 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
There is a commercial requirement to extend the shelf-life of chestnuts. This study aimed to determine the effect of different packages (modified atmosphere packaging (MAP) (0.3% O2 and 32% CO2), polyethylene (PE) and vacuum (VAC)) on physicochemical and microbial properties of raw chestnuts during storage (1, 2, 3 and 6 months). The results showed that the storage time and packaging type influence chestnuts properties. VAC and MAP showed a decrease in the aw, maximum force, and hardness values of the fruits at the end of storage. All treatments may also cause color modifications in the fruit. Total acidity and total soluble solids values remained similar between samples, although they increased in the first two months of storage. During the storage period, there was a significant increase in microorganism counts in the control and PE samples, being observed germination after one month in the latter situation. On the contrary, the use of MAP and VAC could significantly control the proliferation of the microorganisms; however, after 3 to 6 months, the chestnuts lost quality, showing a fermented smell.
Introduction
The chestnut (Castanea sativa Mill.) tree is a species that is exploited for wood and fruit. In Portugal, chestnuts are mainly cultivated for fruit production. China is the world’s largest producer, responsible for more than 80% of the total world production in 2010, while Portugal is only the seventh-largest producer (FAO, Citation2018; Pereira et al., Citation2011). This crop has a substantial economic impact in the Trás-os-Montes region of Portugal and makes an essential contribution in the combat against desertification, a trend occurring in these regions. According to the Portuguese Statistics Institute, the annual chestnuts production in 2017 in the North of Portugal was 25652 tons (INE, Citation2017). Chestnuts are known as carriers of nutritious elements and rich in fiber, amino acids, polyunsaturated fatty acids and free sugars (Borges et al., Citation2008; Vasconcelos et al., Citation2010), as well as, contain considerable amounts of potassium (K) and magnesium (Mg) (Borges et al., Citation2008). Due to its economic and nutritional value, the production of chestnuts in Portugal is increasing as new groves are being planted in various regions. However, the quality of chestnut fruit can be affected in postharvest by insects damages and growth of microorganisms, which reduces the nut quality and can lead to severe economic losses. So, the storage conditions are one of the problems faced by the chestnuts industry. Usually, the chestnuts are stored at ambient temperature or in refrigerated chambers in bulk. Particularly high temperature, air relative humidity, and moisture inside the fruit are the main reasons for enhancing the growth of the microorganisms. So, storage is one of the stages in the chestnuts industry, which will influence the final quality of the product. Furthermore, chestnuts have a short shelf-life due to their high carbohydrate (starch and simple sugars) and moisture contents (Navarro et al., Citation2011).
In order to reduce these losses, the use of suitable packaging materials may be a possible solution to the chestnuts industry to reduce the risk of losses and maintain the safety of chestnuts. One of the principal roles of food packaging is to protect the food products from outside influences and damage (Mangaraj et al., Citation2009). Modified atmosphere packaging (MAP) is a technique of sealing the product in different polymeric film packages that will modify the O2 and CO2 levels within the package atmosphere. These modifications in the air inside package influence the metabolism of the product and decrease the growth of microorganisms, which induce an increase in the shelf life and retain the quality and appearance of the product (Mangaraj et al., Citation2009). Vacuum packaging (VAC) is another way to increase the shelf-life of food products (Randel et al., Citation1997). The product is placed in an air-tight pack, the air sucked out, and the package sealed. By removing air from around the product, the levels of O2 in the packaging are reduced, impeding the ability of aerobic microorganisms to grow and spoil the product. Polyethylene bags are a conventional packaging used in food industry to protect the product from outside damages (Marsh and Bugusu, Citation2007).
The use of MAP has been already documented to retain the quality of chestnuts over time. Peano et al. (Citation2014) studied the application of MAP (80% CO2) and two types of material during 90 days of storage for two species of chestnut: C. sativa × C. mollissima Blume and C. sativa; Kim et al. (Citation2012) evaluated the effect of MAP (2–3% O2 and 15–20% CO2) with variable micro-perforations in C. crenata, var. Seokchu; Tzortzakis and Metzidakis (Citation2012) investigated the effect of heat stress (HS) and ultra-low oxygen (ULO) in controlled (CA) or modified (MA) atmosphere on chestnut (C. sativa cv. Rodiana) during 90 days; and Bhisanbut et al. (Citation2008) tested the influence of MAP and VAC during 26 days in colossal cultivars of chestnut. It was found that the MA has the dual effect of maintaining fruit quality and protecting fruits from spoilage due to molds (Peano et al., Citation2014). The micro-perforations number can be a useful variable for controlling the package atmosphere around the chestnuts, and it can be optimized according to the retail temperature (Kim et al., Citation2012). In the heat stress (HS) and ultra-low oxygen (ULO) modified atmosphere (MA) treatments, an increase in the respiration rate was observed (Tzortzakis and Metzidakis, Citation2012), and MAP and VAC maintained the moisture content and helped to extend the shelf life of the packaged chestnuts for 26 days (Bhisanbut et al., Citation2008). Although some works are dealing with the effect of MAP in chestnuts, few of them have compared different packaging, as PE and VAC. Furthermore, there is a lack of studies on chestnuts from Portugal. Some of the studies that evaluated the effect of packaging on chestnuts (Castanea sativa) were from Switzerland (Jermini et al., Citation2006); Italy (Cecchini et al., Citation2011; Peano et al., Citation2014); USA (Bhisanbut et al., Citation2008); and Greece (Tzortzakis and Metzidakis, Citation2012).
Thus, the present work aimed to determine the effects of different packaging systems on the physicochemical composition and microbial quality of fresh chestnuts (Castanea sativa), throughout six months of storage.
Material and Methods
Samples
Approximately 15 kg of fresh and shell chestnuts (Castanea sativa) were provided by a local enterprise of Trás-os-Montes, Portugal. After transportation to the laboratory, the chestnuts were subjected to a hot-water treatment (immersion in water at 48–50°C for 45 minutes, and then in water at room temperature) to prevent the growth of microorganisms and reduce the percentage of insect damage. This treatment is compulsory for chestnuts that will be exported (DGAV, Citation2018). Then, the samples were placed in trays with absorbent paper for 36 h at room temperature, in order to absorb the exterior water present on chestnuts.
Packaging
Three packaging systems were applied, namely: 1) vacuum packaging (VAC) (polyamide and polyethylene – high barrier packaging) (Alfa, Spain); 2) modified atmosphere packaging (MAP) (polyester) (TECNOPACK, Portugal) (32% CO2, 0.3% O2 and N2 as filler) and 3) conventional packaging (polyethylene bag) (PE). Concerning the sizes of the plastic packages used in the present work, the VAC bags had a size of 22 cm×13 cm; MAP 15 cm×30 cm; while PE had dimensions of 35 cm×15.5 cm.
VAC and MAP were done in a vacuum machine (Original Henkelman vacuum systems, Boxer 42, Netherlands). The MAP atmosphere was selected because some studies indicate that treatment with 40–50% CO2 for 5–7 days at 0°C (Kader, Citation2003; Mencarelli, Citation2001) was adequate to treat chestnuts. So, we wanted to try a lower CO2 percentage for a higher period. Several packs in VAC, MAP and PE, with 300 g of fresh raw chestnuts each, were stored for six months at 0°C, 90% RH, in a refrigeration chamber of the chestnuts industry. As a control, 300 g of chestnuts were maintained in individual baskets, without packaging, in the same conditions (temperature, RH, and time of storage) and space (chamber of chestnuts industry), as the packed samples. At 0, 1, 2, 3, and 6 months of storage, three packs of each treatment, and three individual baskets (control) were taken from the chestnuts industry to the laboratory.
Headspace Concentrations in Packages
The CO2 and O2 concentrations inside the packages (three samples of each type of packaging at each sampling time) were measured using a gas analyzer (OXYBABY, WittGas Santander, Spain). These measurements were done in order to know the change of CO2/O2 inside each packaging over time. First, we put a rubber seal on the package and then we introduced the needle, linked to the gas analyzer, in the packaging. The O2 and CO2 levels were determined immediately. After doing these measurements, the packages were aseptically open, and the microbiological analyzes were performed, followed by the physicochemical determinations.
All measurements were done in triplicate.
Physicochemical Analysis
Moisture content of the chestnuts was measured by the conventional drying method as described in the AOAC method 925.40 (AOAC, Citation1995), being the results expressed in %. Water activity (aw) was determined in a portable water activity meter (Novasina, LabSwift-aw, Lachen, Switzerland). Color parameters of chestnuts shell and inside the fruit were analyzed using an automatic colourimeter (Minolta CR-400, Osaka, Japan). The parameters lightness (L*), a* that measures the red to green (+red and −green), b* that measures the yellow to blue (+yellow and −blue) and Chroma or saturation of the color (C) were determined.
Furthermore, the total color difference (ΔE*) was also calculated according to the following equation:
where Δ was the difference of the parameters’ values along with the storage and at the beginning (fresh sample, day 0). Texture profile analysis was performed on a TA.XT. Plus texture analyzer (Stable Microsystems, Godalming, UK). The maximum penetration force (N), the distance at which it occurs (elasticity) (mm) and the area below the curve between force and time (indicative of hardness) were determined by penetration with a 2 mm cylinder probe and a test speed of 1.0 mm/s. These parameters were determined on fruit without dark brown outer shell. Ten grams of chestnuts and 50 ml of distilled water were heated in a reflux condenser for 30 min. The obtained solution was used to determine the titratable acidity (TA) and total soluble solids (TSS). TA was determined by titrimetric analysis, and the values were expressed on mg citric acid/100 g dry weight (dw), following the Portuguese Normative (NP-Citation1431, 1977). A few drops of the solution were placed in an Abbe refractometer (Optic Ivymen System, Madrid, Spain) to measure the TSS.
Microbiological Analysis
Three chestnuts (approx. 25–30 g) of each sample were aseptically taken and weighed in a sterilized bag, and the primary decimal dilution was prepared by adding an appropriate volume of sterile peptone water (Liofilchem, Italy) solution. After shaking, serial dilutions were prepared, and aliquots of 0.2 mL were inoculated onto Plate Count Agar (PCA, Liofilchem, Italy), for quantification of mesophilic aerobic count after incubation at 30°C for 48–72 h h (ISO Citation4833:2, 2013), and onto Dichloran Rose Bengal Chloramphenicol Agar (DRBC, Liofilchem, Italy) for molds and yeasts enumeration, after incubation at 25°C for 5 days (ISO 21527-1, Citation2008). All counts were expressed as log10 cfu/g fresh sample.
Statistical Analysis
SPSS statistical software version 18.0 (SPSS Inc., Chicago, IL) was used for data analysis. Results are presented as means ± SD (n = 3). Levene’s test verified the homogeneity of the variances, and the Shapiro-Wilk test verified the normality of the data. As the data followed a normal distribution, analyses of variance (ANOVA) or ANOVA Welch were carried out to evaluate if there were significant differences (p < 0.05) between samples. ANOVA was applied when homogeneity of variances was observed, while ANOVA Welch was applied for the other cases. Additionally, significant post hoc analyses were performed (Tukey HSD test if variances in the different groups were identical or Games-Howell test if they were not).
Results and Discussion
Visual Appearance and Headspace Concentration inside Packages
represents the visual appearance of packaged and control chestnuts during storage (0, 3, and 6 months). At the end of storage, VAC and MAP samples presented similar appearance to fresh samples, although some fermented odor began to appear (), due to the inexistence or deficient O2 levels inside the bags that might have induced fermentative metabolism. Soliva-Fortuny et al. (Citation2002) also detected a production of fermentative metabolites (ex. ethanol and acetaldehyde) in minimally processed pears stored in MAP after three weeks of storage. Furthermore, these metabolites can impart off-flavors that would be the cause of these undesirable sensory changes. In particular, in VAC, the O2 remained very low (0.1–0.2%) throughout storage, while in MAP, it increased (0.3 to 3.0% between 0 and 6 months of storage, respectively). This increase probably happened because some very small holes (invisible to human eye) appeared on the bags and changed the headspace inside the MAP.
Table 1. Oxygen (O2), carbon dioxide (CO2) values, and observations of chestnuts packaged and the control during storage
Figure 1. Visual appearance of packaged (PE – polyethylene; VAC – vacuum; MAP – modified atmosphere packaging) and control chestnuts throughout storage
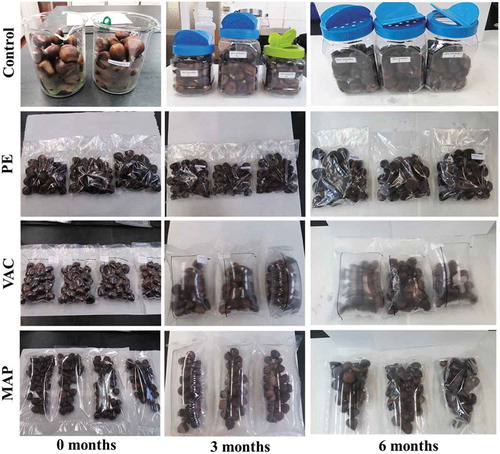
Along with storage, in VAC and MAP packages, the inside volume expanded (), due to chestnuts respiration. This fact explained the increase in CO2 values observed in VAC and MAP packages until three months when compared to day 0. In other fruit studies, it has also been observed an increase in CO2 levels along storage (González-Aguilar et al., Citation2003; Gorny et al., Citation1999).
Visually the control chestnuts showed molds growth after one month of storage, while PE fruits began to germinate at the end of storage (). The germination of chestnuts may be due to the presence of some water droplets inside the pouches, caused by the respiration process. In general, only in VAC and MAP, the chestnuts maintained an excellent appearance for six months, while in PE only for three months of storage. Nevertheless, after three months, the VAC and MAP bags were bloated with a slight fermentative smell.
Color
summarizes the changes in the color parameters in the brown shell and interior of the fruit during the storage period. Regarding the shell color, the L* values increased significantly (p < 0.05) at PE and VAC, when comparing the beginning with the end of storage. Additionally, the highest L* value (39.5) was observed in the PE chestnuts. However, fluctuations in the results were detected in all samples without any definite trend. In almost all situations, the a*, b*, and C values of all samples decreased significantly along with storage. Also, after one month of storage and subsequently, the total color variation values (ΔE*) were always higher than 8, indicating possible color changes outside the nuts when subjected to the proposed packaging. At the end of storage, the lowest total color variation (ΔE*) was observed in chestnuts packaged in MAP (10.9), followed by the VAC package. So, MAP induced a delay on the color change on shell chestnuts, probably due to a decrease in the metabolic processes responsible for pigments degradation. Furthermore, MAP has been reported to maintain the color of fruits, such as mangos (Githiga, Citation2012; Pesis et al., Citation2000), loquat (Amoros et al., Citation2008) and peaches (Artes, Citation2006).
Table 2. Changes in the color of shell and inside chestnuts packaged and the control throughout storage
As the average values for PE, VAC, and control were greater than 12, according to Cecchini et al. (Citation2011) may even lead to a different color.
Regarding the color inside the fruit, in almost all situations, the L* values decreased throughout storage. So, the color inside the fruit showed a slight darkness, being more significant in the samples packed in PE (80.2). This fact may probably be due to a higher enzymatic activity, linked to a higher metabolic activity. During storage, the a* values increased, while b* and C decreased when comparing the beginning with six months of storage. These results suggest the occurrence of some color changes inside the fruit in all situations. These results led to ΔE* values greater than 4 for all treatments throughout storage, which can cause noticeable color changes in the fruit. However, the ΔE* was lower in control (7.0) than the packaged chestnuts. Furthermore, it was interesting to see that the variation of color was higher in the shell than inside the fruit.
Some of these changes in the shell and shelled chestnuts could be due to cold injuries. However, Ekman et al. (Citation2014) referred to the storage, at close to 0°C, can extend chestnut storage life without risking freezing injury. Furthermore, according to the UCDavis recommendations for retaining quality of chestnuts, the optimum storage temperature for chestnuts is ‐1 to 0°C (Ekman et al., Citation2014). So, the probability of having occurred cold injuries is low.
Moisture Content, aw, TA and TSS
In all samples, moisture contents () of chestnuts slightly increased along the storage time, with no significant differences observed between samples at the end of storage (p = 1.62) (). This fact shows that the storage conditions applied by the industry allow the maintenance of the moisture content of the fruits. In more detail, no significant differences were observed at the end of storage, even though the different packaging are expected to have distinct water vapor transmission rates (WVTR). Nevertheless, the packaging with higher barrier properties, namely VAC and MAP, presented the highest moisture contents at the end of storage. On the contrary, the control was the condition that causes the lowest moisture contents due to the inexistence of any barrier to water vapor transmission.
Figure 2. Moisture content and water activity (aw) of packaged and control chestnuts throughout storage
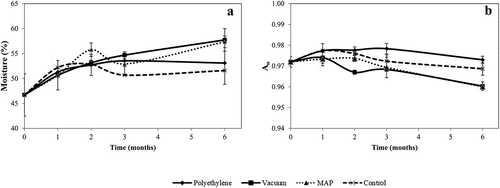
Similar values to ours of moisture were reported by Barreira et al. (Citation2009), between 51.9 to 54.6% for raw chestnuts. Peano et al. (Citation2014) also detected an increase in water values after 90 days of storage, particularly in chestnuts under MAP (from 48 to 60% for Bouche de Betizac variety and 40 to 59% for Garrone Nero variety). However, Bhisanbut et al. (Citation2008) reported different results to ours. They observed a decrease in the moisture values for samples packaged in VAC (51.62 to 46.82%) and MAP (51.62 to 50.98%), during storage.
The aw value of chestnuts before packaging (day 0) was 0.972 (). However, after two months of storage, significant differences were observed between samples. Mainly, the chestnuts packaged in VAC and MAP decreased the aw values when compared to control and PE samples. Moreover, in all samples, the value of aw was still high, allowing the growth of the microorganisms.
Regarding titratable acidity () and total soluble solids (), no significant differences were detected between the packaging types throughout storage. On the other hand, the storage time showed a more significant influence on these two parameters than the packaging type.
Figure 3. Titratable acidity and total soluble solids values determined on packaged and control chestnuts along their storage
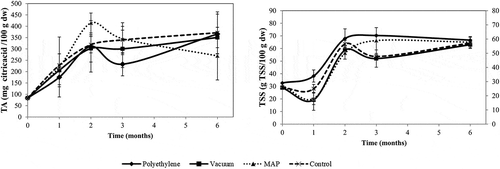
In the first two months, there was an increase in TA and TSS contents for all samples, and then the values slightly decreased or maintained constant. Tzortzakis and Metzidakis (Citation2012) also detected an increase in the TSS in MAP and controlled atmosphere conditions (CA) during 90 days of storage, as well as, Rana et al. (Citation2018) in guava packaged in MAP and VAC (18 days of storage). Other works also reported an increase of sucrose at low temperatures during storage, but this phenomenon has not yet been clarified (Jermini et al., Citation2006; Nomura et al., Citation1995). The increase observed in TSS during the two first months might be due to the hydrolysis of polysaccharides, for example, conversion of starch into sugars by metabolic activities and loss of water from fruit surface. After this time, the rates of formation and consumption of these sugars may have evened out, remaining the values constant, probably due to the occurrence of some physiological processes, such as seed respiration, post-maturation, and germination. Concerning packaging, it was expected that in particular VAC and MAP slowed down the rise in TSS, as well as, minimized the reduction in TA during storage because the CO2 and O2 concentrations in the pack, may reduce the metabolic activities, delaying the ripening process. However, in the present work, these behaviors were not observed clearly.
Texture
The texture parameters in chestnuts were somewhat affected by the type of packaging and storage period (). Some fluctuation in maximum force, elasticity, and hardness values were observed along with storage. However, chestnuts are different from the other nuts because they do not have a hard involucre/shell seed. So, one of the harmful commercial quality factors of chestnut is the softening, which is not due to the softening of the seed (unless it is decayed) but to the air remaining between peel and flesh because of seed water loss through the peel (Mencarelli., Citation2001).
Table 3. Texture parameters of chestnuts packaged and the control throughout storage
Furthermore, the loss of moisture from the surface is the main cause for the cells to lose turgidity and breakdown of pectin leading to the degradative changes in cell wall structure and composition (Rana et al., Citation2018). However, as mentioned previously, chestnuts during storage did not show significant losses in moisture contents in all samples. The low temperature used in the present work may have reduced the metabolic activities and reduce the evapotranspiration loss of water, explaining the slower changes in texture (Rana et al., Citation2018).
It was expected that the values of hardness and maximum force in PE and control samples decreased further since these samples had shown molds growth, which could result in a softer skin and fruit; however, this was not observed. Only in MAP and VAC samples, a slight decrease in the maximum force was observed after six months of storage, as well as an increase in elasticity in MAP samples. However, Martín–Belloso and Soliva–Fortuny (Citation2006) mentioned that O2 and CO2 concentrations do not usually have much effect on the texture of fruits. Nevertheless, the maintenance of CO2/O2 balance is essential for avoiding damage to the fruit tissue due to the phytotoxicity produced by excessive CO2 concentrations or under anoxic environments (Martín–Belloso and Soliva–Fortuny, Citation2006).
Microbial Quality
describes the microbial quality (mesophilic aerobic, molds, and yeasts) of the packaged chestnuts and the control during storage at 0°C and RH 90%.
Table 4. Microbial counts in chestnuts packaged in PE, VAC, and MAP and control throughout storage (log CFU/g ± standard deviation)
Significant differences (p < 0.05) on microbial growth were detected during the storage time, as well as on the packaging type. The microbial populations increased throughout storage in the control samples, while the use of MAP and VAC remarkably inhibited the microbial growth population. MAP and VAC can help to control the proliferation of microorganisms in comparison to PE and control samples because low O2 atmospheres effectively reduce the proliferation of aerobic bacteria (Martín–Belloso and Soliva–Fortuny, Citation2006) and high CO2 atmospheres inhibit most aerobic microorganisms, especially gram-negative bacteria and molds (Al–Ati and Hotchki, Citation2002).
Furthermore, PE samples showed no statistical differences for mesophilic aerobic count, between 0 and 6 months. However, as detected in visual appearance, PE presented higher counts of molds and yeasts at the end of storage (5.22 log CFU/g) than at the beginning (0 days) (3.74 log CFU/g). Bhisanbut et al. (Citation2008) also reported that chestnuts packaged in MAP showed a decrease in the counts on total aerobic bacteria (3.29 to 3.12 log CFU/g, between 0 and 26 days, respectively) and molds (6.91 to 5.81 log CFU/g), along storage at 4 ± 1°C, 85 ± 5% RH. However, they detected an increase in the yeasts (1.89 to 3.11 log CFU/g), as well as in molds and yeasts in the VAC packages. Furthermore, VAC packages also showed a decrease in total aerobic bacteria (3.29 to 2.88 log CFU/g), as in our study concerning the mesophilic aerobic count. These differences may be due to Bhisanbut et al. (Citation2008) have used a different air composition inside the MAP packages (20.8% O2 and 0.03% CO2), a different chestnut species and a short period of storage (26 days). In summary, the use of MAP and VAC could significantly control the proliferation of the microorganisms during six months of storage.
Conclusion
The present study reports important results for the chestnuts industry regarding the best packages to use in order to maintain the safety and quality of these nuts during storage. All types of packaging, as well as the control, showed color variation (ΔE*), both in the chestnuts shell and shelled fruit (interior), but the smallest differences in the shell were observed in MAP. The moisture contents did not differ between samples. TA and TSS increased in the first two months of storage, for all samples. The texture was low affected by the type of packaging and storage period. VAC and MAP were the most effective packaging to inhibit the microbial growth because their use decreased the values of yeast, molds, and aerobic bacteria during storage.
On the contrary, PE and control samples presented molds growth during storage. However, after one month, PE may induce germination, and after three months, VAC and MAP showed a slight fermented smell. Thus, the results showed that chestnuts remained with acceptable microbiological, physical, and chemical quality for three months when packed in MAP or VAC.
Additional information
Funding
References
- Al–Ati, T., and J.H. Hotchkiss. 2002. Application of packaging and modified atmosphere to fresh-cut fruits, p. 305–338. In: O. Lamikanra (ed.). Fresh-cut fruits and vegetables: Science, technology, and market. CRC Press, Boca Raton.
- Amorós, A., M.T. Pretel, J. Zapata, M.A. Botella, F. Romojaro, and M. Serrano. 2008. Use of Modified Atmosphere Packaging with Microperforated Polypropylene Films to Maintain Postharvest Loquat Fruit Quality. Food Sci. Tech. Int. 14(1):95–103. doi: 10.1177/1082013208089985.
- AOAC. (1995). Official Methods of Analysis, 16th ed. Association of Official Analytical Chemists, Arlington, VA, USA.
- Artes, F., P.A. Gomez, and F. Hernandez. 2006. Modified atmosphere packaging of fruits and vegetables. Stewa. Posth Rev 5:2–13.
- Barreira, J.C.M., S. Casal, I.C.F.R. Ferreira, and M.B.P.P. Oliveira, J.A.Pereira (2009). Nutritional, Fatty Acid and Triacylglycerol Profiles of Castanea sativa Mill. Cultivars: A Compositional and Chemometric Approach. Journal of Agricultural and Food Chemistry, 57, 2836-2842.doi: doi:10.1021/jf803754u
- Bhisanbut, A., J. Shin, J. Harte, D. Fulbright, K. Dolan, and B. Harte. 2008. The extension of chestnut product quality using Modified Atmosphere Packaging and Vacuum Skin Packaging. 16th IAPRI World Conference on Packaging, 8th to 12th June, Bangkok, Thailand.
- Borges, O., B. Gonçalves, J.L.S. Carvalho, P. Correia, and A.P. Silva. 2008. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 106(3):976–984. doi: 10.1016/j.foodchem.2007.07.011.
- Cecchini, M., M. Contini, R. Massantini, D. Monarca, and R. Moscetti. 2011. Effects of controlled atmospheres and low temperature on storability of chestnuts manually and mechanically harvested. Postharvest Biol. Technol. 61(2–3):131–136. doi: 10.1016/j.postharvbio.2011.03.001.
- DGAV. 2018. Procedure manual –Export of fresh chestnuts subjected to treatment with hot water in a continuous system - procedure to be adopted at the storage and packing industrial units of chestnuts (version 01). Direção-Geral de Alimentação e Veterinária. Portugal. pp. 13.
- Ekman, J. 2014. Improved postharvest management of chestnuts. Final Report CH13005, Horticulture Innovation Australia Ltd, Sydney, 1-33. (ISBN 0 7341 3461 4).
- FAO. (2018). FAOSTAT - Data for chestnut production in the year 2018 http://www.fao.org/faostat/en/#dataAccessed on 05th January 2020.
- Githiga, R.W. 2012. Effect of 1- Methylcyclopropene and Activebag packaging on the postharvest characteristics of mango fruit (Mangifera indica) cv. Tommy Atkins. M.Sc. Thesis. University of Nairobi, Nairobi, Kenya.
- González-Aguilar, G.A., J.G. Buta, and C.Y. Wang. 2003. Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain postharvest quality of papaya ‘Sunrise’. Postharvest Biol. Technol. 28(3):361–370. doi: 10.1016/S0925-5214(02)00200-4.
- Gorny, J.R., B. Hess-Pierce, and A.A. Kader. 1999. Quality changes in fresh-cut peach and nectarine slices as affected by cultivar, storage atmosphere and chemical treatments. J. Food Sci. 64(3):429–432. doi: 10.1111/j.1365-2621.1999.tb15057.x.
- INE. 2017. Estatísticas Agrícolas 2017. Instituto Nacional de Estatística (ISBN 978-989-25-0445-2). Portugal.
- ISO 21527-1. 2008. Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of yeasts and moulds — Part 1: Colony count technique in products with water activity greater than 0,95. (1st edition).
- ISO 4833-2. 2013. Microbiology of the food chain – Horizontal method for the enumeration of microorganisms – Part 2: Colony count at 30 degrees C by the surface plating technique.
- Jermini, M., M. Conedera, T.N. Sieber, A. Sassella, H. Scharer, G. Jelmini, and E. Hohn. 2006. Influence of fruit treatments on perishability during cold storage of sweet chestnuts. J. Sci. Food Agric. 86(6):877–885. doi: 10.1002/jsfa.2428.
- Kader, A.A. 2003. Produce facts chestnuts recommendations for maintaining postharvest quality. Postharvest Technology Research and Information Center, University of California, Davis.
- Kim, H.K., D.S. An, S.J. Lee, and D.S. Lee. 2012. Dependence of individual primary package atmosphere on retail display temperature and micro-perforations in a master packaging system for chestnuts. J. Food Agricul. Enviro. 10:168–172.
- Mangaraj, S., T.K. Goswami, and P.V. Mahajan. 2009. Applications of plastic films for modified atmosphere packaging of fruits and vegetables: A review. Food Engin. Rev 1(2):133–158. doi: 10.1007/s12393-009-9007-3.
- Marsh, K., and A. Bugusu. 2007. Food packaging—roles, materials, and environmental issues. J. Food Sci. 72(3):39–55. doi: 10.1111/j.1750-3841.2007.00301.x.
- Martín–Belloso, O., and R. Soliva–Fortuny. 2006. Effect of modified atmosphere packaging on the quality of fresh-cut fruits. Stewa. Posth Rev 2:1–8.
- Mencarelli, F. 2001. Section 1 - Postharvest physiology and pathology of chestnuts. In: Postharvest handling and storage of chestnuts. Working document of the project: TCP/CPR/8925 “Integrated Pest Management and Storage of Chestnutsin XinXian County, Henan Province, China„. Food and Agriculture Organization of the United Nations (FAO) (ed.). Rome (Italy). p.1
- Nazzaro, M., C. Barbarisi, F. La Cara, and M.G. Volpe. 2011. Chemical and biochemical characterisation of an IGP ecotype chestnut subjected to different treatments. Food Chem. 128(4):930–936. doi: 10.1016/j.foodchem.2011.03.121.
- Nomura, K., Y. Ogasawara, H. Uemukai, and M. Yoshida. 1995. Change of sugar content in chestnut during low temperature storage. Acta Hortic. 398(398):265–276. doi: 10.17660/ActaHortic.1995.398.28.
- NP-1421 Standard. 1977. Foodstuffs derived from fruit and vegetables. Determination of acidity. In: Portuguese. 1st, D.-G. da Qualidade. ed. Portuguese Institute of Quality; Lisbon, Portugal: 3.
- Peano, C., C. Baudino, N.R. Giuggioli, and V. Girgenti. 2014. The use of a modified atmosphere for the storage of chestnut fruits. Ital. J. Food Sci. 24:74–80.
- Pereira, M.G., L. Caramelo, C. Gouveia, J. Gomes-Laranjo, and M. Magalhães. 2011. Assessment of weather-related risk on chestnut productivity. Nat. Hazard. Earth Sys. Sci. 11(10):2729–2739. doi: 10.5194/nhess-11-2729-2011.
- Pesis, E., D. Aharoni, Z. Aharon, R. Ben-Arie, N. Aharoni, and Y. Fuchs. 2000. Modified atmosphere and modified humidity packaging alleviates chilling injury symptoms in mango fruit. Postharvest Biol. Technol. 19(1):93–101. doi: 10.1016/S0925-5214(00)00080-6.
- Rana, S., S. Siddiqui, and K. Gandhi. 2018. Effect of individual vacuum and modified atmosphere packaging on shelf life of guava. Int. J. Chem. Stud. 6:966–972.
- Randell, K., T. Hattula, and R. Ahvenainen. 1997. Effect of packaging method on the quality of rainbow trout and baltic herring fillets. LWT - Food Sci. Tech 30(1):56–61. doi: 10.1006/fstl.1996.0131.
- Soliva–Fortuny, R.C., M. Biosca-Biosca, N. Grigelmo-Miguel, and O. Martín–Belloso. 2002. Browning, polyphenol oxidase activity and headspace gas composition during storage of minimally processed pears using modified atmosphere packaging. J. Sci. Food Agric. 82(13):1490–1496. doi: 10.1002/jsfa.1209.
- Tzortzakis, N., and I. Metzidakis. 2012. Determination of heat stress and ultra low oxygen in chestnut storage under control and modified atmospheres. Food Nut. Sci. 3(3):387–393. doi: 10.4236/fns.2012.33055.
- Vasconcelos, M.C.B.M., R.N. Bennett, E.A.S. Rosa, and J.V. Ferreira-Cardoso. 2010. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food. Agricult. 90(10):1578–1589. doi: 10.1002/jsfa.4016.
