?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Many researches have reported plant growth promotion activity of irradiated chitosan and sodium alginate (Na-alginate) individually. However, there is no study on the combined effect of irradiated chitosan and Na-alginate on plant growth and quality of the produces. In this study, individual effects of gamma-radiation-treated chitosan and Na-alginate were compared with the mixed solution of irradiated chitosan and Na-alginate by the means of growth and taste of pineapple. Gamma-irradiated Na-alginate treatment with an interval of 30 days showed maximum plant growth while the best pineapple fruit growth was found for the Na-alginate treatment with an interval of 15 days. On the other hand, gamma-irradiated chitosan treatment showed better fruit quality with respect to taste and flavor. However, in terms of both growth and quality, the mixed solution (90:10 irradiated Na-aliginate:chitosan) was found to be the best. A significant increase of pineapple size, sweetness, aroma, and flavor was recorded in case of the mixed treatment with 30 days of interval.
Introduction
The uses of hormones and pesticides for increasing crop’s yield have become popular in recent years. However, the plants and foods that are produced using chemical plant growth promoters and pesticides are the reason of countless healthcare-associated problems (Alewu and Nosiri, Citation2011; Hayes et al., Citation2006; Nicolopoulou-Stamati et al., Citation2016; Organization WH, Citation1990; Pimentel, Citation2005; Sanborn et al., Citation2007; Zheng et al., Citation2016). So, in the broad scenario, although the synthetic plant growth promoters and pesticides enhance economic potential in terms of increased production of food and fiber, there are also serious health implications to man and his environment due to the residual chemicals and pesticides (Forget, Citation1993; Igbedioh, Citation1991). This ultimately leads to overall economic loss by reducing total average work hours of a person and also by increasing healthcare costs. The worldwide deaths and chronic diseases due to pesticide poisoning is about 1 million per year (Amartey, Citation2013). Besides, indiscriminate uses of these chemicals or pesticides exert deleterious effects on soil microorganism, affect fertility status of soil, and also pollute environment (Youssef and Eissa, Citation2014) as well as other life forms (Aktar et al., Citation2009; Nicolopoulou-Stamati et al., Citation2016). Moreover, random use of chemicals might work for a few years, but after a while, they are not enough beneficial for soil organisms to hold onto the nutrients (Savonen, Citation1997).
Pineapple (Ananas comosus) is an important fruit and due to its great economic importance as well as nutritional value, it is much popular fruit in all over the world especially in Bangladesh. During 2014–15, total production of pineapple in Bangladesh was 4,20,000 metric tons. Like the common cultivation practices in other countries, pineapple growers of Bangladesh use harmful chemical agents like as ethrel, ethopen, ethylene, calcium carbide, combination of potash and salt, etc. as ripening agent and pranofix, boxal, cropscare, PGR gold, ocazin, etc. as growth promoter as no other commercial safe alternative is available in Bangladesh for pineapple. Due to the uses of these chemicals in high doses, now a days food safety and food security is a burning issue in the third-world country like Bangladesh. To overcome these chemical hazards in agriculture, natural biomaterials like chitosan and Na-alginate can play an indispensable role for increasing yield and quality of plants and fruits as these are well known for safe bioactive compounds.
Chitosan is a commercially available linear polysaccharide derived from chitin, a major component of the shell of crustaceans and the second most abundant biopolymer in nature next to cellulose (Islam et al., Citation2015). In addition to its low cost of production, chitosan possesses some interesting characteristics such as biocompatibility, nontoxicity, low allergenicity, and biodegradability which allow it to be used in various applications (Kumar et al., Citation2004). Moreover, it was reported that chitosan treatment made plants more tolerant to a wide range of soil and foliar pathogens and induced root nodulation (Hamel and Beaudoin, Citation2010) thus proposing chitosan as a useful tool for agricultural sustainability (Acemi et al., Citation2018; Dzung et al., Citation2017; Malerba and Cerana, Citation2016). It was also reported that sodium alginate (which is a gum, extracted from the cell walls of brown algae) possessed growth promotion, antifungal and disease-defensive effect for plants (Hien et al., Citation2000; Hossain et al., Citation2013; Parvin et al., Citation2013). In case of foliar application, sprayed oligomers are absorbed the plants by stomatal absorption and then transferred inside the cells by binding with cell membrane receptors (González et al., Citation2013; Idrees et al., Citation2011). This cellular uptake of the oligomers has been found to have a positive effect in increasing the total alkaloid content compared to the control plants. This increased alkaloid content may be ascribed to leaf nitrogen content which ultimately promotes the plant growth (AbdEl-Mohdy, Citation2017; Idrees et al., Citation2011).
On the other hand, although there are several methods like enzymatic method, chemical degradation, radiation processing, etc. to prepare these oligomers through reducing molecular weight, radiation processing is the most promising method. The process is simple and it does not require any external chemical and thus no chance of chemical residue. It reduces molecular weight through chain scission mechanism without causing alteration to the main backbone structure of biopolymers like chitosan and alginate (Chmielewski and Haji-Saeid, Citation2004; El-Sawy et al., Citation2010). Because of this chain scission, availability of the bioactive groups of these molecules greatly increases and thus these possess better functionality (Islam, Ismail, et al., Citation2015; Rashid et al., Citation2014). This chain scission also leads to lower molecular size and thus increases stomatal absorption which ultimately facilitates bioactivity. Radiation processing is mostly done by four kinds of radiation, i.e. ultrasonic, ultraviolet, and gamma irradiation and electron beam radiation. Wasikiewicz et al. (Citation2005) applied and studied the effect of ultrasonic, ultraviolet, and gamma irradiation on the degradation of sodium alginate and chitosan in aqueous solutions. Molecular weights were monitored by GPC measurements with respect to the radiation doses which suggested that the gamma radiation was the most effective method for the degradation of chitosan and alginate.
So, gamma-radiation-processed chitosan and sodium alginate could be used as natural plant growth promoter and antifungal agents instead of synthetic growth hormones and pesticides without showing any detrimental effect on human health and environment. There are many studies on the plant growth promoting effect of chitosan and sodium alginate individually (Acemi et al., Citation2018; Hossain et al., Citation2013; Parvin et al., Citation2013). However, no study focused on the combined-effect-irradiated chitosan and sodium alginate on pineapple plants as well as its fruit quality. Therefore, this research was designed to evaluate the combined effect of radiation-processed chitosan and sodium alginate solution on the growth promotion of pineapple plants as well as the fruit. The effect on the taste and flavor of the fruit was also assessed.
Materials and methods
The experiment was conducted in the natural field conditions at Madhupur, Tangail from June 2015 to Sept. 2016. Required chemicals were purchased from Merck, Germany.
Preparation of chitosan and sodium alginate solution
Chitosan was extracted from prawn shells according to Dutta et al. (Citation2004). Chitosan solution (2%) was prepared by dissolving powdered chitosan in 2% acetic acid. A 2% Na-alginate solution was prepared using distilled water as solvent. The chitosan and Na-alginate solutions were then irradiated by gamma ray from a Co-60 source at 40 and 12 kGy radiation doses, respectively.
Experimental plots designing
The study was conducted on 216 pairs of pineapple plants at Madhupur, Tangail, Bangladesh. The starting age of pineapple plants was 3 months and the sizes were approximately same. Total seven experimental plots were prepared in which plot 1 served for control (without any growth promoter treatment). Each parameter like irradiated chitosan solution, irradiated Na-alginate solution, and irradiated mixture solution were designated with two plots. One plot was for the solution spraying at ~15 days interval and the other was at ~30 days interval. The final concentration of chitosan solution was 300 ppm and the concentration of Na-alginate solution was 500 ppm. The mixture solution was prepared by mixing irradiated sodium alginate and chitosan solution at 9:1 ratio.
Foliar application
The solutions were sprayed in the early morning using normal hand sprayer. Cloudy or rainy environment was avoided while spraying.
Evaluation of growth promoting activity of plants and their fruits
Growth promotion effect of the foliar spray was evaluated by observing different parameters like growing leaves length, average number of mature leaves as well as the length and diameter of growing and mature fruits.
Biochemical analysis of mature fruits
Determination of moisture and ash content
Moisture and ash content were calculated following the method suggested by Association of Official Agricultural Chemists (AOAC), 1980. Briefly, pineapple was peeled and sliced. The slices were then dried in the drying oven for 48 h at 105°C. A dried sample of 1 g was then taken in a crucible and heated at 600°C for 2 h in presence of oxygen. Moisture and ash content were then calculated using the following formulae.
Percentage of moisture content
Percentage of ash content
Where, Wf = Weight of fresh pineapple sample
Wd = Weight of dry pineapple sample
Wash = Weight of the obtained ash
Determination of vitamin C concentration
Vitamin C was measured according to the protocol of redox titration using an iodine solution (www.outreach.canterbury.ac.nz, University of Canterbury, Christchurch, New Zealand). Briefly the pineapple juice was strained by cheesecloth and was titrated by 0.005 mol L−1 iodine solution in presence of starch indicator. The concentration of vitamin C was then calculated from the moles of reacting iodine using the reaction equation.
Determination of total soluble solid (TSS) and pH
The TSS of pineapple juice was determined by using HI-98192 professional waterproof EC/TDS/salinity/resistivity meter, Hanna, UK. pH of pineapple juice was directly determined by using HI-2020 edge multiparameter pH meter, Hanna (UK). The meter was first calibrated with different standard solutions. The juice of treated and untreated pineapples was squeezed and the probe sensor was immersed in the juice to record the values. The experiments were done in triplicates.
Assessment of the sensory properties of mature pineapples
The sensory analysis of the oligomer-treated and -untreated (control) pineapples fruit was carried out according to the Hedonic scale utilized by Ibrahim et al. (Citation2014) with some modifications. The sensory quality was evaluated by color, taste, texture, flavor, and overall acceptability for all of the samples. Sliced fresh samples of pineapples were randomly presented to 10 judges with age ranging from 25 to 40 years (selected on their consistency and reliability of judgment) and were asked to rate the differences between samples by allotting the numbers from 0 to 9, where 0–2 represent dislike extremely, 3–5 for dislike, 6–8 for good, and 9 for excellent aroma, taste, flavor, and overall acceptability.
Statistical analysis
The data were analyzed statistically using SPSS-17 statistical software (SPSS Inc., Chicago, IL, USA) by the means of mean and standard deviation.
Results and discussion
Measurement of growing leaves
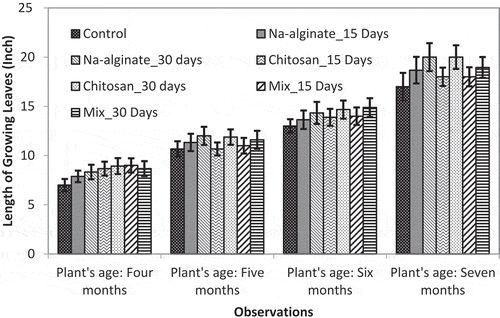
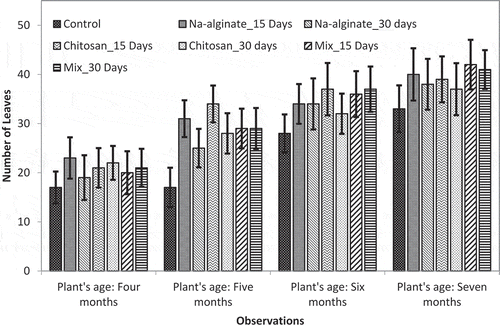
The average length (in inch) growing pineapple plant’s leaves and total number of leaves of a single pineapple plant were compared between the number of groups in field trial. and illustrate the data of plant leaves length and leaves count, respectively.
Figure 2. Effects of radiation-processed alginate, chitosan, and mixture solution on number of leaves of pineapple plants
It was observed that all the treatments induced leaf growing throughout the experimental period compared to the untreated pineapple plants (). Samples sprayed at 15-day and 30-day intervals showed similar leaf growing patterns initially but surprisingly, 30-day interval showed better growth after 7 months of plant age. This event might be occurred because of the frequent mechanical stress faced by the pineapple plants while spraying the treatment solutions in shorter intervals. As the pineapple plants are sowed in rows with very narrow gap between the rows (1–2 ft.), leaves of the plants faced mechanical stress while the sample spraying personnel moving between the rows. And the higher is leaf length the higher was the stress. That might be a reason for higher leaves length at 30 days interval spraying compared to the 15 days interval spraying in older ages of plants. At the 7 months of plant age, leaves length was found to be 20 ± 1.2 in. for 30-day interval irradiated chitosan spraying and 18.95 ± 1.06 in. for the mixed sample spraying at same days of interval. Whereas, the untreated control plants showed leaves length of 17.0 ± 1.4 in. at the same plant age.
Similarly, both irradiated chitosan and Na-alginate treatment led to growing of more leaves in pineapple plants (). However, in this case, sample spraying at 15-day interval showed higher leaf counts compared to the 30-day interval sample spraying in the most cases. However, no significant difference was found between leaf counts of irradiated chitosan and Na-alginate or mixed solution sprayed samples. But all the treated plants showed significant higher number of leaves count compared to the untreated control plants. At the 7 months of plants age, the most leaves count (42 ± 5.05) was found for mixed solution spraying at 15 days of interval whereas the control plants had leaves count of 33 ± 4.77 at the same age.
Similar results were found by the other researchers who used individual chitosan or alginate spraying. Dutta et al. (Citation2004) showed foliar application of irradiated sodium alginate 25–100 mg L−1 concentration, resulted promotive effects on all the growth attributes, physiological activities, herbage yield, content and yield of essential oil, and oil-quality parameters of Mentha arvensis L. Similarly, Idrees et al. (Citation2011), revealed that irradiated sodium alginate applied as leaf sprays at concentrations from 20 to 100 ppm improved growth, photosynthesis, physiological activities, and alkaloid production in C. roseus L. significantly. In another research conducted by Salachna and Zawadzińska (Citation2014), chitosan-treated plants had more leaves and shoots, flowered earlier, formed more flowers and corns. Similar results were also reported by Malerba and Cerana (Citation2016), Wanichpongpan et al. (Citation2001) as well as by Obsuwan et al. (Citation2010).
Measurement growing pineapple
Pineapple size was monitored up to 45 days of crown growing. In the field trial it was found that both untreated and treated plants developed crown (juvenile pineapple) at more and less similar periods of time. Data collection was started after most of the treated and untreated control pineapple plants developed crown. At the first observation, no significant difference was found for both of the length and perimeter of pineapple fruits ( and ). However, pineapple sizes were varied as they grew.
Figure 3. Effects of radiation-processed alginate, chitosan, and mixture solution on the length of growing pineapple fruits
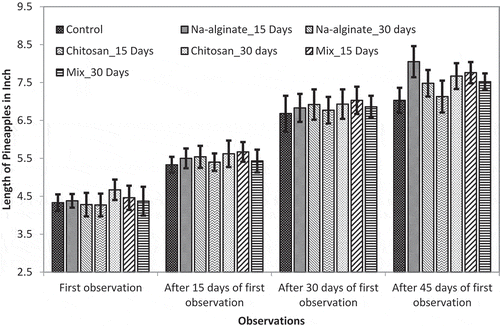
Figure 4. Effects of radiation-processed alginate, chitosan, and mixture solution on the perimeter of growing pineapple fruits
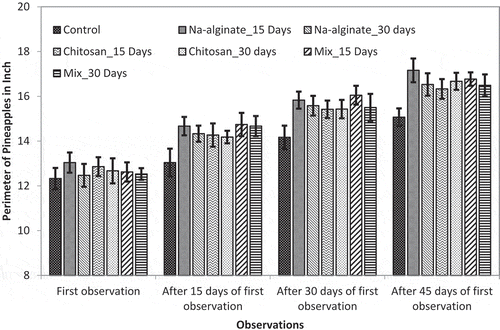
At 45 days of observation, control pineapple had length of 7.03 ± 0.33 in. and perimeter of 15.07 ± 0.39 in. On the other hand, Na-alginate treatment at 15-day interval showed best activity in terms of both length and perimeter of the growing fruit. Fruit length was found 8.05 ± 0.41 in. and fruit perimeter was found 17.16 ± 0.53 in. However, mixed treatment showed moderate growth promotion compared to the Na-alginate treatment. Overall, 10.38% and 6.97% length increases were found for 15-day interval mixed treatment and 30-day interval mixed treatment, respectively (). Similarly, 11.28% and 9.42% perimeter increases were observed for 15-day interval mixed treatment and 30-day interval mixed treatment, respectively ().
So, the experimental data suggested that although treatments with 15-day interval resulted slightly less plant growth compared to the treatment of 30-day interval, the treatment of 15-day interval is more suitable for increased fruit growth. However, the differences were negligible and considering the effort and cost of treatments it was predicted that the treatment with 30-day interval would be more suitable for the largescale farming of pineapple. Moreover, only Na-alginate treatment would be more preferable if only the size of produced pineapple was considered.
Mechanism of action
It is established that the oligomers induce plant growth mainly by enhancing nitrogen assimilation and basal metabolism. In some plants like tobacco they also increase photosynthesis and cell division (González et al., Citation2013). In addition, oligo chitosan has reported antiviral, antifungal, and antibacterial activities which are mainly mediated by increased accumulation of several phenylpropanoid compounds (PPCs) as well as increased cellulose and essential oil content (González et al., Citation2013; Zaman et al., Citation2016).
The actual mechanism of action is still unclear. It is assumed that these oligomers may interact with a specific receptor structurally related to toll like receptor 4 (TLR4) and/or TLR2 or with another similar yet unidentified receptor having protein kinase activity (González et al., Citation2013). Besides, if we consider the incredible double effect of stimulating plant growth and boosting self-defense against pathogens, it has been reported that brassinosteroids (a group of polyhydroxylated plant steroidal hormones involved in leaf development, stem elongation, photomorphogenesis, and pollen tube growth) interacts to a specific receptor of the plasma membrane which eventually activates the signal transduction mediated by a coreceptor named BRI1-associated receptor kinase (BAK)1 (Nam and Li, Citation2002). On the other hand, bacterial microbial-associated molecular patterns (MAMPs) such as elongation factor (EF)-Tu, flagellin, and peptidoglycan also exerts their signal transduction for enhancing self-defense by interacting with the same BAK1 (Chinchilla et al., Citation2007; Postel et al., Citation2010). So, although there are many separate pathways to induce plant growth and self-defense individually, both of the growth promotion and enhanced resistance to pathogens could be achieved if a common coreceptor (BAK1) can be activated. In our research as the polysaccharide oligomers stimulate plant growth as well as pathogenic resistance simultaneously, it can be assumed that the activities are mediated by the stimulation of BAK1 coreceptor.
Biochemical analysis
Biochemical parameters were assessed for the mature (ripen) pineapple with respect to moisture content, ash content, TSS, vitamin C (vit C) content, and pH of the pineapple juice. represents the data of the biochemical analysis.
Table 1. Physicochemical properties of mature pineapples
The results of moisture content of treated and untreated pineapples have shown in . The data demonstrated that the pineapples from the treated plots had slightly more moisture content compared to the untreated control. This suggests that chitosan and Na-alginate treatment led to increased juiciness of the fruits. This might be happened due to increased nutrient uptakes by the treated pineapple plants (Parvin et al., Citation2013). On the other hand, reduced ash content was found for the treated pineapples. It can be assumed that increased juiciness of the mature pineapples led to lower concentration of mineral content per unit volume which ultimately resulted lower ash content.
As shown in , decreased amount of vit C was found for almost all of the treatments. However, the samples from the plots of Na-alginate treatment at 15-day interval showed slightly higher content of vit C (21.13 ± 0.91 mg/100 mL) compared to the untreated control (20.96 ± 0.86 mg/100 mL). It is a matter of fact that the vit C content gradually decreases as the fruit ripens due to a direct action of ascorbic acid oxidase enzyme (ascorbinase), which leads to change of ascorbic acid into 2, 3-dicetogulonic acid. So, there could be an increased production of ascorbinase in the fruits from chitosan- and Na-alginate-treated plots which might also affected on the taste and flavor of pineapple.
Similarly, increased TSS values were also found for the treated pineapple fruits which indicated higher sugar content (glucose and fructose). On the other hand, no significant change in pH was monitored.
Sensory quality evaluation
The sensory quality was evaluated by color, taste, texture, flavor, and overall acceptability for all of the samples. Sliced fresh samples of pineapples were randomly presented to 10 judges with age ranging from 25 to 40 years (selected on their consistency and reliability of judgment) and were asked to rate the difference between samples by allotting the numbers from 0 to 9, where 0–2 represent dislike extremely, 3–5 for dislike, 6–8 for good, and 9 for excellent aroma, taste, flavor, and overall acceptability.
delineated overall scores on color, flavor, texture, appearance, and taste of the mature pineapples. The table demonstrated that almost all features were found better for the pineapples treated by irradiated chitosan and alginate. However, only chitosan-treated samples possessed the best quality in terms of color, texture, and appearance. However, mixed treatment was the best for flavor and overall taste. Overall, alginate and chitosan spraying with 30-day intervals showed better results compared with the samples that were subjected to the spraying with 15-day interval.
Table 2. Sensory quality of harvested mature pineapple at ambient environment (30 ± 1°C/75 ± 5% RH)
According to the table, the quality of pineapple improved significantly for the mixed treatment of alginate and chitosan at 30 days of interval. Specially, the impact on flavor and taste, the most appealing features of pineapple was excellent. The score for flavor improved to 8.2 ± 1.45 from 7.1 ± 1.16 and the score for taste improved to 8.5 ± 1.21 from 7.2 ± 1.38. These results indicated that the chitosan and alginate mix treatment not only increased the size of pineapple but also improved pineapple quality to a great extent.
Conclusion
The results presented in this paper revealed the performance of gamma-radiated chitosan and Na-alginate for growth promotion and quality enhancement of pineapple fruits. It was found that alginate and chitosan mixed treatment increased size as well as the taste and flavor of the pineapple fruits whereas the steroid hormones increase size but decrease taste and flavor. The foliar treatment with an interval of 30-days was the most suitable treatment considering the cost, efforts, and overall growth and taste increment. So, there is a huge potentiality of gamma-radiation-treated chitosan, Na-alginate, and their mixture solutions to increase crop yield as it is natural, low cost, and have no detrimental effect on human or animal health. Thus, the prepared natural growth promoter can play a very important role to meet the emerging food demands.
Acknowledgments
The author would like to acknowledge Access to Information (a2i) unit of Prime Minister Office, Bangladesh for the partial funding of the research. The authors would like to show their sincere gratitude to Mr. Mahbub Hossain, honorable Deputy Commissioner (DC), Tangail district for his support throughout the research period. The authors would also like to thank Mr. Mannan, local pineapple farmer, Modhupur, Tangail for his kind support and valuable suggestions regarding pineapple farming.
References
- AbdEl-Mohdy, H.L. 2017. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arabian J. Chem. 10:S431–S438. doi: 10.1016/j.arabjc.2012.10.003.
- Acemi, A., Bayrak, B., Çakır, M., Demiryürek, E., Gün, E., El Gueddari, N.E. and Özen, F., 2018. Comparative analysis of the effects of chitosan and common plant growth regulators on in vitro propagation of Ipomoea purpurea (L.) Roth from nodal explants. In Vitro Cellular & Developmental Biology-Plant, 54:5, 537-544. doi:10.1007/s11627-018-9915-0
- Aktar, W., D. Sengupta, and A. Chowdhury. 2009. Impact of pesticides use in agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2:1–12. doi: 10.2478/v10102-009-0001-7.
- Alewu, B., and C. Nosiri. 2011. Pesticides and human health. In Margarita Stoytcheva (ed.) Pesticides in the Modern World–Effects of Pesticides Exposure, p. 231-50. InTechOpen.
- Amartey, J. 2013. Estimation of postharvest losses and analysis of insecticide residues in some selected vegetable crops in the greater accra region of Ghana. Accra, Ghana: University of Ghana.
- Chinchilla, D., C. Zipfel, S. Robatzek, B. Kemmerling, T. Nürnberger, J.D.G. Jones, G. Felix, T. Boller, et al. 2007. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 448:497–500. doi: 10.1038/nature05999.
- Chmielewski, A., and M. Haji-Saeid. 2004. Radiation technologies: Past, present and future. Radiat. Phys. Chem. 71:17–21. doi: 10.1016/j.radphyschem.2004.05.040.
- Dutta, P.K., J. Dutta, and V. Tripathi (2004) Chitin and chitosan: Chemistry, properties and applications.
- Dzung, P.D., D.V. Phu, B.D. Du, L.S. Ngoc, N.N. Duy, H.D. Hiet, D.H. Nghia, N.T. Thang, B.V. Le, N.Q. Hien, et al. 2017. Effect of foliar application of oligochitosan with different molecular weight on growth promotion and fruit yield enhancement of chili plant. Plant Prod. Sci. 20:389–395. doi: 10.1080/1343943X.2017.1399803.
- El-Sawy, N.M., H.A.A. El-Rehim, A.M. Elbarbary, E.-S.A. Hegazy, et al. 2010. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr. Polym. 79(3):555–562. doi:10.1016/j.carbpol.2009.09.002.
- Forget, G. 1993. Balancing the need for pesticides with the risk to human health. Impact of pesticide use on health in developing countries: proceedings of a symposium held in Ottawa, Canada, 17-20 Sept. 1990. IDRC, Ottawa, ON, CA.
- González, A., J. Castro, J. Vera, A. Moenne, et al. 2013. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 32:443–448. doi: 10.1007/s00344-012-9309-1.
- Hamel, L.-P., and N. Beaudoin. 2010. Chitooligosaccharide sensing and downstream signaling: Contrasted outcomes in pathogenic and beneficial plant–microbe interactions. Planta. 232:787–806. doi: 10.1007/s00425-010-1215-9.
- Hayes, T.B., P. Case, S. Chui, D. Chung, C. Haeffele, K. Haston, M. Lee, V.P. Mai, Y. Marjuoa, J. Parker, et al. 2006. Pesticide mixtures, endocrine disruption, and amphibian declines: Are we underestimating the impact? Environ. Health Perspect. 114:40. doi: 10.1289/ehp.8051.
- Hien, N.Q., N. Nagasawa, L.X. Tham, F. Yoshii, V.H. Dang, H. Mitomo, K. Makuuchi, T. Kume, et al. 2000. Growth-promotion of plants with depolymerized alginates by irradiation. Radiat. Phys. Chem. 59:97–101. doi: 10.1016/S0969-806X(99)00522-8.
- Hossain, M.A., M.M. Hoque, M.A. Khan, et al. 2013. Foliar application of radiation processed chitosan as plant growth promoter and anti- fungal agent on tea plants. Int. J. Sci. Eng. Res. 4:1693.
- Ibrahim, S.M., Nahar, S., Islam, J.M., Islam, M., Hoque, M.M., Huque, R. and Khan, M.A., 2014. Effect of low molecular weight chitosan coating on physico-chemical properties and shelf life extension of pineapple (Ananas sativus). J. Forest Prod. Ind. 3:161–166.
- Idrees, M., M. Naeem, M. Alam, T. Aftab, N. Hashmi, M.M.A. Khan, L. Varshney, et al. 2011. Utilizing the γ-irradiated sodium alginate as a plant growth promoter for enhancing the growth, physiological activities, and alkaloids production in catharanthus roseus L. Agric. Sci. China. 10:1213–1221. doi: 10.1016/S1671-2927(11)60112-0.
- Igbedioh, S. 1991. Effects of agricultural pesticides on humans, animals, and higher plants in developing countries. Arch. Environ. Health. 46:218–224. doi: 10.1080/00039896.1991.9937452.
- Islam, J., M. Ismail, M. Islam, M.F. Hossain, H.U. Shekhar, et al. 2015. Boosting the food functionality (In Vivo and In Vitro) of spirulina by gamma radiation: An inspiring approach. Int. J. Food Eng. 11:579–585. doi: 10.1515/ijfe-2014-0342.
- Islam, J.M.M., M.F. Rahman, and M.S. Rahaman. 2015. Development of Oligo-Chitosan by ionizing radiation and its application on agricultural sector, In: F.D. Alsewailem, (ed.) Applied researches in polysaccharides, p. 1–19. Research Signpost, Kerala, India.
- Kumar, M.R., R.A. Muzzarelli, C. Muzzarelli, H. Sashiwa, A.J. Domb, et al. 2004. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104:6017–6084. doi: 10.1021/cr030441b.
- Malerba, M., and R. Cerana. 2016. Chitosan effects on plant systems. Int. J. Mol. Sci. 17:996. doi: 10.3390/ijms17070996.
- Nam, K., and J. Li. 2002. BRI1/BAK1, a receptor kinases pair mediating brassinosteroid signaling. Cell. 110:203–212. doi: 10.1016/S0092-8674(02)00814-0.
- Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P. and Hens, L., 2016. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health. 4, 148. doi: 10.3389/fpubh.2016.00148
- Obsuwan, K., Sawangsri, K., Ukong, S. and Uthairatanakij, A. 2010. Effects of chitosan concentration on in vitro growth of dendrobium hybrid seedlings. I Int. Orchid Symp. 878:289–294.
- Organization WH. 1990. Public health impact of pesticides used in agriculture.
- Parvin, F., Yeasmin, F., Islam, J.M.M, Molla, E. and Khan, M.A. 2013. Effect of Gamma irradiated sodium alginate on Malabar Spinach (Basella alba) and Spinach (Spinacia oleracea) as Plant Growth Promoter. Am. Acad. Scholarly Res. J. 5(5):63.
- Pimentel, D. 2005. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustainability. 7:229–252. doi: 10.1007/s10668-005-7314-2.
- Postel, S., I. Küfner, C. Beuter, S. Mazzotta, A. Schwedt, A. Borlotti, T. Halter, B. Kemmerling, T. Nürnberger, et al. 2010. The multi-functional leucine-rich repeat receptor kinase BAK1 is implicated in Arabidospsis development and immunity. Eur. J. Cell Biol. 89:169–174. doi: 10.1016/j.ejcb.2009.11.001.
- Rashid, T.U., S.M. Shamsuddin, M.A. Khan, M.M. Rahman, et al. 2014. Evaluation of fat binding capacity of gamma irradiated chitosan extracted from prawn shell. Soft Mater. 12:262–267. doi: 10.1080/1539445X.2014.880720.
- Salachna, P., and A. Zawadzińska. 2014. Effect of chitosan on plant growth, flowering and corms yield of potted freesia.
- Sanborn, M., K. Kerr, L. Sanin, D.C. Cole, K.L. Bassil, C. Vakil, et al. 2007. Non-cancer health effects of pesticides. Can. Family Physician. 53:1712–1720.
- Savonen, C. (1997) Soil microorganisms object of new OSU service. Good Fruit Grower. http://www.goodfruit.com/archive/1995/6other html.
- Wanichpongpan, P., K. Suriyachan, and S. Chandrkrachang. 2001. Effect of chitosan on the growth of Gerbera flower plant (Gerbera jamesonii), p.198–201. In Tadashi Uragami (ed.) Chitin and chitosan: Chitin and Chitosan in Life Science, p. 198-201. Yamaguchi, Japan.
- Wasikiewicz, J.M., F. Yoshii, N. Nagasawa, R.A. Wach, H. Mitomo, et al. 2005. Degradation of chitosan and sodium alginate by gamma radiation, sonochemical and ultraviolet methods. Radiat. Phys. Chem. 73:287–295. doi: 10.1016/j.radphyschem.2004.09.021.
- Youssef, M., and M. Eissa. 2014. Biofertilizers and their role in management of plant parasitic nematodes. A review. E3 J. Biotechnol. Pharm. Res. 5:1–6.
- Zaman, K., Quoc, H., Van Phu, D., Quang, L., Clozzal, M.N., Divo, M.D., Giardina, E.B., Vilella, F., Smolko, E.E. and Cerchietti, L. 2016. Radiation processing of alginate, chitosan and carrageenan and their applications as plant growth promoters chapter 8.
- Zheng, S., Chen, B., Qiu, X., Chen, M., Ma, Z. and Yu, X. 2016. Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China. Chemosphere. 144:1177–1192. doi: 10.1016/j.chemosphere.2015.09.050.