ABSTRACT
Temporal population changes of Oriental fruit flies (Bactrocera dorsalis) and false codling moths (Thaumatotibia leucotreta) were monitored for 2 years using their respective para-pheromone lures that were placed in small-scale avocado orchards at Taita Hills, southeastern Kenya, and Mount Kilimanjaro, northeastern Tanzania. The two avocado pest species were recorded throughout the year with the highest mean populations occurring in dry season (December–February). The seasonal populations differed significantly (P = .002 and P < .0001 for B. dorsalis at Taita Hills and Mount Kilimanjaro, respectively) and (P = .01 and P < .0001 for T. leucotreta at Taita Hills and Mount Kilimanjaro, respectively). Correlation analysis of dry season datasets revealed that temperature strongly influenced seasonal abundance of B. dorsalis but not that of T. leucotreta. These findings can be utilized by government agencies and farmers to develop efficient control strategies that can contribute toward creating a zone of low pest prevalence.
Introduction
Avocado (Persea americana Mill.) (Lauraceae) fruit is a nutritious food and a major source of income for the small-holder growers (Wasilwa et al., Citation2004) in East Africa. However, false codling moth Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) (Gilligan et al., Citation2011; Venette et al., Citation2003) and Oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) (Leblanc et al., Citation2015; Nugnes et al., Citation2018; Schutze et al., Citation2015) infests immature and ripened avocado fruits (Odanga et al., Citation2018), respectively, in small-scale farmlands at Taita Hills in southeastern Kenya and Mount Kilimanjaro in northeastern Tanzania. Cyclic infestation of fruits by B. dorsalis and T. leucotreta negatively affects the livelihood of stakeholders involved in avocado farming, production, and marketing. The stakeholders incur major revenue losses as a result of direct damage to fruits leading to phytosanitary quarantine restrictions that diminish international and local market opportunities (Ekesi et al., Citation2016; Mwatawala et al., Citation2009; Otieno, Citation2011; Schutze et al., Citation2015; Ware et al., Citation2012). The most affected, at Taita Hills and Mount Kilimanjaro, are the small-holder avocado farmers because majority of them do not practice efficient integrated pest management procedures, unlike the large commercial orchards in Kenya and Tanzania where infestation of the fruits is minimal (Ware et al., Citation2016, Citation2012) under standard phytosanitary rules (FAO, Citation2006).
T. leucotreta is an African nocturnal native moth, whereas B. dorsalis is an invasive diurnal exotic pest. Both are polyphagous pests with specialized infestation traits on avocado fruits. T. leucotreta lay eggs onto young avocado fruits (Du Toit et al., Citation1979; Prinsloo and Uys, Citation2015; Whiley, Citation2002), emergent larvae burrow tunnels beneath the fruit skin where they develop further before dropping onto the ground to pupate under leaf litter, thereby, leaving ugly triangle black scars on exocarp of the infested fruits (Du Toit et al., Citation1979). The triangle black scars on the exocarp make mature avocado fruits unattractive to both local and exportation markets. However, most of the infested fruits fall off the trees before maturity. Conversely, B. dorsalis oviposits eggs into ripened avocado fruits where feeding of emergent larvae accelerates rotting of the fruits (Ware et al., Citation2016), before reaching market. Avocadoes do not ripen while still on tree; however, the ripening process commences after the fruits are harvested or fall off on to the ground (Crane et al., Citation2007) and that is when infestation may occur. In small-holder orchards at Taita Hills and Mount Kilimanjaro, the infestation of ripened avocado fruits is enhanced by poor orchard sanitation (Odanga et al., Citation2018), especially during peak harvesting period in May and June. Hence, understanding seasonal population dynamics and geographical distribution of T. leucotreta and B. dorsalis is a prerequisite to develop efficient integrated pest management strategies that can enhance the livelihood of the small-holder farmers in East Africa.
Spatial distribution of B. dorsalis and T. leucotreta and their infestation of avocado fruits, in small-holder orchards, along altitudinal gradient at Taita Hills and Mount Kilimanjaro is well researched. Odanga et al. (Citation2018) reported the spatial abundance of B. dorsalis and its infestation, recorded mainly in ripened avocado fruits that were collected on the ground during the harvesting period, was higher in warmer lowlands and insignificant at chilly highlands above 1,500 m a.s.l., whereas the geographical distribution of T. leucotreta did not vary with altitude at Taita Hills and Mount Kilimanjaro. However, there is limited information on temporal population dynamics of the two insect pest species in avocado farming systems of Kenya and Tanzania. This study was, specifically, initiated to (i) assess seasonal and monthly population variation of B. dorsalis and T. leucotreta in the small-scale avocado orchards at Taita Hills and Mount Kilimanjaro, (ii) evaluate relationship between seasonal abundance of each of the two avocado insect pest species with corresponding mean temperature and rainfall and (iii) further, generate annual population trends of pest species for lowland, midland, and highland altitudinal zones of each study area.
Materials and Methods
Description of Study Areas and Weather Seasonality
This study was carried out in small-holder avocado orchards along Taita Hills (from 03° 481’S, 38° 378ʹE to 03° 402’S, 38° 296ʹE) in southeastern Kenya and Mount Kilimanjaro (from 03° 378’S, 37° 450ʹE to 03° 481’S, 37° 456ʹE) in northeastern Tanzania. Taita Hills and Mount Kilimanjaro are situated 90 km apart from East Africa and both are about 150 km from shores of the Indian Ocean. Both study areas have four distinct weather seasons in a year and these are designated as (i) Short rain season that occur during months of September, October, and November, (ii) Dry season (December, January, and February), (iii) Long rain season (March, April, and May) and (iv). Cold season (June, July, and August) ().
Table 1. Description of weather seasons of the study areas along Taita Hills and Mount Kilimanjaro. The seasonal mean rainfall and temperature ranges were generated from field information that was recorded at Taita Hills and Mount Kilimanjaro for two consecutive years commencing August 2012 to July 2014
Avocado plant is the most dominant and important fruit crop cultivated by small-holder growers in farmlands along the slopes of Taita Hills and Mount Kilimanjaro. At Taita Hills, avocado orchards are intertwined between a few relics of the original Eastern Arc Mountain cloud forests, forming a strong agro-ecosystem along the study area. Conversely, the study area along the slopes of Mount Kilimanjaro is a homogenous farmland with avocado trees being the leading fruit crop. At both study areas, avocado crop thrives well in areas between 900 m a.s.l. and 1800 m a.s.l. On the contrary, mango (Mangifera indica Linnaeus) (Anacardiaceae) is a dominant fruit crop in areas below 900 m a.s.l. at Taita Hills and Mount Kilimanjaro.
Study Design and Monitoring of Insect Pests, Temperature, and Rainfall
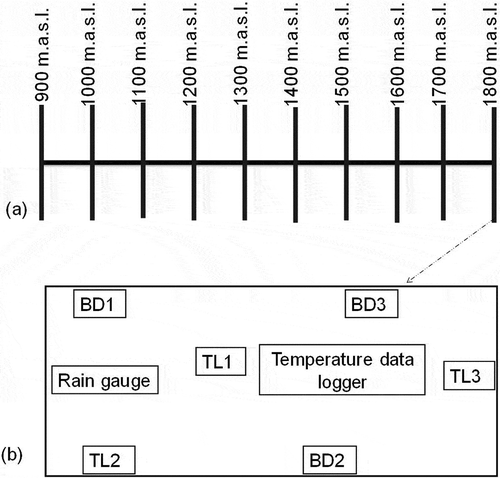
B.dorsalis and T. leucotreta, in avocado orchards at Taita Hills and Mount Kilimanjaro, were monitored weekly for two consecutive years from August 2012 to July 2014, using their respective para-pheromone lures. Ten study blocks were set up along the south-east slopes of Taita Hills and Mount Kilimanjaro with the lowest altitude of a study block being 900 m a.s.l. whereas the highest was at 1800 m a.s.l. (). The minimum distance between any two adjacent study blocks was at least 1 km.
Figure 1. Schematic drawing showing a study area with (a) 10 sampling blocks along altitudinal gradient and (b) a study block with six insect trap lures, one temperature data logger and a rain gauge. BD and TL are the insect trap lures for B. dorsalis and T. leucotreta, respectively. The distance between any two adjacent insect traps within a study block ranged between 100 m and 120 m
A sampling block consisted of a mini-orchard with at least 20 mature avocado trees, out of which three trees were randomly selected for trapping each insect pest species. On lower branch of each of the three selected avocado trees, a trap with species-specific para-pheromone lure for either B. dorsalis or T. leucotreta was suspended at a height of 2 m and monitored weekly. The random selection process was repeated every month at each block, on separate sets of trees, for each of the two insect pest species to avoid cross-contamination of respective para-pheromone lures. In a nutshell, Lynfield trap (Lynfield Plant Protection Center, Auckland, New Zealand) baited with methyl eugenol (ME) charged on cotton wick at a ratio of 4:1 of the ME: othothion was used for monitoring B. dorsalis. For T. leucotreta, Yellow Delta traps baited with dodecenyl acetate lures (Chempac, Suider-Paarl, Western Cape, South Africa) was used for their monitoring. Taxonomic identification of B. dorsalis and T. leucotreta was done using Ekesi and Billah (Citation2007) and Gilligan et al. (Citation2011), respectively.
Daily air temperature (ºC) was recorded using data loggers (Maxim Integrated Products, San Jose, CA, USA) whereas General Wireless Rain Gauges (Gempler, Janesville, WI, USA) recorded rainfall (mm) in avocado orchards along each study area. At each of the 10 sampling blocks, one data logger and a single rain gauge were set up on separate-dried wooden poles measuring 2 m in height, in an open space within a min-orchard (). Seasonal and monthly temperature (ºC), rainfall (mm), and abundance of the pest species were assessed per study area.
Data Analysis
Data on trap catch lures for both B. dorsalis and T. leucotreta were analyzed using Kruskal–Wallis Chi-square (Kruskal and Wallis, Citation1952) and Wilcoxon tests (Wilcoxon, Citation1946). Kruskal–Wallis Chi-square test was used to evaluate differences among the four weather seasonal datasets, whereas Wilcoxon signed-rank test was employed to analyze differences between paired datasets of the two study areas as described by Crawley (Citation2005), Verzani (Citation2018), and Crawley (Citation2007). Spearman’s rank correlation (Crawley, Citation2007) was used to evaluate the relationship between mean number of each insect pest species with average temperature and rainfall. For every avocado pest species in a study area, mean annual population trend was generated for each of the three distinct altitudinal zones; lowland (900–1199 m a.s.l.), midland (1200–1499 m a.s.l), and highland (1500–1799 m a.s.l.). In the analyses, a single dataset comprising of 12-onth information, averaged from the field data of two consecutive years, was used. The analyses were performed using statistical software R, version 3.1.2 (R Development Core Team, Citation2016).
Results
B.dorsalis Seasonal Abundance
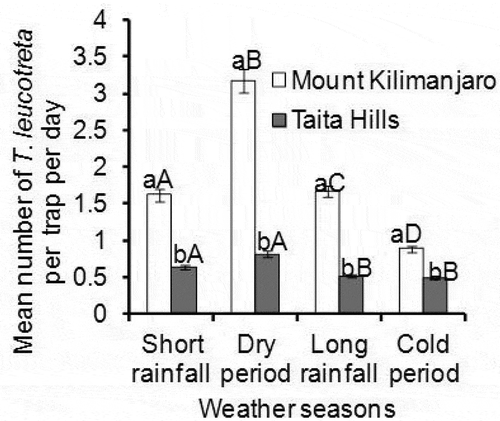
Mean number of B. dorsalis was significantly different among the weather seasons for both study areas; Taita Hills (P = .002, df = 3, χ2 = 15.24) () and Mount Kilimanjaro (P < .0001, df = 3, χ2 = 53.01) (), being highest during dry season (12.66 ± 2.2 and 31.26 ± 4.2 B. dorsalis/trap/day for Taita Hills and Mount Kilimanjaro, respectively) (). Comparing the same season between the two study areas, B. dorsalis were significantly higher at Mount Kilimanjaro for all weather seasons than Taita Hills (V = 863.5, n = 45, p < .0001; V = 1021, n = 45, p = .0001; V = 966, n = 45, p < .0001 and V = 254.5, n = 45, p = .0083 for Short rain, Dry, Long rain, and Cold season, respectively) (). The Dry season was hottest with a mean temperature of 21.7ºC ± 0.31ºC and 21.9ºC ± 0.28ºC for Taita Hills and Mount Kilimanjaro, respectively, whereas the Cold season had lowest (17.7ºC ± 0.29ºC and 17.8ºC ± 0.4ºC for Taita Hills and Mount Kilimanjaro, respectively).
Figure 2. Mean number of B. dorsalis across weather seasons at Taita Hills and Mount Kilimanjaro areas. Bars capped with differing lower and upper case letters within the same weather season are significantly different. The weather season and related months are; Short rainfall (September, October, November), Dry (December, January, February), Long rain (March, April, May) and Cold (June, July, August)
Seasonal mean number of B. dorsalis at both Taita Hills and Mount Kilimanjaro had a strong positive correlation against corresponding temperatures with highest being revealed during dry season (R = 0.94 and R = 0.82 for Taita Hill and Mount Kilimanjaro, respectively) and subsequent long rainfall period (R = 0.88 and R = 0.92 for Taita Hill and Mount Kilimanjaro, respectively) (). Conversely, a strong negative correlation was recorded between mean number of B. dorsalis and average rainfall at both Taita Hills and Mount Kilimanjaro ().
Table 2. Spearman’s rank correlation coefficient, R, depicting relationship between seasonal abundance of Bactrocera dorsalis and corresponding mean air temperature (ºC) and average rainfall (mm), in a weather season
B. dorsalis Monthly Abundance and Zonal Trends
Least mean number of B. dorsalis was witnessed between August and September, in both study areas. Mean number of B. dorsalis per trap/day was significantly different among calendar months for both study areas; Taita Hills (P = .03, df = 11, χ2 = 21.03) () and Mount Kilimanjaro (P < .0001, df = 3, χ2 = 68.68) (), being highest in February (15.25 ± 1.3 and 46.07 ± 4.1 B. dorsalis trap/day for Taita Hill and Mount Kilimanjaro, respectively) ().
Figure 3. Mean number of B. dorsalis trapped per month at Taita Hills and Mount Kilimanjaro study areas
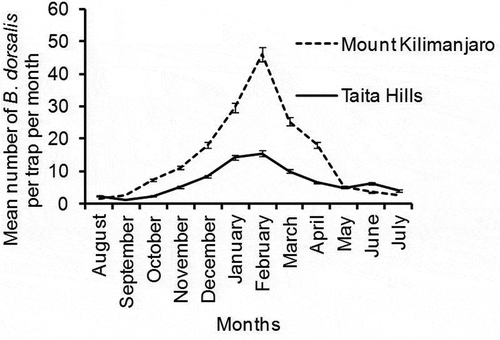
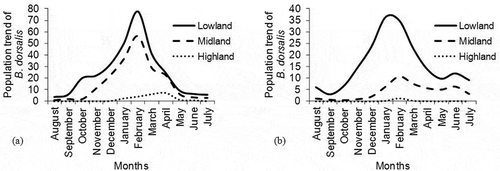
In both study areas, the peak population of B. dorsalis occurred from December to February, during the warmer dry season, that follows the short rains of September and October. Annual population trends for B. dorsalis at both Mount Kilimanjaro and Taita Hills were more pronounced in lowland (900–1199 m a.s.l.) and midland zones (1200–1499 m a.s.l.) but very minimal variation was witnessed in highland region (1500–1799 m a.s.l) (). Peak mean population density at both lowland and midland zones was between November and March (). However, there was no significant difference in monthly mean abundance of B. dorsalis at the uppermost elevated zone of Taita Hills, Kenya (P = .16) ().
Figure 4. Population density trend of B. dorsalis describing a twelve-month variation in mean abundance between August and July at different altitudinal zones of (a) Mount Kilimanjaro and (b) Taita Hills. The zones were categorized as lowland (900–1,199 ma.s.l), midland (1,200–1,499 m a.s.l) and highland region (1,500–1,799 m a.s.l)
T.leucotreta Seasonal Abundance
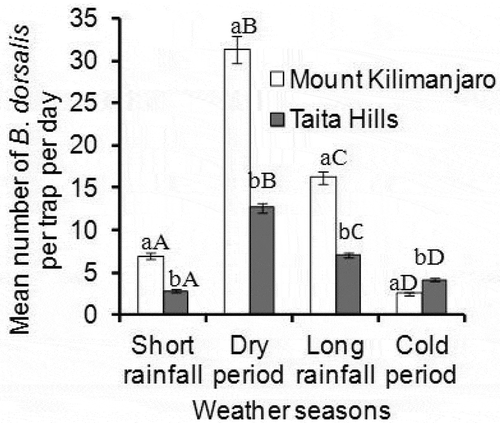
For T. leucotreta, the population density was significantly different among the seasons for both study areas; Taita Hills (P = .01, df = 3, χ2 = 34.99) () and Mount Kilimanjaro (P < .0001, df = 3, χ2 = 100.10) (), being highest also during dry season (0.82 ± 0.05 and 3.18 ± 0.10 mean number of T. leucotreta/trap/day for Taita Hill and Mount Kilimanjaro, respectively) (). Comparing the same season between the two study areas, T. leucotreta were significantly higher at Mount Kilimanjaro for all weather seasons (V = 1033, n = 45, p < .0001; V = 1035, n = 45, p < .0001; V = 1032, n = 45, p < .0001 and V = 986, n = 45, p < .0001 for Short rain, Dry, Long rain, and Cold season, respectively) (). For mean number of T. leucotreta, weak correlations were recorded against corresponding seasonal mean temperature and average rainfall in both study areas ().
Table 3. Spearman’s rank correlation coefficient, R, depicting relationship between seasonal abundance of Thaumatotibia leucotreta and corresponding mean air temperature (ºC) and average rainfall (mm), in a weather season
T.leucotreta Monthly Abundance and Zonal Trends
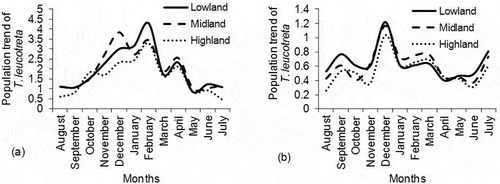
Mean number of T. leucotreta per trap/day was significantly different among calendar months for both study areas; Taita Hills (P < .0001, df = 11, χ2 = 88.71) () and Mount Kilimanjaro (P < .0001, df = 11, χ2 = 146.51) (), being highest in February (3.69 ± 0.23 T. leucotreta/trap/day, for Mount Kilimanjaro) (). Similar to B. dorsalis, the peak population density of T. leucotreta at both Taita Hill and Mount Kilimanjaro occurred between December and March during the warmer dry season that follows the short rains of September and October. Annual population density trend for T. leucotreta at each agro-ecological zone of each study area was comparable ().
Figure 6. Mean number of T. leucotreta trapped per month at Taita Hills and Mount Kilimanjaro study areas
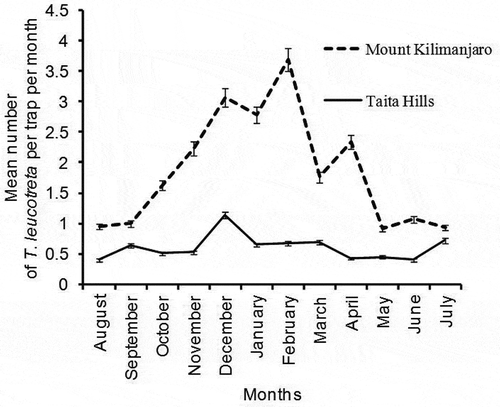
Figure 7. Population density trend of T. leucotreta describing a twelve-month variation in mean abundance between August and July at different altitudinal zones at (a) Mount Kilimanjaro and (b) Taita Hills. The zones were categorized as lowland (900–1,199 m a.s.l), midland (1,200–1,499 m a.s.l) and highland region (1,500–1,799 m a.s.l)
Discussion
This study provides detailed temporal information on T. leucotreta and B. dorsalis in Taita Hills and Mount Kilimanjaro, to already accessible geographical distribution information (Odanga et al., Citation2018). These findings can be utilized by government agencies and farmers to develop efficient pest control strategies that can contribute toward creating a zone of low prevalence of the avocado pest species in East Africa. In the light of these findings, management of B. dorsalis and T. leucotreta in small-holder avocado farming systems of Kenya and Tanzania is discussed.
B. dorsalis Seasonal Abundance
Peak abundance of B. dorsalis occurred during warmer dry season (December, January, and February), immediately after the short rains, instead of the peak avocado harvesting months in May and June. The ambient warmer temperatures during the dry season could have enhanced reproductive ability and hence high numbers of B. dorsalis, specifically, in the lowlands of Taita Hills and Mount Kilimanjaro. Another likely contributing factor to the peak abundance was the presence of mango host fruits during the dry season in lowland areas adjacent to avocado orchards. The lures in avocado orchards possibly attracted B. dorsalis from the mango fruits. Rwomushana et al. (Citation2008a) and Mwatawala et al. (Citation2009) reported that the tropical mango fruits are the most preferred host of B. dorsalis in lowland areas of Kenya and Tanzania, respectively, whereas the sub-tropical avocado fruits are one of the alternate hosts (Mwatawala et al., Citation2009). Moreover, Geurts et al. (Citation2012) reported that B. dorsalis re-invades mango orchards, in lowlands of central Tanzania, with high population density during the dry season that coincides with ripening period of the mango fruits from November to January of the following year.
The comparatively higher number of B. dorsalis at Mount Kilimanjaro than Taita Hills can be explained by the fact that weather seasons at the former homogenous study area had slightly warmer and ambient temperatures for better survival of B. dorsalis. The seasonal population fluctuations of B. dorsalis, in the two study areas, were probably influenced most by temperature hence population peak occurring in the warmer dry season; December to February. These views are in agreement with those of Geurts et al. (Citation2012) and Odanga et al. (Citation2018) who reported that the distribution of B. dorsalis, in Kenya and Tanzania, is strongly influenced by temperature.
B.dorsalis Monthly Abundance and Zonal Trends
Monthly population of B. dorsalis was marginally reduced at the onset of long rains in March and drastically during the peak of the heavy rain season in April and May. The decline in numbers was probably because the heavy rains could have contributed to the drowning of several immature stages of B. dorsalis resulting in low population density during the long rain season. However, small incline in population of B. dorsalis during harvesting period, in May and June, implied that fallen ripened avocado fruits provide reservoirs for survival of immature stages of the pest in avocado orchards at Taita Hills and Mount Kilimanjaro, a scenario that has also been reported by Ware et al. (Citation2016).
Monthly sampling of the two pest species revealed that few individuals of B. dorsalis were occasionally recorded at the highland zone; 1500–1799 m a.s.l, of both Taita Hills and Mount Kilimanjaro transects implying that poor survival and development of B. dorsalis at the uppermost-elevated zones in East Africa. The seasonal incidences of B. dorsalis in the highlands occurred mainly during dry season, in areas that were adjacent to open markets or public facilities. This suggests that fruits infested with B. dorsalis could have possibly been transported from lowlands to uplands. Moreover, the unfavorable environmental conditions could have contributed to very low population density of B. dorsalis in the highlands at both Taita Hills and Mount Kilimanjaro. In both study areas, the highland zones above 1,600 m a.s.l are not conducive for the survival of this poikilothermic B. dorsalis (Odanga et al., Citation2018) due to very low temperatures, extremely high rainfall, insufficient ultra-violet heat, soaring relative humidity, and ever cloudy environment throughout the year. These views corroborate with those of Cammell and Knight (Citation1992), Vargas et al. (Citation1997), Ye and Liu (Citation2005), Yang et al. (Citation1994) and Rwomushana et al. (Citation2008b) who, also, reported that low temperatures slow down the developmental, oviposition, and survival rates of B. dorsalis.
T. leucotreta Seasonal Abundance
The presence of T. leucotreta throughout the year, in the small-scale farmlands, confirmed that this pest can thrive in any weather season at Taita Hills and Mount Kilimanjaro. This also implies that T. leucotreta has high thermal tolerance; hence, it may survive and infests host florae in diverse agro-ecological conditions with extreme weather parameters. For example, Stotter (Citation2009), Newton (Citation1998), Stotter and Terblanche (Citation2009), Boardman et al. (Citation2012) and Terblanche et al. (Citation2017) reported that T. leucotreta thrives well in both warmer lowlands and colder uplands of South Africa, where it infests both cultivated crops and wild plants. These previous reports corroborate with the present findings that T. leucotreta has weak correlations with the corresponding mean temperature at both Taita Hills and Mount Kilimanjaro, implying that distribution of the pest is not determined by weather seasonality.
T. leucotreta Monthly Abundance and Zonal Trends
Peak monthly population density of T. leucotreta occurred between October and February at both Taita Hill and Mount Kilimanjaro due to availability of the preferred host young avocado fruits that are abundant from November to January. However, the small incline in population trend of T. leucotreta during the peak harvesting period in May and June, that coincided with the coldest season at elevations below 1,500 m a.s.l, implied that ripened avocado fruits in orchards could have provided reservoirs for the survival of immature stages of the pest; a scenario that has been reported by Ware et al. (Citation2016). Ware et al. (Citation2016) reported that fallen ripened avocado fruits can be re-infested by T. leucotreta in Kenya and Tanzania. Moreover, the annual population density trend for T. leucotreta at altitudinal zone of each study area did not vary with elevation and the moths were recorded in all weather seasons. For this reason, further research studies should be carried out to identify alternate host plants of T. leucotreta at Taita Hills and Mount Kilimanjaro, that enable this polyphagous pest species to maintain viable population throughout the year, especially when there are no young avocado fruits to infest.
Conclusion
The lower numbers of B. dorsalis recorded during the main avocado harvesting period; May and June, compared to the peak abundance during the dry season in December and February implied that avocado fruits are not the most preferred primary host of the invasive fruit flies at both study areas. Moreover, the annual peak population of T. leucotreta occurred between October and February due to availability of host young avocado fruits at the time. In light of these findings, it is recommended that environmental friendly control measures for T. leucotreta and B. dorsalis should be enhanced at the onset of short rains (September–November), before population buildup in subsequent dry season, and during avocado harvesting period, in May and June. However, routine-integrated pest management measures including orchard sanitation, bio-control techniques, and cold treatment of fruits should be carried out whenever required. It is further recommended to carry out additional research on insect phenology and future prediction studies that can advance our knowledge on how climate will influence seasonal population and fruit infestation in East African small-holder avocado farming systems.
Acknowledgments
The authors would like to thank International Centre of Insect Physiology and Ecology, Dar es Salaam University, MIS, Taita-Taveta county government, National Museums of Kenya, Shadrack Mwakalila, Mary Gikungu, Peterson Nderitu, Martin Gathendu, Granton Mwadime, Filbert Mlingi, and Karen Wambui for providing advice during data collection period in Kenya and Tanzania. This research was carried out under “Climate change impacts on ecosystem services and food security in eastern Africa” project/work package five/avocado.
Additional information
Funding
References
- Boardman, L., T.G. Grout, and J.S. Terblanche. 2012. False codling moth (Thaumatotibia leucotreta) (Lepidoptera, Tortricidae) larvae are chill-susceptible. Insect Sci. 19:315–328. doi: 10.1111/j.1744-7917.2011.01464.x.
- Cammell, M., and J. Knight. 1992. Effects of climatic change on the population dynamics of crop pests. Adv. Ecol. Res. 22:117–162.
- Crane, J.H., C.F. Balerdi, and I. Maguire. 2007. Avocado growing in the Florida home landscape. Hort. Sci. Dept., Florida Coop. Ext. Serv., Inst. Food Agr. Sci., Univ. Florida. Circular 1034: 1–12.
- Crawley, M. 2005. Statistics, an Introduction to R. John Wiley & Sons Ltd, West Sussex, UK.
- Crawley, M.J. 2007. The R book. JohnWiley and Sons Ltd, Chichester, UK.
- Du Toit, W.J., E.A. De Villiers, and A. Tuffin. 1979. The identification of typical surface lesions on avocado fruit. S. Afr. Avocado Grow. Assoc. Yearbook 3:52–53.
- Ekesi, S., and M.K. Billah. 2007. A field guide to the management of economically important tephritid fruit flies in Africa. ICIPE Science Press, Nairobi, Kenya.
- Ekesi, S., M. De Meyer, S.A. Mohamed, M. Virgilio, and C. Borgemeister. 2016. Taxonomy, ecology, and management of native and exotic fruit fly species in Africa. Annu. Rev. Entomol. 61:219–238. doi: 10.1146/annurev-ento-010715-023603.
- FAO. 2006. Requirements for the establishment of pest free places of production and pest free production sites. International Standards for Phytosanitary Measures. ISPM No. 10. 2005 Edition: 105-111. Food and Agriculture Organization, Rome, Italy.
- Geurts, K., M. Mwatawala, and M. De Meyer. 2012. Indigenous and invasive fruit fly diversity along an altitudinal transect in Eastern Central Tanzania. J. Insect Sci. 12:12. doi: 10.1673/031.012.1201.
- Gilligan, M., M.E. Epstein, and K.M. Hoffman. 2011. Discovery of false codling moth, Thaumatotibia leucotreta (Meyrick), in California (Lepidoptera: Tortricidae). Proc. Entomol. Soc. Wash.. 113:426–435. doi: 10.4289/0013-8797.113.4.426.
- Kruskal, W.H., and W.A. Wallis. 1952. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 47(260):583–621. doi: 10.1080/01621459.1952.10483441.
- Leblanc, L., M. San Jose, N. Barr, and D. Rubinoff. 2015. A phylogenetic assessment of the polyphyletic nature and intraspecific color polymorphism in the Bactrocera dorsalis complex (Diptera, Tephritidae). ZooKeys. 540:339–367. doi: 10.3897/zookeys.540.9786.
- Mwatawala, M.W., M. De Meyer, R.H. Makundi, and A.P. Maerere. 2009. Host range and distribution of fruit-infesting pestiferous fruit flies (Diptera Tephritidae) in selected areas of Central Tanzania. Bull. Entomol. Res. 99:629–641. doi: 10.1017/S0007485309006695.
- Newton, P.J. 1998. False codling moth, p. 192–200. In: E.C.G. Bedford, M.A. Van Den Berg, and E.A. De Villiers eds. Citrus pests in the Republic of South Africa. second. ARC - Institute for Tropical and Subtropical Crops and Outspan International, Nelspruit, South Africa.
- Nugnes, F., E. Russo, G. Viggiani, and U. Bernardo. 2018. First record of an invasive fruit fly belonging to Bactrocera dorsalis Complex (Diptera Tephritidae) in Europe. Insects 9(4):182. doi: 10.3390/insects9040182.
- Odanga, J.J., S. Mohamed, S. Mwalusepo, F. Olubayo, R. Nyankanga, F. Khamis, I. Rwomushana, T. Johansson, and S. Ekesi. 2018. Spatial Distribution of Bactrocera dorsalis and Thaumatotibia leucotreta in smallholder avocado orchards along altitudinal gradient of Taita Hills and Mount Kilimanjaro. Insects. 9:71. doi: 10.3390/insects9020071.
- Otieno, W. 2011. EPHIS experience with market access and compliance with official standards. Acta Hortic. 911:73–76. doi: 10.17660/ActaHortic.2011.911.8.
- Prinsloo, G.L., and V.M. Uys. 2015. Insects of cultivated plants and natural pastures in Southern Africa. Entomological Society of Southern Africa, Hatfield, South Africa.
- R Development Core Team. 2016. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 19 Jan. 2019. http://www.R-project.org/.
- Rwomushana, I., S. Ekesi, I. Gordon, and C. Ogol. 2008a. Host plants and host plant preference studies for Bactrocera dorsalis (Diptera: Tephritidae) in Kenya, a new invasive fruit fly species in Africa. Ann. Entomol. Soc. Am.. 101:331–340. doi: 10.1603/0013-8746(2008)101[331:HPAHPP]2.0.CO;2.
- Rwomushana, I., S. Ekesi, C.K.P.O. Ogol, and I. Gordon. 2008b. Effect of temperature on development and survival of immature stages of Bactrocera invadens (Diptera: Tephritidae). J. Appl. Entomol. 132(9‐10):832–839. doi: 10.1111/j.1439-0418.2008.01318.x.
- Schutze, M.K., N. Aketarawong, W. Amornsak, K.F. Armstrong, A.A. Augustinos, N. Barr, W. Bo, K. Bourtzis, L.M. Boykin, C. Cáceres, et al. 2015. Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data. Syst. Entomol. 40:456–471. doi: 10.1111/syen.12113.
- Stotter, R.L. 2009. Spatial and temporal distribution of False codling moth across landscapes in the Citrusdal area. University of Stellenbosch, Western Cape Province, South Africa, Master’s Thesis.
- Stotter, R.L., and J.S. Terblanche. 2009. Low-temperature tolerance of false codling moth Thaumatotibia Leucotreta (Meyrick) (Lepidoptera Tortricidae) in South Africa. J. Therm. Biol. 34:320–325. doi: 10.1016/j.jtherbio.2009.05.002.
- Terblanche, J.S., K.A. Mitchell, W. Uys, C. Short, and L. Boardman. 2017. Thermal limits to survival and activity in two life stages of false codling moth Thaumatotibia leucotreta (Lepidoptera: Tortricidae). Physiol. Entomol. 42(4):379–388. doi: 10.1111/phen.12210.
- Vargas, R.I., W.A. Walsh, D. Kanehisa, E.B. Jang, and J.W. Armstrong. 1997. Demography of four Hawaiian fruit flies (Diptera Tephritidae) reared at five constant temperatures. Ann. Entomol. Soc. Am. 90(2):162–168. doi: 10.1093/aesa/90.2.162.
- Venette, R.C., E.E. Davis, M. Dacosta, H. Heisler, and M. Larson. 2003. Mini risk assessment: False codling moth, Thaumatotibia (=Cryptophlebia) leucotreta (Meyrick) (Lepidoptera Tortricidae). University of Minnesota, Department of Entomology, Falcon Heights, MN, USA.
- Verzani, J. 2018. Using R for introductory statistics. Chapman & Hall/CRC, New York, USA.
- Ware, A.B., C.L.N. Du Toit, E. Du Toit, R. Collins, R. Clowes, S. Ekesi, and S. Mohamed. 2016. Host suitability of three avocado cultivars Persea americana (Miller) (Lauraceae) to oriental fruit fly (Bactrocera (invadens) dorsalis (Hendel) (Diptera Tephritidae). Crop Prot. 90:84–89. doi: 10.1016/j.cropro.2016.08.024.
- Ware, A.R., C.L.N. Du Toit, S.A. Mohamed, P.W. Nderitu, and S. Ekesi. 2012. Cold tolerance and disinfestation of Bactrocera dorsalis (Diptera Tephritidae) in ‘Hass’ avocado. J. Econ. Entomol. 105:1963–1970. doi: 10.1603/EC12041.
- Wasilwa, L.A., J.K. Njuguna, E.N. Okoko, and G.W. Watani. 2004. Status of avocado production in Kenya. Kenya Agricultural Research Institute, Nairobi, Kenya.
- Whiley, A.W. 2002. Crop management, p. 231–258. In: A.W. Whiley, B. Schaffer, and B.N. Wolstenholme (eds.). Avocado: Botany, production and uses. CABI Publishing, Oxon, UK.
- Wilcoxon, F. 1946. Individual comparisons of grouped data by ranking methods. J. Econ. Entomol. 39(2):269–270. doi: 10.1093/jee/39.2.269.
- Yang, P., J.R. Carey, and R.V. Dowell. 1994. Temperature influences on the development and demography of Bactrocera dorsalis (Diptera Tephritidae) in China. Environ. Entomol. 23(4):971–974. doi: 10.1093/ee/23.4.971.
- Ye, H., and J. Liu. 2005. Population dynamics of Bactrocera dorsalis (Diptera Tephritidae) in Xishuangbanna of southern Yunnan. Ying Yong Sheng Tai Xue Bao. J. Appl. Ecol. 16(7):1330–1334.