ABSTRACT
Macrophomina phaseolina causes charcoal rot in strawberry. The pathogen has a wide host range, is favored by high soil temperatures, and current fumigants are not as effective as methyl bromide. Breeding strawberry cultivars resistant to M. phaseolina has become an important focus. Eleven cultivars were evaluated in a glasshouse trial for resistance to an isolate of M. phaseolina. Plants were inoculated by drenching the potting medium with a suspension of microsclerotia. Plant mortality was recorded for up to 23 weeks. Based on plant mortality and survival analyzes, ‘Albion’ was similarly susceptible as ‘Camarosa’ and a number of historical and current cultivars showed tolerance and/or resistance to the pathogen. The preliminary findings in this study can assist in the development of new strawberry genotypes against M. phaseolina.
Introduction
Macrophomina phaseolina is a soil-borne fungal pathogen that causes the strawberry crown rot disease charcoal rot. Within Australia, charcoal rot in strawberry has been reported in commercial farms in Queensland, Victoria and Western Australia (Golzar et al., Citation2007; Hutton et al., Citation2013). These three states account for 88% of the strawberry production in Australia, where the industry is valued at A$445M (Hort Innovation, Citation2019). Apart from Australia, the disease has also been reported in other strawberry producing countries including Argentina (Baino et al., Citation2011), Chile (Sanchez et al., Citation2013), France (Baudry and Morzieres, Citation1993), Iran (Sharifi and Mahdavi, Citation2012), Israel (Zveibil and Freeman, Citation2005), Spain (Aviles et al., Citation2008), Tunisia (Hajlaoui et al., Citation2015) and the United States (Koike, Citation2008a; Mertely et al., Citation2005). Sanchez et al. (Citation2016) described M. phaseolina as an emerging and devastating pathogen of strawberry.
At least 500 plant species are known hosts of M. phaseolina (Wyllie, Citation1988) including cotton where the disease is also known as charcoal rot (Ghaffar and Erwin, Citation1969), on soybeans where it causes ashy-stem blight (Dhar and Sarbhoy, Citation1987) and in rice, causing dry root rot (Than et al., Citation1991). Sorghum is also a known host of M. phaseolina in Australia (Ryley et al., Citation2008). This is a particular concern as sorghum is commonly used as a cover crop between strawberry seasons in many of the production areas in Australia. In 2015, M. phaseolina was isolated from sorghum grown as a cover crop in Wamuran, Queensland and was subsequently found to be pathogenic to strawberry (Gomez, unpublished data). Similarly, in Israel, isolates of M. phaseolina from other host plant species rotated with strawberry were found to be pathogenic to strawberry and thus highlighted the importance of avoiding rotation of crops that may host the pathogen (Zveibil et al., Citation2012). Weeds may also serve as alternative hosts as was reported in a study in which various weeds found in mung bean fields in Queensland were also hosts of M. phaseolina (Fuhlbohm et al., Citation2012).
Strawberry plants affected with charcoal rot show progressive symptoms of wilting of foliage, drying and death of older leaves with the younger leaves persisting initially but later succumbing to complete plant collapse leading to plant death (Koike et al., Citation2013). When affected crowns are cut open longitudinally, dark brown necrotic areas in the internal cortex and vascular tissues are observed (Koike et al., Citation2013).
The pathogen produces round to irregularly shaped structures, called microsclerotia that are made up of aggregations of hyphae held together by a melanized rind (Dhingra and Sinclair, Citation1978; Gangopadhyay and Wyllie, Citation1974). These are resting structures that allow the pathogen to survive in soil or in infected material as it senesces (Short and Wyllie, Citation1978). In soil, microsclerotia may allow persistence of the fungus for up to 15 years (Short et al., Citation1980).
M. phaseolina microsclerotia remained viable in bean crop residue buried in soil for 21 months (Songa and Hillocks, Citation1998), and 18 and 16 months on corn and sorghum, respectively (Cook et al., Citation1973). It is not known how long the pathogen survives in buried strawberry crop residues, but incorporating infected strawberry material into the soil may increase the microsclerotium concentration in the soil.
Microsclerotia are considered resistant to environmental variables (Olaya and Abawi, Citation1996). Soil temperature and moisture content are the two most important factors that can affect the survival of microsclerotia (Papavizas, Citation1977); however, Zveibil et al. (Citation2012) demonstrated that survival of microsclerotia of M. phaseolina was dependent on soil temperatures with soil moisture only having limited effects. High soil temperatures (greater than 27°C) have been shown to promote charcoal rot (Persley et al., Citation2010). Such high temperatures occur in the Australian strawberry growing seasons. In 2016, the monthly maximum air temperatures in the production period in South East Queensland (winter) and Victoria (summer) ranged from 27°C to 31°C and 33°C to 42°C, respectively (Bureau of Meteorology, Citation2017). In addition, the use of black-colored plastic mulch can raise soil temperatures. Under this mulch at a depth of 5 cm, soil temperatures can be 6.5°C to 9.0°C higher than air temperature (Hutton and Gomez, Citation2006). This may create more favorable conditions for the development of charcoal rot.
Historically in Australia, the majority of production fields used methyl bromide as a soil fumigant to control soil-borne pathogens, and charcoal rot was only a very minor disease. In 1997 however, under the Montreal Protocol, a global phase-out of methyl bromide was agreed upon due to the emissions being associated with the degradation of the ozone layer. Consequently, methyl bromide was phased out of Australian strawberry fruit production in 2006. Hutton et al. (Citation2013) reported the association of M. phaseolina causing crown rots in fruit production farms in Australia not long after methyl bromide was phased out. Several reports also attributed the increase in charcoal rot incidence in the last decade to the withdrawal of methyl bromide and the ineffectiveness of current soil fumigant alternatives (Koike, Citation2008b; Mertely et al., Citation2005; Zveibil and Freeman, Citation2009). In a field study, Hutton et al. (Citation2013) found soil fumigants chloropicrin and 1, 3-dichloropropene were ineffective in eradicating M. phaseolina in buried infected strawberry crowns. A recent study in California showed fungicides alone were not effective at controlling the pathogen (Carter, Citation2016).
Cultural management of M. phaseolina is difficult, with a wide host range, an ability to produce survival structures and withstand high soil temperatures, current fumigants are not as effective as methyl bromide and there are no effective fungicides against the pathogen. The management strategy of developing resistant cultivars has therefore become increasingly important. Identifying and developing pathogen-resistant strawberry cultivars is considered the most cost effective and sustainable strategy for control of crown and root diseases (Mackenzie et al., Citation2006). Consequently, the attainment of cultivars resistant to M. phaseolina is now the focus of many breeding programs internationally, with great importance put on the identification of resistance in existing strawberry genotypes to soil-borne diseases in general (Holmes et al., Citation2017).
Previous studies characterizing strawberry genotype response against M. phaseolina have used the toothpick-inoculated and microsclerotial suspension methods predominantly. Koike et al. (Citation2016) described the M. phaseolina-colonized toothpick method to be a very severe inoculation method, and as a result only showed differences in susceptibility early and for a short time after inoculations. Drenching the growing medium with a suspension of microsclerotia was considered a closer representation of what occurs in the field (Zveibil and Freeman, Citation2005). Aviles et al. (Citation2009) compared the colonized toothpick and microsclerotial suspension methods of inoculation and found that symptoms were expressed earlier in the plants inoculated using the colonized toothpick method, but no cultivar by isolate interactions were detected. In contrast, with the microsclerotia method, cultivars were found to respond differently depending on the isolate.
Strawberry cultivars that have been reported to show tolerance and/or resistance to M. phaseolina include ‘Seascape’ (Koike, Citation2008b), ‘Albion’ (Fang et al., Citation2012), ‘Aromas’ (Fang et al., Citation2012), ‘Coral’ (Aviles et al., Citation2012) and ‘Splendor’ (cited in De Los Santos et al., Citation2016). Holmes et al. (Citation2017) tested several cultivars from different breeding programs in California and found cultivars that were highly and moderately resistant, as well as those that were susceptible to M. phaseolina. However, there is limited information on the response of strawberry cultivars currently grown commercially in Australia to M. phaseolina infection. Apart from ‘Albion’, those that have been reported previously to have resistance are either no longer grown in significant numbers in Australia or were not available for testing.
In this study, 11 strawberry cultivars were screened for resistance to M. phaseolina by drenching the potting medium with a microsclerotial suspension of M. phaseolina isolate BRIP 66625. Current commercial cultivars grown in Australia and internationally, and a number of historical cultivars from the Australian collection were evaluated. The results of this study will add knowledge of cultivar response to M. phaseolina and aid in developing commercial strawberry breeding lines with potential resistance to M. phaseolina.
Materials and Methods
M. phaseolina Isolate from Infected Strawberry Plants
M. phaseolina BRIP 66625 (Queensland Plant Pathology Herbarium) was isolated from a strawberry plant (cv. Florida Radiance) showing symptoms of crown rot from Glenview in Queensland in 2009. These symptoms had included an internal necrotic rot in the crown and discolouration along the vascular tissues when cut longitudinally. The isolation was conducted using methods described by Hutton et al. (Citation2013) by placing small crown tissue pieces with necrotic symptoms on a Petri dish containing quarter-strength potato dextrose agar (PDA) amended with 50 ppm streptomycin sulfate (Sigma-Aldrich, St. Louis, MO, USA). M. phaseolina was identified based on production of microsclerotia and colony morphology as described by Holliday and Punithaligam (Citation1970). The isolate, which was hyphal-tipped prior to storage in sterile-deionized water (SDW), was subsequently verified as M. phaseolina by DNA sequencing of the internal transcribed spacer region.
Inoculum Preparation
BRIP 66625 was revived from storage by sub-culturing onto PDA amended with streptomycin, as described. The plates were incubated at 24°C in continuous near-UV light for 3 weeks. A microsclerotial suspension was prepared based on methods described by Aviles et al. (Citation2009) with the following modifications. PDA imbedded with microsclerotia was blended for 45 s (two Petri dishes with 200 mL SDW at a time) to produce a microsclerotial suspension. As indicator of concentration, the final stock of inoculum suspension was mixed thoroughly, and microsclerotia of M. phaseolina were counted from 10 samples of 0.1 mL samples of the suspension under a stereo microscope. This was repeated an additional two times to obtain an average of 1.4 × 103 microsclerotia per mL.
Strawberry Cultivars
Current commercially grown cultivars ‘Albion’, ‘Camarosa’, ‘Strawberry Festival’, ‘Florida Radiance’, ‘Red Rhapsody’, ‘Rubygem’ and ‘Suncoast Delight’ along with historical cultivars ‘Earlibrite’, ‘Kabarla’, ‘Phenomenal’ and ‘Sweet Charlie’ were utilized in the trials. Runners of ‘Albion’ and ‘Camarosa’ were purchased as certified commercial runners from Toolangi Certified Strawberry Runner Grower’s Co-operative Ltd., Victoria, Australia. Runners of all other cultivars were produced as part of the Australian National Strawberry Varietal Improvement Program at Maroochy Research Facility, Nambour. All plants were grown in 1:1 sterile peat: sand mix in 100 mm Spacesaver® pots and maintained prior to pathogen inoculations.
Screening of Cultivars
To study the response of strawberry cultivars to M. phaseolina, 50 mL of the microsclerotial suspension was poured into each pot containing a strawberry plant. Ten plants of each cultivar, with the exception due to limited availability of ‘Kabarla’ (nine plants), were inoculated. Plants from different cultivars were inoculated in a randomized block design and afterward placed in the same order in an evaporatively cooled glasshouse set to a maximum of 40°C. The pots were placed on a heated bench set at 30°C. Non-treated controls consisted of ten plants each of ‘Camarosa’ and ‘Albion’, treated with 50 mL of SDW per pot and located separate from the inoculated plants in the same glasshouse and were not included in the analysis. The two cultivars were chosen based on reported susceptibility and resistance respectively in a study in Western Australia (Fang et al., Citation2012).
All plants were assessed weekly for up to 23 weeks post inoculation during which time the incidence of plant mortality, represented by the symptom of complete plant collapse or wilt due to M. phaseolina isolate BRIP 66625, was recorded. Those plants showing symptoms were collected and isolations made for recovery of the pathogen, using the method of Hutton et al. (Citation2013) as described previously. When M. phaseolina and/or a mixed culture with M. phaseolina was present on the PDA plate, it was considered that plant death was due to M. phaseolina. If M. phaseolina was not recovered it was assumed the plant died from other causes and any such plants were eliminated from the final analysis. Control plants were similarly monitored for development of any symptoms and were also not included in the final analysis. Assessments were carried out in November to April at the Maroochy Research Facility, Nambour.
Data Analysis
First, an initial analysis of the mortality of plants from each cultivar to BRIP 66625 was performed based on whether plants were alive or dead at the final time point (week 23). The mortality data were coded as 0 (alive) and 1 (dead) for each plant. The standard analysis approach for such data is to fit a Generalized Linear Model (GLM) assuming a binomial distribution and logit link (logistic regression), however there were some cultivars with 0% mortality and others with 100% mortality showing complete separation (Albert and Anderson, Citation1984). A logistic regression model was therefore fitted, applying Firth’s correction to the likelihood (Firth, Citation1993). The R (R Core Team, Citation2015) package logistf (Heinze and Schemper, Citation2002) was used for the analysis.
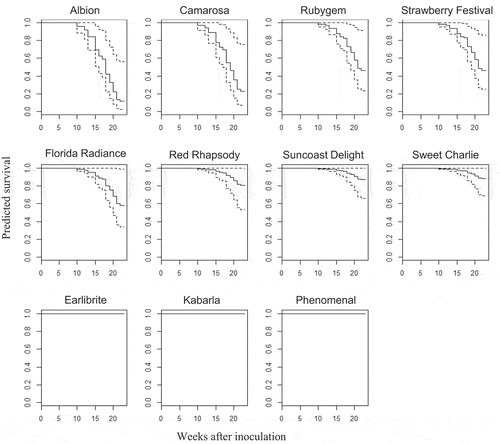
In the second analysis, strawberry plant survival data over time was analyzed using the Cox Proportional Hazards model (Cox, Citation1972) using the Survival package (Therneau, Citation2015) in R (R Core Team, Citation2015). The data consisted of a vector of times (time to event for each plant) and a vector indicating which times where deaths and which were censored (plant still alive at the end of the trial). The survival analysis was based on the hazard function for each cultivar. The hazard function is the predicted instantaneous risk of death at time t, conditional on survival to that time. It may vary over time but the proportional hazards model assumes the hazard for one genotype is a constant proportion of the hazard in other genotypes, and this proportion is called the hazard ratio (Duerden, Citation2014). Hazard ratios were predicted for each cultivar relative to the hazard of a standard reference cultivar. For this study, ‘Camarosa’ was used as the standard susceptible reference cultivar, based on reported susceptibility to M. phaseolina (Fang et al., Citation2012; Koike, Citation2008a; Sanchez et al., Citation2013). A hazard ratio for a cultivar equal to or greater than 1 suggests equal or greater susceptibility than ‘Camarosa’, i.e. higher risk of plant deaths. Hazard ratios less than 1 suggest lower susceptibility (therefore lower risk of death at any given time) than ‘Camarosa’. Cultivars that had 0% plant mortality were not included in the analysis, and would have a nil hazard. Predicted survival plots, which show the proportion of plants that survive at each time, were presented for ‘Camarosa’ and the other cultivars for resistance against M. phaseolina.
Results
Yellowing and early stage of necrosis of leaves were first observed on plants 2 weeks after inoculation. The first signs of plant wilt due to M. phaseolina were recorded on an ‘Albion’ plant 10 weeks following inoculation. At the final time point (23 weeks after inoculation), plant mortality varied significantly between cultivars (P < .001) based on Firth’s method for logistic regression. The predicted proportion dead at week 23 for each cultivar is given in . Based on confidence intervals, the proportion dead at the final time point for ‘Albion’ and ‘Camarosa’ were significantly higher than that of ‘Phenomenal’, ‘Earlibrite’ and ‘Kabarla’. ‘Sweet Charlie’ and ‘Suncoast Delight’ have significantly lower mortality than ‘Albion’ but not ‘Camarosa’. The proportion dead for ‘Red Rhapsody’ is significantly lower than ‘Albion’. The proportion dead for ‘Albion’ at the final time point was higher than ‘Camarosa’, but not statistically different.
Table 1. Regression coefficients, standard errors and 95% confidence intervals (CI) together with predicted proportion dead (%) on the backtransformed original scale for each cultivar at week 23 after inoculating with M. phaseolina isolate BRIP 66625 from the logistic regression model using Firth’s method
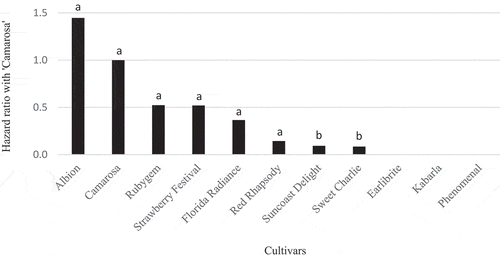
Hazard ratios from the survival analysis for each cultivar are shown in . ‘Phenomenal’, ‘Kabarla’ and ‘Earlibrite’ had 0% mortality, therefore hazard ratios of zero. ‘Sweet Charlie’ and ‘Suncoast Delight’ had significantly lower hazard ratios than the reference cultivar ‘Camarosa’ (P ≥ 0.05). The hazard ratios of other cultivars were not significantly different from ‘Camarosa’. ‘Albion’ had a hazard ratio greater than 1 (1.447) however was not significantly different from ‘Camarosa’.
Figure 1. Hazard ratios for cultivars tested compared to the reference cultivar ‘Camarosa’ when challenged with M. phaseolina isolate BRIP 66625. A hazard ratio of 1 or more implies equal or greater susceptibility than the control ‘Camarosa’. Hazard ratios less than 1 imply greater resistance than ‘Camarosa’. Columns with the same subscript are not significantly different in response to the pathogen (P ≥ 0.05)
The predicted survival proportion for each cultivar over time is presented in . As expected, for the reference (susceptible) cultivar ‘Camarosa’, the analysis predicted a very low proportion of survival at 23 weeks after inoculation. The survival prediction for ‘Albion’ was similar to ‘Camarosa’. Predicted survival proportions for ‘Florida Radiance’, ‘Strawberry Festival’ and ‘Rubygem’ ranged between 0.4 and 0.6 at 23 weeks. ‘Suncoast Delight’ and ‘Sweet Charlie’ had a predicted survival proportion of over 0.8, followed closely by ‘Red Rhapsody’. With 0% mortality in this study, the predicted survival for ‘Phenomenal’, ‘Kabarla’ and ‘Earlibrite’ was 1.
Discussion
In this study, cultivars ‘Camarosa’ and ‘Albion’ were the most susceptible to M. phaseolina. As described earlier, ‘Camarosa’ was used as a reference cultivar based on several reports of susceptibility to M. phaseolina. One of those was a study by Fang et al. (Citation2012) in Western Australia, where the authors also reported that ‘Albion’ was resistant. This however differs from the results observed here where ‘Albion’ was rated as having similar susceptibility to ‘Camarosa’. Reports from California (Koike et al., Citation2016) and Chile (Sanchez et al., Citation2016) also showed that ‘Albion’ plants exhibited a high mortality rate of 100% and 60%, respectively, when challenged with M. phaseolina. Additionally in California, Holmes et al. (Citation2017) ranked ‘Albion’ as highly susceptible to M. phaseolina after screening several existing cultivars and breeding lines from different strawberry breeding programs. The result for ‘Albion’ in this glasshouse study is supported by observations in Queensland, Australia’s Granite Belt region during summer production, where high plant losses of ‘Albion’ in recent years have been attributed to M. phaseolina (Gomez, unpublished data). In Western Australia, Fusarium oxysporum f. sp. fragariae is regarded as the major pathogen associated with crown rot diseases (Fang et al., Citation2011). However, M. phaseolina has been isolated from ‘Albion’ from a Western Australian fruit farm (Gomez, unpublished data). Assessment of ‘Albion’ in Western Australia warrants further investigation to determine if current isolates may be able to overcome the resistance and so exhibit a shift in the pathogen profile, or if indeed M. phaseolina from Western Australia is distinct from that in the eastern states of Australia.
Based on the hazard ratios, ‘Strawberry Festival’, ‘Rubygem’, ‘Florida Radiance’ and ‘Red Rhapsody’ were not statistically different to the reference cultivar ‘Camarosa’. A study by Sanchez et al. (Citation2016) reported ‘Florida Radiance’ and ‘Strawberry Festival’ plant mortality greater than 70% and 40%, respectively, when inoculated with M. phaseolina. Aviles et al. (Citation2012) also found ‘Florida Radiance’ was one of the most susceptible cultivars to M. phaseolina in Spain. Mertely et al. (Citation2005) in Florida reported 100% mortality in ‘Strawberry Festival’ when inoculated with M. phaseolina. ‘Rubygem’ is a cultivar developed by the national breeding project in Australia (Herrington et al., Citation2007), made available commercially by mid-2000s, and is currently grown for winter production. The pathogen has previously been isolated from wilting plants of this cultivar from commercial farms in the Sunshine Coast, Queensland (Gomez, unpublished data). ‘Rubygem’ is also grown in other parts of the world, such as Turkey. M. phaseolina has been reported in Turkey (Yildiz et al., Citation2010), but it is not known if the pathogen has an association with ‘Rubygem’ in production fields outside Australia at the time of this study.
Interestingly, no plant mortality was recorded due to M. phaseolina isolate BRIP 66625 on the historical cultivars ‘Phenomenal’, ‘Kabarla’ and ‘Earlibrite’. ‘Phenomenal’ was developed in Queensland in the early 1900s and by 1946 was the basis for the strawberry industry in Queensland (Barnes et al., Citation2017). ‘Kabarla’ and ‘Earlibrite’ were available commercially between 1990 and early 2000. All three cultivars are no longer available commercially, but are in the Australian National Strawberry Varietal Improvement Program germplasm collection. In contrast, cultivars ‘Suncoast Delight’ and ‘Red Rhapsody’ are currently grown commercially for the Australian winter production. Both of these cultivars were developed by the Australian varietal improvement program and while the hazard ratios of each cultivars were low, it was only ‘Suncoast Delight that was significantly lower, implying greater resistance to charcoal rot, than ‘Camarosa’. ‘Sweet Charlie’ also showed high resistance, and according to our pedigree charts is a distant ancestor of both ‘Suncoast Delight’ and ‘Red Rhapsody’. ‘Phenomenal’ is a progenitor of both ‘Kabarla’ and ‘Red Rhapsody’ (Barnes et al., Citation2017). This may suggest that possible resistance from the oldest cultivar ‘Phenomenal’ may have been inherited through the crossings and development of past and current cultivars.
Differences in cultivar responses in this study compared with other studies may be due to the inoculation methods and the resistance levels of the cultivars to one local isolate of M. phaseolina. As described earlier, considering the very severe nature of jabbing a M. phaseolina-colonized toothpick into the crown of a strawberry plant, the method used in this study was to drench the growing medium with a suspension of microsclerotia, which is considered to be a closer representation of natural infection. The use of one strawberry isolate of M. phaseolina from Queensland may also explain the difference between responses of ‘Albion’ in this study compared with the study done in Western Australia (Fang et al., Citation2012). It is feasible that the two isolates differ in virulence.
Hence, further work is needed to investigate variation in virulence to strawberry by using a more extensive range of M. phaseolina isolates obtained throughout Australia. This includes testing a wide range of isolates originating from strawberry and non-strawberry (alternative) hosts.
Host-pathogen studies are integral to understanding the behavior of existing cultivars in breeding programs to develop M. phaseolina resistant genotypes (Sanchez et al., Citation2016). This preliminary study has demonstrated that current strawberry cultivars grown in Australia, including cultivars now in the germplasm collection, have varying degrees of resistance to M. phaseolina. Cultivars that showed the lowest hazard ratio and high predicted survival proportion could be used in future crossing strategies to develop new elite breeding lines with resistance to M. phaseolina in Australia, to help minimize economic losses to charcoal rot.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Albert, A., and J.A. Anderson. 1984. On the existence of maximum likelihood estimates in logistic regression models. Biometrika 71:1–10. doi: 10.2307/2336390.
- Aviles, M., S. Castillo, J. Bascon, T. Zea-Bonilla, P.M. Martin-Sanchez, and R.M. Perez-Jimenez. 2008. First report of Macrophomia phaseolina causing crown and root rot of strawberry in Spain. Plant Path 57:382. doi: 10.1111/j.1365-3059.2007.01717.x.
- Aviles, M., S. Castillo, C. Borrero, M.L. Castillo, T. Zea-Bonilla, and R.M. Perez-Jimenez. 2009. Response of strawberry cultivars: ‘Camarosa’, ‘Candonga’ and ‘Ventana’ to inoculation with isolates of Macrophomina phaseolina. Acta Hort 842:291–294. doi: 10.17660/ActaHortic.2009.842.51.
- Aviles, M., S. Castillo, C. Borrero, and A. Refoyo. 2012, February 18-22. Strawberry cultivar susceptibility to charcoal rot caused by Macrophomina phaseolina. In: Z. Yun-Tao (ed.). Book of abstracts, p. 224, 7th ISHS international strawberry symposium, Beijing, China. China Agricultural Press, Beijing, P. R. China.
- Baino, O.M., S.M. Salazar, A.C. Ramallo, and D.S. Kirschbaum. 2011. First report of Macrophomina phaseolina causing strawberry crown and root rot in North-western Argentina. Plant Dis. 95:1477. doi: 10.1094/PDIS-03-11-0193.
- Barnes, A.J., A.O. Gomez, J. Neal, and M.E. Herrington. 2017. The phenomenal ‘Phenomenal’. The cultivar developed in Queensland over a hundred years ago used in disease resistance research today. ASHS 2017 Annual Conference, Hawaii, USA, Poster Board #366. <https://ashs.confex.com/ashs/2017/webprogramarchives/Paper26832.html>.
- Baudry, A., and J.P. Morzieres. 1993. First report of charcoal rot of strawberry in France. Acta Hort 348:485–488. doi: 10.17660/ActaHortic.1993.348.99.
- Bureau of Meteorology. 2017, June 19. Daily maximum temperature 2016. <www.bom.gov.au>.
- Carter, M. 2016. Investigating novel approaches for the control of the soilborne strawberry pathogens Macrophomina phaseolina and Fusarium oxysporum f. sp. fragariae. A Thesis presented to the Faculty of California Polytechnic State University. https://digitalcommons.calpoly.edu/cgi/viewcontent.cgi?article+270&context=theses
- Cook, G.E., M.G. Boosalis, L.D. Dunkle, and N.G. Ovody. 1973. Survival of Macrophomina phaseolina in corn stalk and sorghum residue. Plant Dis. Rep. 57:873–875.
- Cox, D.R. 1972. Regression models and life tables (with discussion). J. R. Stat. Soc. Series B 34:187–220.
- De Los Santos, B., M. Chamorro, J.J. Medina-Minguez, N. Capote, A. Aguado, and F. Romero. 2016. Emerging diseases in strawberry crop: Charcoal Rot and Fusarium wilt, p. 230. In: A.M. Husaini and D. Neri (eds.). Strawberry growth, development and diseases. CAB International, Boston, MA.
- Dhar, V., and K. Sarbhoy. 1987. Ashy stem blight of soybean in India. Curr. Sci. 56:1182–1183.
- Dhingra, O.D., and J.B. Sinclair. 1978. Biology and pathology of Macrophomina phaseolina. Universidade Federal de Vicosal, Brazil.
- Duerden, M. 2014. What are hazard ratios? Hayward Medical Communications, Hayward Group, Ltd, Newmarket, United Kingdom. doi: 10.13140/RG.2.1.4447.8884.
- Fang, X.L., D. Phillips, H. Li, K. Sivasithamparam, and M.J. Barbetti. 2011. Severity of crown and root diseases of strawberry and associated fungal and oomycete pathogens in Western Australia. Australas. Plant Pathol. 40:109–119. doi: 10.1007/s13313-010-0019-5.
- Fang, X.L., D. Phillips, G. Verheyen, H. Li, K. Sivasithamparam, and M.J. Barbetti. 2012. Yields and resistance of strawberry cultivars to crown and root diseases in the field, and cultivar responses to pathogens under controlled environment conditions. Phytopathol. Mediterr. 51:69–84. doi: 10.14601/Phytopathol_Mediterr-9746.
- Firth, D. 1993. Bias reduction of maximum likelihood estimates. Biometrika 80:27–38. doi: 10.1093/biomet/80.1.27.
- Fuhlbohm, M.J., M.J. Ryley, and E.A.B. Aitken. 2012. New weed hosts of Macrophomina phaseolina in Australia. Australas. Plant Dis. Notes 7:193–195. doi: 10.1007/s13314-012-0082-6.
- Gangopadhyay, S., and T.D. Wyllie. 1974. Melanin like compound in the sclerotia of Macrophomina phaseolina. Indian Phytopathol. 27:661–663.
- Ghaffar, A., and D.C. Erwin. 1969. Effect of soil water stress on root rot of cotton caused by Macrophomina phaseoli. Phytopathology 59:95.797.
- Golzar, H., D. Phillips, and S. Mack. 2007. Occurrence of strawberry root and crown rot in Western Australia. Australas. Plant Dis. Notes 2:145–147. doi: 10.1071/DN07057.
- Hajlaoui, M.R., M. Mnari-Hattab, M. Sayeh, I. Zarrouk, A. Jemmali, and S.T. Koikie. 2015. First report of Macrophomina phaseolina causing charcoal rot of strawberry in Tunisia. New Dis. Rep. 32:14. doi: 10.5197/j.2044-0588.2015.032.014.
- Heinze, G., and M. Schemper. 2002. A solution to the problem of separation in logistic regression. Stat. Med. 21:2409–2419. doi: 10.1002/sim.1047.
- Herrington, M.E., C.K. Chandler, J.A. Moisander, and C.E. Reid. 2007. ‘Rubygem’ strawberry. Hort Sci. 42:1482–1483. doi: 10.21273/HORTSCI.42.6.1482.
- Holliday, P., and E. Punithaligam. 1970. Macrophomina phaseolina. In: (Commonwealth Mycological Institute)CMI descriptions of pathogenic fungi and bacteria. Commonwealth Mycological Institute, Kew, Surrey, England. No. 275, pp. 1–2.
- Holmes, G., K. Ivors, R. Brantley, J. Winslow, and T. Gordon. 2017, 19 Aug. Host plant resistance for management of Verticillium wilt, Fusarium wilt, and Macrophomina crown rot in California strawberries. <https://www.mbao.org/static/docs/confs/2017-sandiego/papers/3holmesg.pdf>.
- Hort Innovation. 2019. Australian horticulture statistics handbook: Fruit 2017-18. Berries – Strawberries. Horticulture Innovation Australia Limited. 8 Mar. <https://www.horticulture.com.au/globalassets/hort-innovation/resource-assets/ah15001-australian-horticulture-statistics-handbook-fruit-.pdf>.
- Hutton, D.G., and A.O. Gomez. 2006. The use of solarisation to control soil-borne diseases and weeds. In: C. Menzel and G. Waite (eds.). Final report horticulture Australia project BS01002 and BS05003, pp. 181–191. Department of Primary Industries and Fisheries, Queensland, Australia.
- Hutton, D.G., A.O. Gomez, and S.W. Mattner. 2013. Macrophomina phaseolina and its association with strawberry crown rot in Australia. Intl. J. Fruit Sci. 13:149–155. doi: 10.1080/15538362.2012.698143.
- Koike, S.T. 2008a. Crown rot of strawberry, caused by Macrophomina phaseolina, in California. Plant Dis. 92:1253. doi: 10.1094/PDIS-92-8-1253B.
- Koike, S.T. 2008b. Macrophomina crown rot - possible new production issue for strawberry in California strawberries. University of California Cooperative Extension Crop Notes July-August. Western Farm Press. http://www.westernfarmpress.com/vegetables/macrophomina-crown-rot-possible-new-production-issue-California-strawberries
- Koike, S.T., R.S. Arias, C.S. Hogan, F.N. Martin, and T.R. Gordon. 2016. Status of Macrophomina phaseolina on strawberry in California and preliminary characterization of the pathogen. Intl. J. Fruit Sci. 16:148–159. doi: 10.1080/15538362.2016.1195313.
- Koike, S.T., T.R. Gordon, O. Daugovish, M. Bolda, and K. Subbarao. 2013. Recent developments on strawberry plant collapse problems in California caused by Fusarium and Macrophomina. Intl. J. Fruit Sci. 13:76–83. doi: 10.1080/15538362.2012.697000.
- Mackenzie, S.J., D.E. Legard, L.W. Timmer, C.K. Chandler, and N.A. Peres. 2006. Resistance of strawberry cultivars to crown rot caused by Colletotrichum gloeosporioides isolates from Florida is nonspecific. Plant Dis. 90:1091–1097. doi: 10.1094/PD-90-1091.
- Mertely, J., T. Seijo, and N. Peres. 2005. First report of Macrophomina phaseolina causing a crown rot of strawberry in Florida. Plant Dis. 89:434. doi: 10.1094/PD-89-0434A.
- Olaya, G., and G.S. Abawi. 1996. Effect of water potential on mycelial growth and on production and germination of sclerotia of Macrophomina phaseolina. Plant Dis. 80:1347–1350. doi: 10.1094/PD-80-1347.
- Papavizas, G.C. 1977. Some factors affecting survival of sclerotia of Macrophomina phaseolina in soil. Soil Biol. Biochem. 9:337–341. doi: 10.1016/0038-0717(77)90006-2.
- Persley, D., T. Cooke, and S. House. 2010. Macrophomina phaseolina, p. 56. In: D. Persley, T. Cook, and S. House (eds.). Diseases of vegetable crops in Australia. The State of Queensland, Department of Employment, Economic Development and Innovation. CSIRO Publishing, Collingwood, VIC.
- R Core Team. 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna Austria. 08 Aug. 2017. <https://www.R-project.org/>.
- Ryley, M.J., D.M. Persley, D.R. Jordan, and R.G. Henzell. 2008. Status of sorghum and pearl millet diseases in Australia, p. 441–448. In: J.F. Leslie (ed.). Sorghum and millets diseases. Iowa State Press, Ames. doi: 10.1002/9780470384923.ch73.
- Sanchez, S., M. Gambardella, J.L. Henriquez, and I. Diaz. 2013. First report of crown rot of strawberry caused by Macrophomina phaseolina in Chile. Plant Dis. 97:996. doi: 10.1094/PDIS-12-12-1121-PDN.
- Sanchez, S., J.L. Henriquez, L.A. Urcola, A. Scott, and M. Gambardella. 2016. Susceptibility of strawberry cultivars to root and crown rot caused by Macrophomina phaseolina. J. Berry Res. 6:345–354. doi: 10.3233/JBR-150114.
- Sharifi, K., and M. Mahdavi. 2012. First report of strawberry crown and root rot caused by Macrophomina phaseolina in Iran. Iran. J. Plant Pathol 47:479–480.
- Short, G.E., and T.D. Wyllie. 1978. Inoculum potential of Macrophomina phaseolina. Phytopathology 68:742–746. doi: 10.1094/Phyto-68-742.
- Short, G.E., T.D. Wyllie, and P.R. Bristow. 1980. Survival of Macrophomina phaseolina in soil and residue of soybean. Phytopathology 70:13–17. doi: 10.1094/Phyto-70-13.
- Songa, W., and R.J. Hillocks. 1998. Survival of Macrophomina phaseolina in bean seed and crop residue. Int. J. Pest Manag. 44:109–114. doi: 10.1080/096708798228400.
- Than, H., M.M. Thein, and S.S. Myint. 1991. Relationship among Rhizoctonia bataticola isolates in rice-based cropping systems on colony fusion types. Int. Chickpea Newsl. 25:29–31.
- Therneau, T. 2015. A package for survival analysis in S. version 2.38. 08 Aug. 2017. <https://CRAN.R-project.org/package=survival>.
- Wyllie, T.D. 1988. Charcoal rot of soybeans- current status, p. 106–113. In: T.D. Wyllie and D.H. Scott (eds.). Soybean diseases of the North Central Region. American Phytopathological Society, St Paul, Minnesota.
- Yildiz, A., S. Benlioglu, O. Boz, and K. Benlioglu. 2010. Use of different plastics for soil solarisation in strawberry growth and time-temperature relationships for the control of Macrophomina phaseolina and weeds. Phytoparasitica 38:463–473. doi: 10.1007/s12600-010-0123-7.
- Zveibil, A., and S. Freeman. 2005. First report of crown and root rot in strawberry caused by Macrophomina phaseolina in Israel. Plant Dis. 89:1014. doi: 10.1094/PD-89-1014C.
- Zveibil, A., and S. Freeman. 2009. Methods for detection of soilborne pathogens affecting strawberry in Israel. Acta Hort 842:191–194. doi: 10.17660/ActaHortic.2009.842.26.
- Zveibil, A., N. Mor, N. Gnayem, and S. Freeman. 2012. Survival, host-pathogen interaction and management of Macrophomina phaseolina on strawberry in Israel. Plant Dis. 96:265–272. doi: 10.1094/PDIS-04-11-0299.