ABSTRACT
The optimal postharvest handling to reduce postharvest decay and maintain quality of ‘Cuello Dama Negro’ fresh dark figs grown in Spain is been studied. Different storage temperatures (0ºC and 4ºC), relative humidity (RH, 75% to 95%) and cooling strategies (delayed and intermittent cooling) were tested. Moreover, different postharvest strategies such as 1-MCP (10 ppm), two different passive modified atmosphere packaging (Xtend® and LifePack MAP), and SO2 generating pads (UVASYS, Grapetek (Pty) Ltd.), were also tested. Storage at 0ºC, 95% RH together with MAP effectively decreased postharvest rots and therefore increased the market life of ‘Cuello Dama Negro’ fresh figs, without altering the fruit quality nor the consumer liking degree. No improvement on the shelf life of the fruit was observed with the application of 1-MCP. The use of SO2 generating pads reduced the decay but detrimentally affected fruit quality by inducing skin bleaching. Low temperature from harvest to consumption is crucial for a good maintenance of quality in fresh fig. In addition, EMAP technology is a low-cost technology able to reduce decay and maintain fruit quality of fresh figs up to 2 weeks.
Introduction
The fig (Ficus carica L.), a member of the Moraceae family, is a pear-shaped infructescence called syconium that is widely marketed and consumed as fresh or dried. Worldwide, fig tree cultivation area exceeds 315,530 ha, with an estimated production of 1,152,799 t (FAOSTAT, Citation2014). Turkey is the first fig producer in the world with 23% of the world’s total production, followed by Egypt, Morocco and Algeria. The fig production in Europe has dramatically decreased from 150,000 tons per year in the 90´s to around 90,000 tons in the last decade, contributing currently with the 7% of the worldwide production. In spite of the dramatic reduction in the production of figs within Europe, fresh figs consumption is increasing during the recent years. An increase in the demand of these fruit is mainly associated with the discovery of figs as a nutritious and nutrient-dense food together with a higher exposure of the consumers to ethnic flavors. Figs are commonly used in high cuisine, for salads, dishes, or marmalades. Figs are an excellent source of minerals, vitamins, fiber and polyphenols, especially anthocyanins and other flavonoids that act as antioxidants and can protect against several common degenerative diseases (Crisosto et al., Citation2010; Pereira et al., Citation2017; Trad et al., Citation2014).
However, marketability of fresh figs is limited due to their high are very perishable at room temperature, showing early senescence, fermentation, and fungal decay that limits their storage period and marketing life (Cantín et al., Citation2011; Coviello et al., Citation2009; Doster and Michailides, Citation2007; Karabulut et al., Citation2009; Michailides et al., Citation2008). The most sensitive part of the fruit to fungal decay is the natural opening of the fruit, called ostiole, which serves as an entry for pathogens to reach the internal cavity. Also, fruit skin side cracking and ostiole-end splitting provide entry sites for fungal decay and moisture loss. In turn, the presence of the ostiole reduces the number of existing strategies that could be used for rot control in figs, since any water-based treatment by dipping or spraying after harvest may leave free water in those open entries, inducing spore germination of pathogens. Low-temperature storage, with high relative humidity (RH), is one of the most common tools currently employed to maintain quality and control spoilage of fresh figs (Cantín et al., Citation2011; Crisosto and Kader, Citation2004; Irfan et al., Citation2013). Storage at −1ºC or 0ºC with 95% RH is considered by some scientific sources to be the optimal conditions (Crisosto et al., Citation2010). Thus said, there is still confusion among growers and retailers worldwide concerning the best storage conditions to be used for different cultivars. Information about postharvest tools to extend the shelf life of fresh figs is scarce, and specially for cultivars grown in limited production areas.
Contrary to the effect shown in different climacteric fruits such as kiwifruit, apple, pear, plum, and litchi (Chiriboga et al., Citation2013; Luo et al., Citation2009; Watkins, Citation2008), postharvest 1-MCP application on fresh figs has been shown to be ineffective (D’Aquino et al., Citation2003) or only slightly delay fruit softening (Gozlekci et al., Citation2008; Sozzi et al., Citation2005). Regarding modified atmosphere packaging (MAP), some studies have shown that optimum O2 levels are between 5% and 10%, while CO2 levels should be between 15% and 20% (Crisosto and Kader, Citation2004). Among passive MAP, equilibrium modified atmosphere packaging (EMAP), is a technology that uses polymeric fims with different numbers and dimensions of microperforations, resulting in decreased levels of O2 and increased levels of CO2 within the package headspace. The use of EMAP is a low-cost alternative to controlled atmosphere storage. This technology has been shown to successfully extend the shelf life of strawberries up to 10 days when combined with low temperature (2ºC) (Sanz et al., Citation2002). However, as far as we know, scarce information exists on the use of EMAP for fresh figs. Villalobos et al. (Villalobos et al., Citation2015) showed an extension of cold storage up to 21 days in ‘San Antonio’ and ‘Banane’ breba fruit. In a recent study, Villalobos et al. (Citation2016) demonstrated also the benefits of using the EMAP technology on the storage potential of ‘Cuello Dama Blanco,’ ‘Cuello Dama Negro’ and ‘San Antonio’ fresh figs.
SO2 generating sheets are widely used worldwide for grapes, especially in the fruit used for export markets (Lichter et al., Citation2008; Palou et al., Citation2010; Zutahy et al., Citation2008). This technology is based on the reaction of the sulfite salt contained in the pads placed inside the boxes with water vapor from environmental humidity, which leads to a continuous emission of low SO2 concentrations within the packages (Nelson, Citation1983). SO2 technology has also been tested to control postharvest decay on other fruit species such as banana (Williams et al., Citation2003), lemon (Smilanick et al., Citation1995) and raspberry (Spayd et al., Citation1984). Cantin et al. (Citation2012) were the first reporting the use of SO2 generating pads alone or in combination with SO2 fumigation on fresh figs, and demonstrated that it was a useful tool to significantly reduce decay in ‘Black Mission,’ ‘Black Turkey,’ ‘Kadota,’ and ‘Sierra’ fresh figs. However, they reported a high incidence of bleaching and browning in the fruit surface.
The purpose of the trials reported herein was to probe that the use of optimal handling operations and postharvest technologies such as temperature, humidity, 1-MCP, EMAP, and SO2 generating pads could minimize postharvest losses and maximize the shelf life of ‘Cuello Dama Negro’ dark fresh figs.
Material and Methods
Fruit Materials and Postharvest Treatments
Freshly harvested fig (Ficus carica L.) of ‘Cuello Dama Negro’ dark-skin commercial cultivar grown in Alguaire (Lleida, Spain) were used in this work. The figs were harvested early in the morning and transported to IRTA-FruitCentre (Lleida) on the same day. Figs were then selected by eliminating defective fruit (bruised, other physical damage, incorrect maturity, odd color). Also, the initial weight of each box containing ca. 3,000 g of fruit was recorded to evaluate fruit loss after storage. Six trays of 30 fruit (a total of 180 fruit per treatment) were used to set up each of the nine following treatments:
0ºC at 90-95% RH
4ºC at 90-95% RH
4ºC at 75-80% RH
Delayed cooling: fruit were kept during 6 h at 20ºC and then stored in the cold room at 0ºC and 90-95% RH.
Intermittent cooling: fruit were immediately stored in the cold room at 0ºC and 90-95% RH for 3 h, then taken out to 20ºC during 3 h, and then reentered into the cold room at 0ºC and 90-95% RH.
Xtend® MAP technology: fruit trays were individually introduced in Xtend® bags (65 cm in length × 55 cm in width, Stepac L.A. Ltd, Tefen, Israel), 20 μm thick with high microperforation of 0.0016% (holes of 0.8 mm each). According to the manufacturer’s specifications, the proprietary blends of polymeric materials composing the Xtend® film had O2 permeance of 24 × 10 − 14 mol s − 1 m − 2 at 5°C and water vapor transmission rate of ~2 × 10 − 10 mol s − 1 m – 2 Pa−1, respectively. Then, trays were immediately stored at 0ºC and 90-95% RH. After transfer to shelf-life conditions at 20°C, the rubber bands were removed and the bags were opened.
LifePack MAP technology: fruit trays were individually introduced in LifePack bags (polyamide-based coextruded transparent bags 20 µm thick with high microperforation, and a size of 72 cm in length × 64 cm in width), provided by Pampols Packaging Integral S.A., Lleida, Spain (material specifications not provided by the manufacturer). Then, trays were immediately stored at 0ºC and 90-95% RH. After transfer to shelf-life conditions at 20°C, the rubber bands were removed and the bags were opened.
1-MCP 10 ppm: immediately after arrival, the fruit were allowed to equilibrate at 20ºC and then treated with 1 μL L−1 1-MCP (SmartFreshTM) during 8 h at 20ºC. 1-MCP treatment was applied using the product SmartFreshTM (Agrofresh Inc, CA, USA) following the manufacturer recommendations. After the 8 h of treatment, fruit boxes were removed stored in a cold room at 0ºC and 90-95% RH.
SO2 generating pads: immediately after arrival, commercial dual release SO2 generating pads (UVASYS, Grapetek (Pty) Ltd., Cape Town, South Africa) were placed directly on top of the tray and each box was then wrapped with a 30 μm linear low-density polyethylene (LLDPE) film. Then, the fruit were stored in the cold room at 0ºC and 90-95% RH. The SO2 pads and bags were removed on the first day of evaluation.
After the establishment of the different treatments, the fruit were kept at the above-described conditions until evaluation.
Weight Loss
Weight loss due to transpiration and respiration of the fruit during storage under different treatments was monitored and calculated by the following equation:
Weight loss (%) = ((W0-Wf)/W0) x 100, where W0 is the initial weight of the packaged fruit (0 days) and Wf is the final weight of the packaged fruit at each sampling day (d from now on).
Ethylene Production
Three repetitions of three fruit per treatment and evaluation time were sampled for ethylene production analysis at harvest. After the storage under different treatments, one repetition of approx. 300 g of fresh figs was evaluated for each treatment and storage period combination. The analysis was performed by enclosing the sample of figs (previously weighed) in a 2-L airtight glass jar for 2 h at 20ºC. Samples (1 mL) of effluent air from the flasks, were taken using a syringe and injected into a gas chromatograph (Agilent Technologies 6890, Wilmington, Germany) fitted with a FID detector and an alumina column F1 80/100 (2 m x 1/8 x 2.1, Tecknokroma, Barcelona, Spain). The injector and detector were kept at 120ºC and 180°C, respectively.
Atmosphere Composition of the Headspace
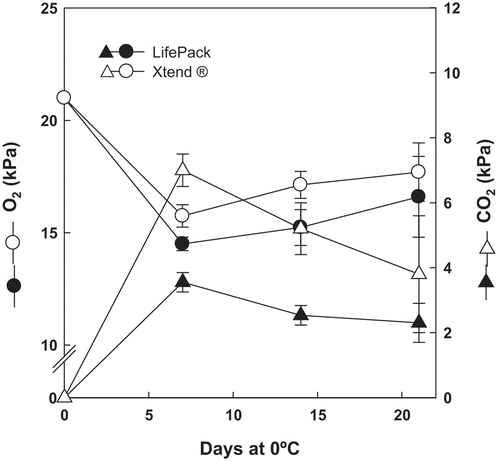
The CO2 and O2 concentrations of the headspace inside the MAP packages (LifePack and Xtend®) were monitored every 7 d during storage (up to 21 d) using an O2/CO2 gas analyzer (CheckPoint O2/CO2, PBI Dansensor, Ringsted, Denmark). The gas analyzer needle was inserted through a septum, Ø15 mm, white, hard-single use (Dansensor A/S 220235) on the outside of the bag. Results were expressed as kPa of O2 and CO2 inside the package. Oxygen concentration inside the packages decreased during storage, reaching levels around 17–18 kPa at the end of the storage time. Contrary, CO2 concentration increased during storage time reaching around 7 kPa in the case of the Xtend® package, and around 4 kPa in the LifePack package. At the end of the storage time (21 days), CO2 concentrations were 4 kPa and 2 kPa for the Xtend® and the LifePack package, respectively.
Fruit Quality Evaluation
Fruit evaluation was carried out at harvest and immediately after 7, 14 and 21 d of cold storage, as well as after 1 and 3 d of simulated shelf display at 20°C (except for treatments at 4ºC where a high incidence of decay forced to end the treatment after 7 d of storage plus 2 d of shelf-life simulation). Three replicates of 10 fruit each were used on every evaluation for each treatment-time combination. Some of the evaluations could not be done after the simulated shelf display due to the high percentage of fruit decay.
Visual fruit quality parameters including percentage of decayed fruit, percentage of fruit with side cracking, percentage of fruit with ostiole cracking and percentage of purple coverage were visually determined.
Total soluble solids (TSS), pH and titratable acidity (TA) were measured for each replicate of 10 fruit. TSS values were measured using a digital hand-held refractometer (Atago, Tokyo, Japan) and results expressed as %. TA and pH were determined in 5 g aliquots diluted to 50 mL with deionized water from a MIli-Q water purification system (Millipore, Bedord, MA) and then titrated with 0.1 mol L−1 NaOH up to pH 7.9. Results expressed as g citric acid 100 g−1 fresh weight (FW).
Fig firmness was measured by compression on two cheeks of each fruit using a TA-XT2 Texture analyzer (Stable Micro Systmes Ltd., England, UK). Ten fruit from each treatment x storage time combination were measured. Force was applied to produce a 6% deformation by a 75 mm aluminum plate. Firmness was assessed as the positive peak force at the first compression cycle, averaged, and expressed in Newtons (N). Chewiness was assessed as the force at the first significant break in the first positive bite area, and it was expressed in N.seg.
Degree of Liking
The degree of liking of suitable samples (only regular cold storage and MAP technologies could be used due to safety issues), was performed using a panel of minimum 30 untrained consumers. Fruit was tasted at harvest, and immediately after 7, 14 and 21d of cold storage. No tasting was carried out during shelf-life simulation to avoid the existence of rotten flavors in the fruit. Each consumer was asked to indicate his/her degree of liking/disliking using a nine point hedonic scale (1, dislike extremely; 9, like extremely). Consumers were volunteers from the staff working at IRTA and the University of Lleida. The samples could be re-tasted as often as desired. All evaluations were conducted in individual booths under white illumination and at room temperature.
Statistical Analysis
Homogeneity of variances was determined using Levene´s test. The quality traits were analyzed by ANOVA. For data expressed as a percentage derived from counts (decayed fruit, side, and ostiole cracking), arcsine-transformation was performed before the analysis of variance. Means were separated by Tukey’s difference test (P ≤ 0.05). Non-transformed means are presented in the tables. Analyses were performed using JMP8.0.1 software (SAS Institure Inc., Cary, NC, USA).
Results
Analysis of Headspace Composition of the EMAP Technologies
The composition of the atmosphere inside both of the packages used in this study (LifePack and Xtend®) was rapidly modified () reaching levels of O2 around 15kPa in both technologies after 7d of storage at 0ºC. Thereafter and until the end of the trial (21d) an equilibrium was reached for both EMAP technologies (ca. 17-18kPa O2). Regarding CO2, the Xtend® package led to a higher accumulation of CO2 during the first week (7kPa) if compared to LifePack (4kPa). Thus said, from 7 to 21d a decrease in the CO2 levels was observed for both packages reaching at the end of the trial 4kPa and 2kPa of CO2 for Xtend® and LifePack technologies, respectively.
Effect of Treatments on Decay Incidence
Just after harvest and also after removal of fruit from cold storage, the percentage of decay fruit rapidly grew up during simulated retail conditions at 20ºC (). After 7d of cold storage and 1d of shelf life, fruit stored at 4ºC (75-80% and 90-95% RH) showed the highest percentage of decay, ranging from 33% to 43% of decayed fruit, although was not significantly different to that observed in fruit stored at 0ºC and 95% RH (23%) or under the LifePack MAP (23%). However, a much lower incidence of decay was observed in the fruit under intermittent cooling (3%), or treated with slow-release SO2 pads (3%) or with 1-MCP (7%).
Table 1. Mean values of quality parameters and decay of ‘Cuello Dama Negro’ fresh fig measured at harvest, and after 1 or 3 days of shelf life at 20ºC
Table 2. Effect of treatments on the percentage of rotten fruit of ‘Cuello Dama Negro’ fresh fig, measured after 7 and 14 d of cold storage at 0°C plus 1, 2, and/or 3 d of shelf life at 20°C, and immediately after 21 d of cold storage at 0°C
One day later on retail simulation conditions (7d + 2d), the incidence of decay increased significantly for all the treatments, except for the fruit stored under LifePack MAP. Both MAP treatments showed the lowest percentages of rotten fruit (20%). The highest incidence was observed in the fruit stored at 4ºC and 75-80% RH (67%), although no significant differences were observed when comparing with fruit stored at 0ºC (60%), at 4ºC and 90-95% RH (53%), under delayed cooling (60%) or intermittent cooling (57%). After 3d of retail simulating conditions, decay incidence was very high for all the treatments.
Fruit stored at 4ºC (both under 80-85% or 90-95% RH) had to be discarded after 10d of storage due to the high incidence of decay affecting the fruit, and therefore was not evaluated after 14d of storage. After 14d of storage plus 1d of retail simulation, the incidence of decay was higher than 50% for all the treatments except for the fruit stored under MAP technology (LifePack and Xtend®), which showed the lowest percentages of decay (33.3 and 23.3%, respectively).
Immediately after 21d of storage (no retail simulation), fruit stored under Xtend® MAP technology showed a decay incidence of only 7%, being by far the best treatment to prevent fungal rots. Also, the LifePack MAP technology showed lower decay incidence (36.7%) than the rest of treatments.
Effect of Treatments on Visual Fruit Quality
After 7d of storage plus 1d of retail simulation ()., percentage of side cracking was statistically higher in the fruit stored under LifePack (63%) than in the fruit stored at 4ºC and 90-95% (30%) and no statistically significant differences were observed among the rest of treatments. After one more day of retail conditions (7d + 2d), side cracking incidence was significantly higher in the fruit stored at 0ºC, 95% RH and in the fruit with SO2 pads than in the rest of treatments. After 14d and 1d of retail conditions, fruit under intermittent cooling showed the lowest incidence of side cracking, whereas fruit treated with 1-MCP and SO2 pads showed the highest incidence of side cracking (80%), although it was not significantly different to the fruit stored under LifePack MAP (63%). After 21d of storage, the best treatments regarding the incidence of side cracking were delayed cooling and Xtend® MAP. No statistically significant differences were found among treatments regarding the incidence of ostiole cracking.
Table 3. Effect of treatments on the percentage of side cracking, ostiole cracking, and color coverage of ‘Cuello Dama Negro’ fresh figs, determined after 7 and 14 d of cold storage at 0°C plus 1 or 2 d of shelf life at 20°C, and immediately after 21 d of cold storage at 0°C
Storage with slow-release SO2 pads resulted in a lower coverage of color after 7d and 14d of storage plus 2d of retail conditions, and immediately after 21d of storage ().
Effect of Treatments on Physicochemical Parameters
Soluble solids content (SSC) after 7d of storage plus 1d of retail simulation ranged between 19.9% and 23.3% (), within the same range than at harvest (). Fruit stored in LifePack MAP showed significantly lower SSC (19.9%) than fruit subjected to either delayed or intermittent cooling (23.3% and 22.8%, respectively). No significant differences were observed for the rest of treatments. However, after 14d + 1d, fruit stored in LifePack MAP showed the highest SSC (23.2%) among all the treatments, whereas fruit under intermittent cooling showed the lowest (16.8%), without differing significantly from fruit cooled with an extra delay (18.3%).
Table 4. Effect of treatments on soluble solids content (SSC, ºBrix) and titratable acidity (TA, g/100 g) of ‘Cuello Dama Negro’ fresh figs, determined after 7 and 14 d of cold storage at 0°C plus 1d of shelf life at 20°C, and immediately after 21d of cold storage at 0°C
Fruit under regular cooling (0ºC and 95% RH) showed the highest value of TA (0.58%) after 7d + 1d compared to the rest of the treatments. This value, as in general for the rest of treatments, decreased during storage significantly. No differences among treatments were observed after 14d +1d.
Firmness and chewiness decreased during retail conditions for fruit at harvest () and after cold storage (). After 7d of storage plus 1d of retail simulation, no significant effect of the treatments was observed in comparison to fruit stored at either 0 or 4ºC. Differences among treatments were only noticeable when comparing fruit treated with 1-MCP and fruit stored under LifePack MAP. Moreover, after one more day of shelf life, those differences faded. After 14d of storage plus 1d of retail simulation, only fruit stored under Xtend® MAP showed significantly higher firmness than cold-stored untreated fruit.
Table 5. Effect of treatments on the hardness (N) and the fracturability (N.seg) of ‘Cuello Dama Negro’ fresh fig, determined after 7 and 14 d of cold storage at 0°C plus 1 and 2 d of shelf life at 20ºC
Regarding chewiness, after 7d+1d significant differences were only observed between 1-MCP treated fruit (which showed the highest value) and fruit under intermittent cooling and LifePack MAP.
Weight loss percentage increased throughout storage for all treatments (), not reaching in any case values higher than 10% after 21d of storage. After 7d of storage, fruit submitted to intermittent cooling showed the highest percentage of weight loss (6.8%), only comparable to the weight loss observed in the fruit stored at 4ºC and 75-80% RH (3.8%) but significantly higher than the remaining treatments. One week later (14d of storage), fruit submitted to intermittent cooling showed again one of the highest weight losses (7.5%) yet in this case not significantly different than that observed in1-MCP-treated fruit (8.3%), LifePack (7.3%) and SO2 (7.0%) treated fruit. The lowest percentage of weight loss after 14d of storage was for fruit stored at 0ºC and 95% RH. Similarly, after 21d of storage, the highest fruit weigh loss was observed on the fruit submitted to intermittent cooling (10.3%) and 1-MCP (10.0%), without being significantly different from fruit treated with SO2 pads (8.9%).
Table 6. Effect of treatments on the percentage of weight loss of ´Cuello Dama Negro´ fresh fig determined immediately after 7, 14 and 21d of cold storage at 0°C
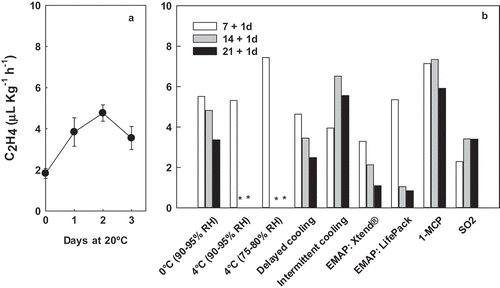
Ethylene production after 7 d of storage was higher in the treatments of 4ºC, 75-80% RH and under 1-MCP treatment than the rest of the treatments. The lowest ethylene production after 7d of storage was observed in fruit stored under the Xtend® MAP or the SO2 pads. For most treatments, ethylene production was highest upon removal from 7d of cold storage than after 14 or 21d except in fruit submitted to intermittent warming (highest at 14d) or in fruit treated with 1-MCP or the SO2 pads ().
Figure 2. (A) Ethylene production (µL Kg−1 h−1) in fruit stored at 20ºC (retail conditions) immediately after harvest. (B) Effect of treatments on the ethylene production capacity (µL Kg−1 h−1) of ´Cuello Dama Negro´ fresh fig determined after 7, 14 and 21d of cold storage at 0°C plus 1d of shelf life at 20ºC
All the treatments obtained a degree of liking score higher than acceptable (5) until the end of the storage time (21d). However, a slight decrease over time was observed for all the treatments. Results showed that consumers did not find statistically significant differences among the treatments for any of the evaluation time.
Discussion
Scarce information is available regarding the optimal postharvest strategies to control decay during shelf life of fresh figs, and therefore to minimize postharvest waste. After trying different postharvest handling operations and postharvest techniques to control decay on ‘Cuello Dama Negro’ fresh figs, the highest percentage of sound fruit was obtained when the fruit was maintained at 0ºC under EMAP up to the moment of consumption. This technology resulted very promising on extending the store time for fresh figs. The effectiveness of a controlled atmosphere on controlling fruit decay has been largely proved in other fruit species (Cantin et al., Citation2012; Serradilla et al., Citation2013) and also in breba and fresh figs (Bouzo et al., Citation2012; Villalobos et al., Citation2016). The results found in this report also highlight the importance of maintaining the temperature close to 0ºC up to the moment of consumption, even in the retail shelf display.
In our study, fruit stored under EMAP technologies (Xtend® and LifePack) showed an important decrease in the ethylene production if compared to the other treatments. Accordingly, previous works in figs have shown that CO2 enriched atmospheres greatly reduced ethylene biosynthesis, both at ambient temperature and under cooling conditions (Colelli et al., Citation1991; Mathooko et al., Citation1993). Weight loss percentages observed in our study are in agreement with previous results observed for other cultivars of figs or brebas (Bouzo et al., Citation2012; Villalobos et al., Citation2015, Citation2016). The results confirm that the microperforated films used in the EMAP Xtend® and LifePack technologies allow the maintenance of high RH inside the package, and therefore limit the weight loss of fresh figs. The lower SSC found in the fruit stored under EMAP might be due to the reduced respiratory and ethylene production rate found in fruit from these treatments and hence is also in agreement with previous studies for other fig and breba cultivars (Bouzo et al., Citation2012; Villalobos et al., Citation2015, Citation2016). We observed a delay on the decrease of TA and firmness in the fruit kept under Xtend® EMAP, which also agrees with results shown by other authors (Crisosto et al., Citation2010; Villalobos et al., Citation2015, Citation2016) and confirms the effect of the atmosphere composition on delaying fruit ripening.
Our results on the application of 1-MCP at the commercial maturity stage are in agreement with previous reports that showed no effect (D’Aquino et al., Citation2003) or just some retardation on the fruit softening (Gozlekci et al., Citation2008; Sozzi et al., Citation2005). The higher weight loss observed in this assay confirmed that ripe figs are very prone to desiccation and, therefore, any postharvest treatment used in figs must provide an appropriate maintenance of humidity for preserving the quality of fresh figs (Duong Van et al., Citation2011). Although both 1-MCP and CO2 are considered inhibitors of ethylene action, their modes of action in regulating the ethylene biosynthesis are different (Mathooko et al., Citation2001). As in our study, Sozzi et al. (Citation2005) showed that storage temperatures have a higher effect on fig firmness and fruit deterioration than 1-MCP application. The increase in ethylene production under 1-MCP treatment observed in our study has also been observed previously (Freiman et al., Citation2012; Sozzi et al., Citation2005). This increase could be due to the advance maturity stage of the fruit at the moment of harvest. Optimal harvest of figs is specific to each variety but is usually done at the beginning of the ripening phase, where changes in color and firmness happen. In our study, commercial harvest of ‘Cuello Dama Negro’ figs occurs at an advanced maturity stage since the ethylene climacteric peak occurs 2 days after harvest when the fruit is kept at 20ºC (data not shown). When 1-MCP is applied in fruit at a post climacteric stage, it may enhance the production of ethylene, as observed for other fruit species (Jeong et al., Citation2003; McCollum and Maul, Citation2007; Pelayo et al., Citation2003). The increase in ethylene production after 1-MCP application at commercial maturity indicates that harvested figs have an auto-inhibitory regulation of ethylene synthesis rather than the typical autocatalytic patter of climacteric fruit. However, Freiman et al. (Citation2012) have demonstrated the benefits of extending fresh fig storage with the pre-harvest application of 1-MCP, yet only if applied before optimal maturity.
In agreement with our results, previous work has described the harmful effect of SO2 on the postharvest quality of fresh figs (Cantín et al., Citation2011) and table grapes (Nelson, Citation1983; Zoffoli et al., Citation2008). The control of decay occurred by the SO2 treatments, agrees with previous results published on table grapes (Miklota-Gabler et al., Citation2010; Palou et al., Citation2010), blueberries (Cantin et al., Citation2012) and fresh figs (Cantín et al., Citation2011). The biocidal effectiveness of SO2 to control decay caused by B. cinerea by killing both mycelia and spores has been previously demonstrated on table grapes and figs (Cantín et al., Citation2011; Palou et al., Citation2010). In agreement with our results, other studies with different fresh fig cultivars have also shown that fruit firmness was not consistently affected by the use of SO2 generating pads (Cantín et al., Citation2011). However, the high incidence of browning and bleaching observed when SO2 generating pads were used makes this technology infeasible for use on fresh figs.
As expected, the results drawn from the consumer test show a decrease in the degree of liking with storage time, which might be related to the relative accelerated senescence of the fruit during storage. Overall, none of the treatments tested altered the consumer degree of liking and hence our results agree with previous studies where fresh figs under CA have received acceptable scores at least for 15 days of storage (Villalobos et al., Citation2015).
Conclusion
The results presented herein shows that control of low temperature from harvest to consumption is crucial for a good maintenance of quality in ‘Cuello Dama Negro’ fresh fig. In addition, we show that EMAP technology (Xtend® and LifePack), is a low cost promising technology able to reduce decay and increase the storage potential of ‘Cuello Dama Negro’ fresh figs up to 2 weeks. On the other hand, postharvest application of SO2 generating pads (UVASYS, Grapetek (Pty) Ltd.) and of 1-MCP technologies (10 ppm) did not provide any benefits to the postharvest life of ‘Cuello Dama Negro’ fresh figs.
Additional information
Funding
References
- Bouzo, C.A., M. Travadelo, and N.F. Gariglio. 2012. Effect of different packaging materials on postharvest quality of fresh fig fruit. Int. J. Agric. Biol. 14:821–825.
- Cantin, C.M., I.S. Minas, V. Goulas, M. Jimenez, G.A. Manganaris, T.J. Michailides, and C.H. Crisosto. 2012. Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 67:84–91. doi: 10.1016/j.postharvbio.2011.12.006.
- Cantín, C.M., L. Palou, V. Bremer, T.J. Michailides, and C.H. Crisosto. 2011. Evaluation of the use of sulfur dioxide to reduce postharvest losses on dark and green figs. Postharvest Biol. Technol. 59:150–158. doi: 10.1016/j.postharvbio.2010.09.016.
- Chiriboga, M.-A., M. Saladie, J. Gine Bordonaba, I. Recasens, J. Garcia-Mas, and C. Larrigaudiere. 2013. Effect of cold storage and 1-MCP treatment on ethylene perception, signalling and synthesis: Influence on the development of the evergreen behaviour in ‘Conference’ pears. Postharvest Biol. Technol. 86:212–220. doi: 10.1016/j.postharvbio.2013.07.003.
- Colelli, G., F.G. Mitchell, and A.A. Kader. 1991. Extension of postharvest life of ‘Mission’ figs by CO2-enriched atmospheres. Hortsci. 26:1193–1195. doi: 10.21273/HORTSCI.26.9.1193.
- Coviello, R., W. Bentley, T. Michailides, L. Ferguson, and B. Westerdahl 2009. UC IPM pest management guidelines: fig, p. 3447. Oakland, CA: University of California Agriculture and Natural Resources Publication.
- Crisosto, C.H., and A.A. Kader. 2004. Fig, In: Gross, K. (Ed.). The commercial storage of fruits, vegetables, florist and nursery stocks. Handb Beltsville, USA: ARS-USDA.
- Crisosto, C.H., V. Bremer, L. Ferguson, and G.M. Crisosto. 2010. Evaluating quality attributes of four fresh fig (Ficus carica L.) cultivars harvested at two maturity stages. Hortscience. 45:707–710. doi: 10.21273/HORTSCI.45.4.707.
- D'Aquino, S., A. Palma, A. Dore, and M Agabbio. 2003. Non conventional treatments to reduce figs decay. Acta Hortic 604:817–821.
- Doster, M.A., and T.J. Michailides. 2007. Fungal decay of first-crop and main-crop figs. Plant Dis. 91:1657–1662. doi: 10.1094/PDIS-91-12-1657.
- Duong Van, H., S. Tong, F. Tanaka, E. Yasunaga, D. Hamanaka, N. Hiruma, and T. Uchino. 2011. Controlling the weight loss of fresh produce during postharvest storage under a nano-size mist environment. J. Food Eng. 106:325–330. doi: 10.1016/j.jfoodeng.2011.05.027.
- FAOSTAT. 2014. http://www.fao.org/faostat/en/
- Freiman, Z.E., V. Rodov, Z. Yablovitz, B. Horev, and M.A. Flaishman. 2012. Preharvest application of 1-methylcyclopropene inhibits ripening and improves keeping quality of ‘Brown Turkey’ figs (Ficus carica L.). Sci. Hortic. 138:266–272. doi: 10.1016/j.scienta.2012.01.007.
- Gozlekci, S., M. Erkan, I. Karasahin, and G. Sahin (2008). Effect of 1-methylcyclopropene (1-MCP) on fig (Ficus carica cv. Bardakci) storage. Acta Horticulturae, Number 798, Volume 1, 325–330.
- Irfan, P.K., V. Vanjakshi, M.N.K. Prakash, R. Ravi, and V.B. Kudachikar. 2013. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 82:70–75. doi: 10.1016/j.postharvbio.2013.02.008.
- Jeong, H., D.J. Huber, and S.A. Sargent. 2003. Delay of avocado (Persea americana) fruit ripening by 1-methylcyclopropene and wax treatments. Postharvest Biol. Technol. 28:247–257. doi: 10.1016/S0925-5214(02)00176-X.
- Karabulut, O. A., K. Ilhan, U. Arslan and C. Vardar. 2009. Evaluation of the use of chlorine dioxide by fogging for decreasing postharvest decay of fig. Postharvest Biol. and Technol., 3:313–315.
- Lichter, A., Y. Zutahy, T. Kaplunov, and S. Lurie. 2008. Evaluation of table grape storage in boxes with sulfur dioxide-releasing pads with either an internal plastic liner or external wrap. Horttechnology. 18:206–214. doi: 10.21273/HORTTECH.18.2.206.
- Luo, Z., J. Xie, T. Xu, and L. Zhang. 2009. Delay ripening of ‘Qingnai’ plum (Prunus salicina Lindl.) with 1-methylcyclopropene. Plant Sci. 177:705–709. doi: 10.1016/j.plantsci.2009.08.013.
- Mathooko, F.M., T. Sotokawa, Y. Kubo, A. Inaba, and R. Nakamura. 1993. Retention of freshness in fig fruit by Co2-enriched atmosphere treatment or modified atmosphere packaging under ambient-temperature. J. Jpn. Soci. Hortic. Sci. 62:661–667. doi: 10.2503/jjshs.62.661.
- Mathooko, F. M., Y. Tsunashima, W.Z.O. Owino, Y. Kubo, A. Inaba. Regulation of genes encoding ethylene biosynthetic enzymes in peach (Prunus persica L.) fruit by carbon dioxide and 1-methylcyclopropene. Postharvest Biol. Technol., 21 (2001), pp. 265–281.
- McCollum, G., and P. Maul. 2007. 1-Methylcyclopropene inhibits degreening but stimulates respiration and ethylene biosynthesis in grapefruit. Hortscience. 42:120–124. doi: 10.21273/HORTSCI.42.1.120.
- Michailides, T.J., D.P. Morgan, D. Felts, and M.A. Doster. 2008. Control of dacay in Caprifigs and Calimyrna figs with fungicides. Acta Hortic. 798:269–275. doi: 10.17660/ActaHortic.2008.798.39.
- Miklota-Gabler, F., J.L. Smilanick, M.F. Mansour, and H. Karaca. 2010. Influence of fumigation with high concentrations of ozone gas on postharvest gray mold and fungicide residues on table grapes. Postharvest Biol. Technol. 55:85–90. doi: 10.1016/j.postharvbio.2009.09.004.
- Nelson, K.E. 1983. Effects on in-package sulfur dioxide generators, package liners, and temperature on decay and desiccation of table grapes. Am. J. Enol. Vitic. 34:10–16.
- Palou, L., M. Serrano, D. Martinez-Romero, and D. Valero. 2010. New approaches for postharvest quality retention of table grapes. Fresh Produce 4:103–110.
- Pelayo, C., S.E. Ebeler, and A.A. Kader. 2003. Postharvest life and flavor quality of three strawberry cultivars kept at 5 degrees C in air or air+20 kPa CO2. Postharvest Biol. Technol. 27:171–183. doi: 10.1016/S0925-5214(02)00059-5.
- Pereira, C., M.J. Serradilla, F. Perez-Gragera, A. Martin, M.C. Villalobos, and M. Lopez-Corrales. 2017. Evaluation of agronomic and fruit quality traits of fig tree varieties (Ficus carica L.) grown in Mediterranean conditions. Spanish J. Agric. Res. 15(3).
- Sanz, C., R. Olias, and A.G. Perez. 2002. Quality assessment of strawberries packed with perforated polypropylene punnets during cold storage. Food Sci. Technol. Inter. 8:65–71. doi: 10.1177/1082013202008002604.
- Serradilla, J.M., M. Del Carmen Villalobos, A. Hernandez, A. Martin, M. Lozano, and M. de Guia Cordoba. 2013. Study of microbiological quality of controlled atmosphere packaged ‘Ambrunes’ sweet cherries and subsequent shelf-life. Int. J. Food Microbiol. 166:85–92. doi: 10.1016/j.ijfoodmicro.2013.06.006.
- Smilanick, J.L., D.A. Margosan, and D.J. Henson. 1995. Evaluation of heated solutions of sulfur dioxide, ethanol, and hydrogen peroxide to control postharvest green mold of lemons. Plant Dis. 79:742–747. doi: 10.1094/PD-79-0742.
- Sozzi, G.O., M.A. Abrajan-Villasenor, G.D. Trinchero, and A.A. Fraschina. 2005. Postharvest response of ‘Brown Turkey’ figs (Ficus carica L.) to the inhibition of ethylene perception. J. Sci. Food Agric. 85:2503–2508. doi: 10.1002/jsfa.2296.
- Spayd, S.E., R.A. Norton, and L.D. Hayrynen. 1984. Influence of sulfur dioxide generators on red raspberry quality during postharvest storage. J. Food Sci. 49:1067. doi: 10.1111/j.1365-2621.1984.tb10393.x.
- Trad, M., C. Le Bourvellec, B. Gaaliche, C.M.G.C. Renard, and M. Mars. 2014. Nutritional compounds in figs from the southern Mediterranean region. Int. J. Food Prop. 17:491–499. doi: 10.1080/10942912.2011.642447.
- Villalobos, M.C., A. Martin, S. Ruiz-Moyano, E. Martin, M.G. Cordoba, and M.J. Serradilla (2015). Effect of modified atmosphere packaging on the antioxidant activity and total phenolic content in ‘albacor’ figs, p. 573–579. In: G.A. Manganaris, P.M. Toivonen, and P. Kalaitzis (Eds.). Lemesos (Cyprus): V International Conference Postharvest Unlimited.
- Villalobos, M.D., M.J. Serradilla, A. Martin, M.L. Corrales, C. Pereira, and M.D. Cordoba. 2016. Preservation of different fig cultivars (Ficus carica L.) under modified atmosphere packaging during cold storage. J. Sci. Food Agric. 96:2103–2115. doi: 10.1002/jsfa.7326.
- Watkins, C.B. 2008. Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. Hortscience. 43:86–94. doi: 10.21273/HORTSCI.43.1.86.
- Williams, O.J., G.S.V. Raghavan, K.D. Golden, and Y. Gariepy. 2003. Postharvest storage of Giant Cavendish bananas using ethylene oxide and sulphur dioxide. J. Sci. Food Agric. 83:180–186. doi: 10.1002/jsfa.1303.
- Zoffoli, J.P., B.A. Latorre, and P. Naranjo. 2008. Hairline, a postharvest cracking disorder in table grapes induced by sulfur dioxide. Postharvest Biol. Technol. 47:90–97. doi: 10.1016/j.postharvbio.2007.06.013.
- Zutahy, Y., A. Lichter, T. Kaplunov, and S. Lurie. 2008. Extended storage of ‘Red Globe’ grapes in modified SO2 generating pads. Postharvest Biol. Technol. 50:12–17. doi: 10.1016/j.postharvbio.2008.03.006.