ABSTRACT
Pecan, [Carya illinoinensis (Wangenheim) C. Koch] is a nut crop of growing worldwide interest and importance. In this research, a micropropagation protocol was developed during 2015–2016 using five pecan cultivars (‘GraTex’, ‘Wichita’, ‘Choctaw’, ‘10 J’ and ‘GraKing’). In the establishment phase, nodal segments of mature pecan trees were collected 9, 13, 17, 21, and 25 days after bud-break and maintained at 4°C for 0, 24, 48, or 72 hours. Next, they were soaked in 2.5% sodium hypochlorite for 1, 2, 3, 4, 5, 6, or 7 minutes. Each explant was cultured on DKW medium individually. For proliferation phase, the best established shoots were cut into nodal segments and cultured on DKW medium supplemented with different BA concentrations (4.44, 13.30, 22.20, and 44.40 µM). Proliferating cultures were established and microshoots were rooted on MS medium containing IBA (4.9, 14.7, 24.5, and 49.0 µM) for 6 days in the dark. Date of cutting field-grown shoots for explanting had a significant effect on disinfection and culture establishment, with optimum success 11–15 days after bud break. Preliminary chilling treatment (4°C for 48 h) prior to cutting into nodal segments improved explant establishment. The best proliferation rate was obtained using 22.2 µM BA. Root induction using 24.5 µM IBA gave the highest rooting percentage. Rooted microshoots were successfully transferred to pots. Although the ‘Wichita’ and ‘10 J’ cultivars had the highest success for acclimatization (89% and 84% respectively) the difference between cultivars was not significant and plantlets of five pecan cultivars were successfully acclimated. The results of this research can be applied by the authors in commercial level.
Introduction
The pecan [Carya illinoinensis (Wangenheim) C. Koch] is an important tree in the Juglandaceae family, witnessing a rapid increase in world-wide consumption (Haroon, Citation2010; INC, Citation2018). Pecan is native to North America, including Texas and northern Mexico (Andersen and Crocker, Citation2009). Pecan trees are generally propagated by budding or grafting in field nurseries (Ajamgard et al., Citation2016; Hancock, Citation1997). Summaries of pecan propagation by conventional methods have already been reported (Ajamgard et al., Citation2016; Brutsch et al., Citation1977; Smith et al., Citation1974). Most commonly, seed-propagated rootstocks are used in the production of grafted pecan trees but this can contribute to variability in pecan orchards. In addition, the nursery cycle for producing salable trees is long and the process requires large amounts of scion material. Advances in methods for vegetative propagation of fruit trees through tissue culture now make it possible to produce large numbers of genetically identical plants economically in a short period of time (Bowman et al., Citation1997). Own-rooted pecans are not available because it has not been technically feasible before now. The main advantage for having self-rooted pecan cultivars is to speed propagation (1 versus 3 years), and most likely lower production costs of the propagated tree. In our knowledge, there is no literature showing self-rooted trees of pecan being more or less vulnerable to biotic or abiotic stresses (Casales et al., Citation2018; Garner, Citation2011; Renukdas et al., Citation2010)
Early reports of pecan cultivars tissue culture include Smith (Citation1977) but neither was successful in establishing plants in soil. Major issues in micropropagation of pecan have included poor rooting and poor survival after transplanting to the greenhouse (Brutsch et al., Citation1977). Knox (Citation1980) obtained a few shoots and plantlets in vitro using inverted nodal cuttings from mature pecans, but they did not survive transplanting. Knox and Smith (Citation1981) and Wood (Citation1982) used nodal stem explants from seedlings to establish shoot proliferation with a combination of 17.6 µM 6-benzyladenine (BA) and 0.49 µM indole-3-butyric acid (IBA), but no consistent shoot proliferation or root development occurred, and plants were not established in soil. Phillips and Ramirez (Citation1983) used apical and axillary buds and obtained shoot elongation, but rooting and establishment were poor. Hansen and Lazarte (Citation1984) obtained single node cuttings from 2-month-old pecan seedlings on liquid WPM and 2% glucose supplemented with 13.2 µM BA. The shoots developed adventitious roots upon transferring to the soil.
Corte-Olivares et al. (Citation1990) reported a procedure for propagating mature pecan trees but encountered severe contamination problems that resulted in data that were not amenable to statistical analysis. Results of this preliminary study indicated that some adult pecan phenotypes had potential for clonal micropropagation. Renukdas et al. (Citation2010) used nodal explants of ‘Desirable’ and ‘Cape Fear’ seedlings and at least nine multiple shoots per explants were obtained after three weeks of culture. The multiple shoots were successfully rooted and transferred to peat pellets and subsequently to the greenhouse. The explants used were of seedling origin.
While there has been considerable publication for pecan, all reports are concerning studies using juvenile explant materials. What is missing are reports using clonal cultivars, which by definition are non-juvenile (mature). We believe it is significant that the publications do not include methods for introduction of mature cultivars into culture. The ability to micropropagate juvenile material has not translated into the ability to micropropagate clonal mature cultivars (Renukdas et al., Citation2010). Thus, we believe this research is relevant to the scientific community, as it describes for the first time methods to micropropagate multiple cultivars. In addition, due to limited availability of pecan clonal plants in the world, the rapid development of its orchards in subtropical regions is only possible through using of micropropagation techniques. In the current study, we present a method for micropropagation of five pecan cultivars, and describe establishment, multiplication, rooting, and acclimatization phases for producing plants.
Materials and Methods
Plant Material
Nodal stem segment explants were taken from pecan cultivars ‘GraTex’, ‘Wichita’, ‘Choctaw’, ‘10 J’ and ‘GraKing’ grown at the Safiabad Agricultural Research Center of Dezful, in southwestern Iran.
Optimizing Establishment Phase
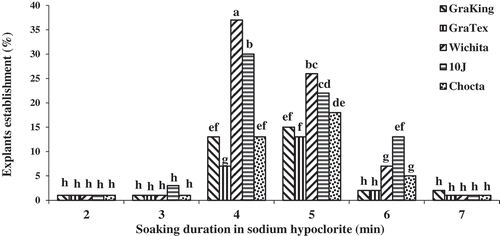
In order to optimize establishment phase, an experiment was conducted as split plot design completely randomized with three replications and 16 jars as plot. In the first step, date of cutting the shoots from the trees (main factor) and chilling treatments of explant (main factor) were evaluated. Shoots of mature pecan trees () were collected 9, 13, 17, 21 and 25 days after bud-break and transferred to the tissue culture lab of Department of Horticulture, Aburaihan Campus, University of Tehran, and maintained at 4°C for 0, 24, 48 or 72 hours. After chilling, nodal segments were excised in a laminar air flow hood, keeping one vegetative leaf bud per segment (). For surface sterilization, excised buds first were submersed briefly in ethanol (70% v/v) for 5 seconds and then rinsed twice in distilled water. After that, plants were disinfected using a commercial bleach solution (5% active chlorine) diluted 50% with distilled water, giving a treatment with a 2.5% active chlorine solution. One droplet of soap was added to the solution as a surfactant. Nodal segments were soaked under agitation for 1, 2, 3, 4, 5, 6, or 7 minutes (subfactor), and triple rinsed with sterile-distilled water.
Figure 1. Preparation of pecan explants and their establishment on DKW medium; (a) Explants were taken from the spring flush of mature pecan trees; (b) Shoots were cut to one vegetative bud per node; (c) Explants were cultured individually in jars containing DKW medium
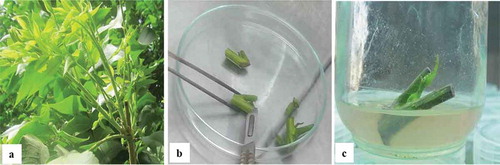
Each explant was transferred to an individual jar (). The establishment phase was repeated 3 times. Therefore, 96 explants were analyzed in each treatment. The DKW culture medium (Driver and Kuniyuki, Citation1984) was supplemented with 4.4 µM 6-benzyladenine (BA), and 0.49 µM indole-3-butyric acid (IBA). The media was solidified with 2.1 g/l Gelrite® (Sigma Co.), pH adjusted to 5.6, and sterilized by autoclaving (121°C for 20 min). Jars were kept in a growth chamber at 25 ± 1°C with a 16-h photoperiod (40–45 μE·m–2 s–1) for an establishment period of 21 days.
Proliferation Phase
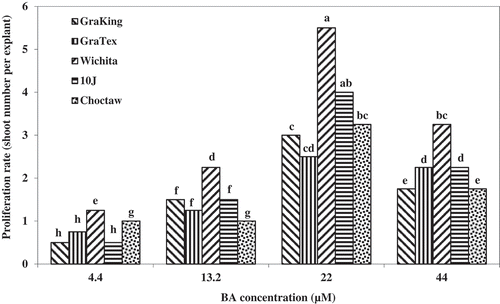
For proliferation, an experiment was conducted as completely randomized design with three replications and 16 jars as plot and four shoots in each. The new tissue culture shoots from healthy, non-contaminated and without brown symptoms of explants from the establishment phase () were excised and cultured on DKW medium supplemented with different concentrations of BA (4.44, 13.3, 22.20, and 44.4 µM) for determination of the medium giving the best proliferation rate (Renukdas et al., Citation2010) (). The average number of shoots was assessed after 28 days of culturing.
Figure 2. Proliferation of pecan on DKW medium supplemented with varying BA concentrations; (a) Healthy explants were selected from the establishment phase, (b) New shoots from establishment phase were excised for proliferation, (c) Multiple shoots were produced after 21 days of culturing during the proliferation phase

In Vitro Rooting
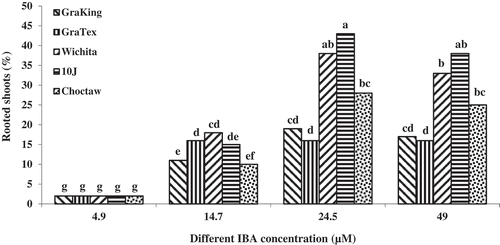
Rooting was accomplished in two phases: root induction and root development (Vahdati et al., Citation2004). In the first phase, shoots 4 to 5 cm in length were excised from cultures of the best proliferation rate treatment that was obtained using 22.2 µM BA. Shoots were cut above the callus and the basal ends stuck in an induction medium consisting of MS medium (Murashige and Skoog, Citation1962) supplemented with IBA (4.9, 14.7, 24.5, and 49.0 µM), 40 g/L sucrose and 2.1 g/L Gelrite® for 6 days in the dark at 25°C (). For root expression, shoots were transferred to a sterilized 1:1.25 (v/v) mix of half strength DKW basal medium and vermiculite for 35 days at 25 ± 1°C with 16 h photoperiod as described for walnut by Vahdati et al. (Citation2004, Citation2009) and Hassankhah et al. (Citation2014). ().
Plant Acclimatization
After root development, rooted plants were transferred to pots containing a mixture of perlite: peat (1:1, v/v, ) and during the first week they were maintained under the plastic bags. During the second week, the plastic covers were removed gradually. Elongation of shoots and growing of new leaves were observed after six weeks. Plantlets were transferred to larger pots at this time () and again repotted after three months ().
Statistical Analysis
Analysis of variance was carried out using SAS software (ver. 9.2; SAS Institute, Inc., Cary, NC) and means of treatments were compared using Duncan’s multiple range test at 5% probability level.
Results and Discussion
Establishment Phase
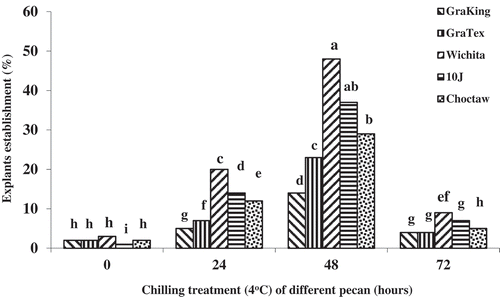
Results showed that a preliminary chilling treatment of rapidly growing shoots of mature pecan trees prior to cutting the shoots into nodal segments improved explant establishment. Treating the shoots at 4°C for 48 h prior to culture was optimum for explant establishment (). Wu et al. (Citation2009) reported that pre-chilling treatment (4°C for 6 weeks) also significantly promoted in vitro initiation of Rubus cultures. Pre-chilling of shoots promoted vigorous growth of new sprouts from the explants and, therefore, resulted in the highest initiation rate.
Figure 5. Effect of chilling duration (4°C) on the establishment of various pecan cultivars in micropropagation. Means of the columns followed by the same letter are not significantly different according to Duncan’s multiple range test (P ≤ 0.05)
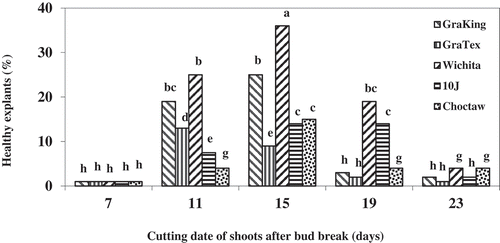
Endogenous contamination and phenolic compounds are two major limitation factors during establishment of mature pecan tissue in culture. These contaminants could not be controlled by surface disinfectants and contaminated the culture media during the establishment phase. It seems that the population of microorganisms in the young shoots after budbreak has increased as they become woody gradually. So procuring explants from younger shoots would reduce contamination and increase the chances of success in the establishment phase. The best source of nodal explant segments from mature pecan trees was rapidly growing, newly flushed shoots taken 11–15 days after bud break (). However, results showed that new shoots younger than 9 days after bud break could not be used because the tissue of these shoots is damaged at the time of sterilization. Corte-Olivares et al. (Citation1990) considered late May and early June to be optimum for taking axillary bud explants for in vitro culture of pecan.
Figure 6. Effect of cutting date of shoots of various pecan cultivars, in days after bud break, on the health of explants (explants without symptom of browning). Means of the columns followed by the same letter are not significantly different according to Duncan’s multiple range test (P ≤ .05)
A soaking treatment of 4–5 minute duration in 2.5% sodium hypochlorite was effective for superficial disinfection of nodal pecan explants (), confirming the results of previous studies (Corte-Olivares et al., Citation1990; Renukdas et al., Citation2010). In contrast, Haroon (Citation2010) used 15% commercial bleach (NaOCl, 3.0% v/v) solution containing Tween 20 (0.1% v/v) for about 10–20 minutes for surface sterilization of pecan explants. ‘Wichita’ had the highest explant establishment percent of cultivars attempted ().
Proliferation Phase
Micropropagation is affected by many factors, including genotype, culture medium, and plant growth regulators (Arab et al., Citation2014). Different cultivars have different responses to micropropagation. The best proliferation rate among pecan cultivars tested in this experiment was obtained using 22.2 µM BA (). Wood (Citation1982) reported that a combination of 17.6 µM BA and 0.49 µM (IBA) was the most effective for pecan shoot proliferation. In addition, Renukdas et al. (Citation2010) used nodal explants of ‘Desirable’ and ‘Cape Fear’ seedlings and obtained at least nine multiple shoots per explant after three weeks of culture using 13.3 µM BA and stem segments of seedling pecan, not cultivars, were multiplied on WPM medium containing 13.3 µM BAP (Hassanen and Gabr, Citation2013).
In Vitro Rooting
The effect of IBA concentration on rooting of mature pecan explants was significant at P ≤ 0.05. Shoots induced to root on MS medium supplemented with 24.5 µM IBA had the highest rooting percentage (43%) (). Renukdas et al. (Citation2010) reported that root induction from pecan micro shoots of seedling origin was observed after three weeks of culture in modified liquid WPM supplemented with 49.2 µM IBA. Similar results were obtained by Hansen and Lazarte (Citation1984). Hassanen and Gabr (Citation2013) reported that the best rooting percentage, using pecan micro shoots of seedlings origin (75%), was obtained on half strength WPM medium supplemented with 14.7 µM IBA and 1.5 mg/l AgNO3. The low rooting percentages achieved in this study seems to be due to the maturity of the mother plants for explant preparation. Whereas in the literatures cited above, the explants were obtained from young seedlings.
Plant Acclimatization
In acclimatization phase, due to careful control of the temperature (23°C −27°C) and humidity (90%-95%) conditions, especially in the first weeks, rooted plants of all five varieties of pecan were successfully transferred to pots and acclimated. Although the ‘Wichita’ and ‘10 J’ cultivars had the highest success for acclimatization (89% and 84%, respectively) the difference between cultivars was not statistically significant ().
Table 1. Plants status of different pecan cultivars at acclimatization phase
In conclusion, the results of this study indicate the potential for producing own-rooted plantlets from mature pecan cultivars. To increase the proliferation rate and rooting percentages, procedures should be more completely optimized. It is expected that each cultivar will respond to individually optimized protocols. This study provides a basis for further exploration of clonal pecan micropropagation. Five commercial cultivars of pecan were successfully introduced to in vitro culture, micropropagated, rooted, and acclimated to establish clonal plants. This procedure not only enables production of large number of own-rooted trees in a relatively short duration but also can play an important future role in micropropagaion and improvement of pecan cultivars and rootstocks.
Acknowledgments
We appreciate Iran National Science Foundation (INSF), University of Tehran, Shiraz University, Center of Excellence for Walnut Improvement, and the Technology of Iran and Safiabad Agricultural Research Center of Dezful, for their support. We also acknowledge Saadat Sarikhani and Charles A. Leslie for technical editing of the final draft.
References
- Ajamgard, F., M. Rahemi, and K. Vahdati. 2016. Development of improved techniques for grafting of pecan. Sci. Hort. 204:65–69. doi: 10.1016/j.scienta.2016.03.030.
- Andersen, P.C., and T.E. Crocker. 2009. The pecan tree. Florida Cooperative Extension Service. Institute of Food and Agricultural Sciences, Gainesville, FL: University of Florida.
- Arab, M.M., A. Yadollahi, A. Shojaeiyan, S. Shokri, and S.M. Ghojah. 2014. Effects of nutrient media, different cytokinin types and their concentrations on in vitro multiplication of G×N15 (hybrid of almond× peach) vegetative rootstock. J. Genet. Eng. Biotechnol. 12(2):81–87. doi: 10.1016/j.jgeb.2014.10.001.
- Bowman, K.D., H.K. Wutscher, R.D. Hartman, and A.E. Lamb. 1997. Enhancing development of improved rootstocks by tissue culture propagation and field performance of selected rootstocks. Proc. Fla. State Hort. Soc. 110:10–12.
- Brutsch, M.O., P. Allan, and B.N. Wolstenholme. 1977. The anatomy of adventitious root formation in adult-phase pecan [Carya illinoinensis (Wangenh) K. Koch] stem cuttings. Hort. Res. 17:23–31.
- Casales, F.G., E. Van der Watt, and G.M. Coetzer. 2018. Propagation of pecan (Carya illinoensis): A review. Afr. J. Biotechnol. 17(18):586–605. doi: 10.5897/ajb2017.16183.
- Corte-Olivares, J., G.C. Phillips, and S.A. Butler-Nance. 1990. Micropropagation of pecan. HortScience 25(10):1308. doi: 10.21273/HORTSCI.25.10.1308.
- Driver, J.A., and A.H. Kuniyuki. 1984. In vitro propagation of Paradox walnut rootstock (Juglans hindsii × Juglans regia). HortScience 19(4):507–509.
- Garner, J.O. 2011. In vitro propagation of elite pecan (Carya illinoinensis (Wangenh.) K. Koch) cultivars. National Institute of Food and Agriculture. https://reeis.usda.gov/web/crisprojectpages/0207971-in-vitro-propagation-of-elite-pecan-carya-illinoinensis-wangenhk-koch-cultivars.html
- Hancock, B.G. 1997. Development of pecan industry, p. 1–7. In: L.A. Stein (ed.). Texas pecan handbook. Texas Agricultural Extension Service, USA.
- Hansen, K.C., and J.E. Lazarte. 1984. In vitro propagation of pecan seedlings. HortScience 19(2):237–239.
- Haroon, A. 2010. Propagation of Pecan (Carya illinoensis) using in vitro techniques. Lahore, Pakistan, University of the Punjab, PhD Dissertation.
- Hassanen, S., and M. Gabr. 2013. In vitro rooting of pecan (Carya illinoinensis Wang-C. Koch). World Appl. Sci. J. 21(3):315–319. doi: 10.5829/idosi.wasj.2013.21.3.2856.
- Hassankhah, A., K. Vahdati, M. Lotfi, M. Mirmasoumi, J. Preece, and M. Assareh. 2014. Effects of ventilation and sucrose concentrations on the growth and plantlet anatomy of micropropagated Persian walnut plants. Int. J. Hort. Sci. Tech. 1(2):111–120. doi: 10.22059/ijhst.2014.52781.
- INC. 2018. Nuts and dried fruits, statistical yearbook, p. 76. Spain: International Nut and Dried Fruits Press.
- Knox, C.A. 1980. Histological and physiological aspects of growth responses and differentiation of pecan [Carya illinoinensis (Wangenh) K. Koch] tissues in vitro. College Station, Texas A and M University, PhD Dissertation.
- Knox, C.A., and R.A. Smith. 1981. Progress in tissue culture methods for production of ‘Riverside’ stocks [Pecans, varieties, propagation]. Pecan Quart. 15:27–34.
- Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x.
- Phillips, G.C., and J.J. Ramirez. 1983. Pecan tissue culture. Proceedings of the 17th Western Pecan Conference, Cooperative Extension Service and Western Irrigated Pecan Growers Association, New Mexico State University, Las Cruces, pp. 101–109.
- Renukdas, N.N., M. Manoharan, and J.O. Garner Jr. 2010. In vitro propagation of pecan [Carya illinoinensis (Wangenh) K. Koch]. Plant Biotech. 27(2):211–215. doi: 10.5511/plantbiotechnology.27.211.
- Smith, I.E., B.N. Wolstenholme, and P. Allan. 1974. Rooting and establishment of pecan stem cuttings. Agroplantae 7:21–28.
- Smith, M.W. 1977. Shoot meristem and callus tissue culture of pecans [Carya illinoinensis (Wangenh) K. Koch]. Texas A and M University College Station, PhD Dissertation.
- Vahdati, K., C. Leslie, Z. Zamani, and G. McGranahan. 2004. Rooting and acclimatization of in vitro-grown shoots from mature trees of three Persian walnut cultivars. HortScience 39(2):324–327. doi: 10.21273/HORTSCI.39.2.324.
- Vahdati, K., R. Razaee, and M. Mirmasoomi. 2009. Micropropagation of some dwarf and early mature walnut genotypes. Biotech. 8(1):171–175. doi: 10.3923/biotech.
- Wood, B.W. 1982. In vitro proliferation of pecan shoots. HortScience 17:890–891.
- Wu, J.H., S.A. Miller, H.K. Hall, and P.A. Mooney. 2009. Factors affecting the efficiency of Micropropagation from lateral buds and shoot tips of Rubus. Plant Cell Tissue Organ Cult. 99(1):17–25. doi: 10.1007/s11240-009-9571-5.