.2011.05.093.
- Franco, B.D.G.M., and M. Landgraf. 2008. Microbiologia de Alimentos. Atheneu, São Paulo, Brasil.
- Georgé, S., P. Brat, P. Alter, and M.J. Amiot. 2005. Rapid determination of polyphenols and vitamin c in plant-derived products. J. Agric. Food Chem. 53:1370–1373. doi: 10.1021/jf048396b.
- Gharsallaoui, A., G. Roudaut, O. Chambin, A. Voilley, and R. Saurel. 2007. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 40(9):1107–1121. doi: 10.1016/j.foodres.2007.07.004.
- Gibson, G.R., E.R. Beatty, X. Wang, and J.H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108(4):975–982. doi: 10.1016/0016-5085(95)90192-2.
- Gomes, F.S., L.M.C. Cabral, S. Couri, M.B.D. Campos, and P.A. Costa. 2014. Lycopene content and antioxidant capacity of watermelon powder. Acta Hortic. 1040:105–110. doi: 10.17660/ActaHortic.2014.1040.13.
- Gouin, S. 2004. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 15(7):330–347. doi: 10.1016/j.tifs.2003.10.005.
- Kha, T.C., M.H. Nguyen, and P.D. Roach. 2010. Effects of spray drying conditions on the physicochemical and antioxidant properties of the gac (Momordica Cochinchinensis) fruit aril powder. J. Food Eng. 98(3):385–392. doi: 10.1016/j.jfoodeng.2010.01.016.
- Letexier, D., F. Diraison, and M. Beylot. 2003. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am. J. Clin. Nutr. 77(3):559–564. doi: 10.1093/ajcn/77.3.559.
- Liu, R.H. 2013. Dietary bioactive compounds and their health implications. J. Food Sci. 78(Suppl 1):A18–25. June. DOI: 10.1111/1750-3841.12101.
- Masibo, M., and Q. He. 2008. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 7(4):309–319. doi: 10.1111/crfs.2008.7.issue-4.
- Mercandante, A.Z., G. Britton, and D.B. Rodríguez-Amaya. 1998. Carotenoids from yellow passion fruit (Passiflora Edulis). J. Agric. Food Chem. 46:4102–4106. doi: 10.1021/jf9801724.
- Pessoa Lemos, Y., P. Henrique Mariano Marfil, and V. Regina Nicoletti. 2017. Particle size characteristics of buriti oil microcapsules produced by gelatin-sodium alginate complex coacervation: Effect of stirring speed. Int. J. Food Prop. 20(S2):S1438–S1447.
- Ramírez, M.J., G.I. Giraldo, and C.E. Orrego. 2015. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 277:89–96. doi: 10.1016/j.powtec.2015.02.060.
- Rao, V.A. 2001. The prebiotic properties of oligofructose at low intake levels. Nutr. Res. 21(6):843–848. doi: 10.1016/S0271-5317(01)00284-6.
- Ribeiro, S.M.R., J.H. Queiroz, M.E.L.R. de Queiroz, F.M. Campos, and H.M.P. Sant’Ana. 2007. Antioxidant in mango (Mangifera Indica L.) pulp. Plant Foods Hum. Nutr. 62(1):13–17. doi: 10.1007/s11130-006-0035-3.
- Rivas, J.C. 2012. Microencapsulamento de Polpa de Goiaba Com Material Encapsulante Prébiótico. Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ/Brasil. Dissertation type.
- Robert, P., and C. Fredes. 2015. The encapsulation of anthocyanins from berry-type fruits. Trends Food. Mol. 20:5875–5888.
- Rodriguez-Amaya, D.B.A. 2001. Guide to carotenoid analysis in foods. Ilsi, Washington.
- Rufino, M.D..S.M., Alves, R.E. de Brito, E.S. de Morais, S.M. Sampaio, C.D.G. Sampaio, Pérez-Jiménez, J. and Saura-Calixto, F.D. 2007. Metodologia Científica : Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH. Embrapa Agroindústria Tropical-Comunicado Técnico (INFOTECA-E).
- Santana, A.A., L.E. Kurozawa, R.A. De Oliveira, Santana, and J.K. Park. 2013. Influence of process conditions on the physicochemical properties of pequi powder produced by spray drying. Drying Technol. 31:825–836. doi: 10.1080/07373937.2013.766619.
- Seifert, S., and B. Watzl. 2007. Inulin and oligofructose: Review of experimental data on immune modulation. J. Nutr. 137(11 Suppl):2563S–2567S. doi: 10.1093/jn/137.11.2563S.
- Singleton, V.L., and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybidicphosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144–168.
- Sofía, T.L., V. Rafael, and Á. Sara. 2014. Stabilization of a functional refreshment from mango nectar and yacon (Smallanthus Sonchifolius) through spray drying encapsulation. Funct. Food. Health Dis. 4(2):77–86. doi: 10.31989/ffhd.v4i2.28.
- Tatar Turan, F., A. Cengiz, and T. Kahyaoglu. 2015. Evaluation of ultrasonic nozzle with spray-drying as a novel method for the microencapsulation of blueberry’s bioactive compounds. Innovative Food Sci. Emerg. Technol. 32:136–145. doi: 10.1016/j.ifset.2015.09.011.
- Tharanathan, R.N., H.M. Yashoda, and T.N. Prabha. 2006. Mango (Mangifera Indica L.), “The King of Fruits”—An overview. Food Rev. Int. 22(no. 2):95–123. doi: 10.1080/87559120600574493.
- Thies, C., and C. Thies. 2005. Microencapsulation. In kirk-othmer encyclopedia of chemical technology. John Wiley & Sons, Inc, Hoboken, NJ, USA.
- Tonon, R.V., C. Brabet, and M.D. Hubinger. 2010. Anthocyanin stability and antioxidant activity of spray-dried Açaí (Euterpe Oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 43(3):907–914. doi: 10.1016/j.foodres.2009.12.013.
- Wang, Y., Z. Lu, F. Lv, and X. Bie. 2009. Study on microencapsulation of curcumin pigments by spray drying. Eur. Food Res. Technol. 229(3):391–396. doi: 10.1007/s00217-009-1064-6.
- Zeraik, M.L., C.A.M. Pereira, V.G. Zuin, and J.H. Yariwake. 2010. Maracujá : Um alimento funcional ? Passion fruit : A functional food ? Rev. Bras. Farmacognosia 20(3):459–471. doi: 10.1590/S0102-695X2010000300026.
