ABSTRACT
Greenhouse trials were conducted to evaluate the effect of several locally available carbon (C) sources on weed suppression using anaerobic soil disinfestation (ASD). Carbon sources included rice bran, sorghum-sudangrass, cowpea, buckwheat, paper mulch, brewer`s spent grain, waste coffee grounds, and peanut shells applied at 4 mg of C/g of soil. All trials were conducted in containers of 0.2-m height and 0.15-m diameter. The germination of common chickweed, redroot pigweed, white clover, and yellow nutsedge was reduced similarly with all C sources used for ASD. The addition of distiller’s yeast at 10 kg/ha to C sources at 4 mg of C/g of soil provided similar or better weed control than ASD treatments with C sources alone. ASD treatments in all trials reduced weed viability from 50% to 100% compared to the non-treated control. Redox potential in all ASD treatments during the 3-week treatment was lower (more anaerobic) than the non-treated control.
Introduction
In many high-value specialty crop production systems where crop rotation is not practical, growers must utilize pre-plant treatments to suppress soil-borne pests, including weeds, plant pathogens, and plant parasitic nematodes (Shennan et al., Citation2009). For the past 40 years, pre-plant soil chemical fumigation has been widely used in many developed countries to achieve good pest control efficacy and higher crop yields relative to non-treated soils. However, fumigation increases the environmental and human health risk, along with the increasing production costs from barrier films and expanding buffer zones (Fennimore et al., Citation2013; Gao et al., Citation2011). The chemical fumigants 1,3-dichloropropene (1,3-D) and chloropicrin are now applied as alternatives to MeBr for pest control in strawberry (Rosskopf et al., Citation2005). However, 1,3-D and chloropicrin are toxic to humans, and chloropicrin is not as effective on weeds as methyl bromide (MeBr) fumigation (Noling and Becker, Citation1994). Due to the health risks and relatively high cost associated with chemical fumigants, it is necessary to evaluate alternative strategies to disinfest soils.
Anaerobic soil disinfestation (ASD) was independently developed in Japan (Shinmura, Citation2000) and in the Netherlands (Blok et al., Citation2000) as alternatives to chemical soil disinfestation. In the U.S and the Netherlands, this method is called “anaerobic” soil disinfestation to emphasize the anaerobic soil condition (Butler et al., Citation2012). The method involves using a large number of decomposable organic materials, applying irrigation to field capacity, and use of polyethylene mulch film to limit gas and create anaerobic condition. Under anaerobic conditions, the carbon source is also decomposed by other microorganisms, which produce organic acids, aldehydes, alcohols, ammonia, metal ions, and volatile organic compounds that are suppressive or toxic to several soil-borne pests and diseases (Huang et al., Citation2015).
In general, the types and amounts of carbon sources have profound influence on the ASD effect. The carbon sources used should contain an adequate supply of labile carbon to support soil microbial growth (Momma, Citation2008), and various labile carbon sources have been studied, such as molasses (Butler et al., Citation2012), rice bran (Shennan et al., Citation2009), wheat bran (Momma, Citation2008), ethanol (Uematsu et al., Citation2007), other forms of plant biomass (Messiha et al., Citation2007) or other agricultural byproducts (Serrano-Pérez et al., Citation2017). Other carbon sources such as ethanol have shown promise in controlling soil-borne pests (Momma et al. Citation2010; Uematsu et al., Citation2007). However, the high cost of ethanol makes it unsuitable for large-scale usage in the US, and the application of ethanol to soil is regulated in US. Bioethanol produced from agricultural waste is a potential way to solve this problem. Honda et al. (Citation2008) and Kitamoto et al. (Citation2011) reported a method to process bioethanol production under field conditions using local forage crops, and Horita and Kitamoto (Citation2015) evaluated the effect of the products and byproducts from such bioethanol fermentation as carbon sources for ASD. This study indicated that residual organic substances in the bioethanol fermentation products had the potential to enhance the effect of ASD treatment. Locally available waste materials, such as brewer`s spent grain (BSG), have been shown to produce bioethanol when mixed with yeast (Saccharomyces cerevisiae) (Liguori et al., Citation2015). Although few field studies on bioethanol fermentation used wastes such as BSG, BSG could provide a possible way to generate low-cost ethanol or even to enhance the effect of ASD treatment by applying yeast during ASD.
In Virginia, strawberries are mostly grown using an annual plasticulture production system with strawberry cultivars transplanted in early fall (Christman and Samtani, Citation2019). Strawberries are planted continuously with limited time to prepare land post-harvest and disinfest the soil before the next transplant season. Pre-plant weed control is necessary because strawberry is sensitive to weed competition. Strawberry yields are depressed due to competition with annual weeds such redroot pigweed (Amaranthus retroflexus), shepherd’s purse (Capsella bursa-pastoris) and common chickweed (Stellaria media); biennials including wild carrot (Daucus carota); and perennials include dandelion (Taraxacum officinale), quackgrass (Elymus repens), white clover (Trifolium repens) and yellow nutsedge (Cyperus esculentus) (Melanson et al., Citation2019).
According to a meta-analysis of 533 ASD experiments, including 88 ASD studies on weed suppression (Shrestha et al., Citation2016), density of common lamb’s quarters (Chenopodium album) and yellow nutsedge were significantly reduced by ASD, while suppression of redroot pigweed was not achieved. Moreover, weed suppression was primarily at high soil temperature during ASD treatment, and only when the C source rate was greater than 1 kg m−2. In addition, liquid C sources showed better effects compared to dry solid C sources in terms of weed suppression.
Strawberries are also very susceptible to soilborne plant pathogens (e.g., Phytophthora, Cylindrocarpon, Colletotrichum, Macrophomina, and Rhizoctonia). Traditionally growers depended on soil fumigation with mixtures of MeBr and chloropicrin to control soilborne pathogens along with weed propagules. Although ASD has been evaluated for strawberry in some geographic sites in the U.S (Shennan et al., Citation2018, Mazzola et al., Citation2018), there are no recommendations (C source types and rates, application protocol, and treatment duration) for strawberry growers in Virginia and the mid-Atlantic region. The objective of this study was to evaluate weed control efficacy of local carbon sources used in ASD treatments, and consisted of three trials, each with separate objectives. The objective of trial 1 was to evaluate the effect of several local C sources on weed control in ASD. The objective of trial 2 was to evaluate the effect of local C sources mixed with ethanol in ASD. The objective of trial 3 was to evaluate if C sources mixed with distiller`s yeast could stimulate bioethanol fermentation during the ASD process, and whether this C source and distiller’s yeast combination could enhance ASD weed control efficacy. Local C sources tested included brewer’s spent grain, paper mulch, waste ground coffee, and cover crops.
Materials and Methods
Trial Setup
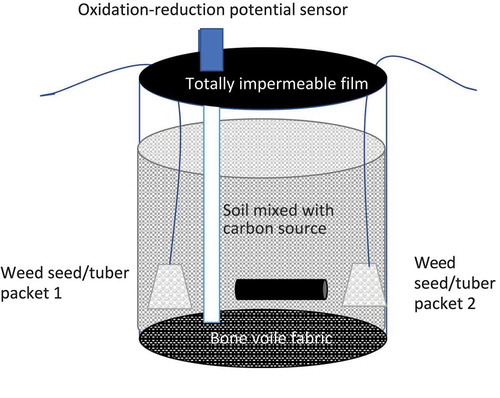
Greenhouse ASD pot trials were initiated at the Southern Piedmont Agricultural Research and Extension Center (AREC), Blackstone, VA beginning in May 2017. In the greenhouse, custom-made “bioreactors” (182 cm2 soil surface area) were constructed of PVC tubing 20 cm tall with a 15 cm diameter, and a piece of bone voile fabric mesh was attached to the bottom of each tube (). The bioreactor was filled with 6.8 kg of field top soil (sandy loam, pH = 6.5) from the Southern Piedmont AREC that was premixed in a tub with treatment appropriate C sources, and 2-liter tap water added to bring soil to 20% moisture content, which was the field capacity for sandy loam soil (Blok et al., Citation2000). The ORP sensors and temperature sensors were buried near the bottom of the bioreactor at 15 cm depth, and bags were placed close to the sensors (details provided in text below). The black 1.25 mil virtually impermeable plastic film (VIF, Raven Industries Engineered Films Division, Sioux Falls, SD, USA) was secured on top of bioreactor with duct tape for all treatments except the non-treated control.
All the carbon source values were calculated based on the recommendations of Butler et al. (Citation2012), with a C rate was 4 mg C/g soil, or 16 t/ha in these trials. These calculations were based on 15 cm soil depth and soil density of 1.08 g/cm3 Carbon sources were chosen considering the availability and costs in our region during the time period of strawberry bed preparation and dry rice bran was included as a positive control. The C sources used in these trials and the amount used (gram/container) were: dry rice bran (64 g), sorghum-sundangrass (Sorghum × drummondii) residue (67 g), cowpea residue (74 g), buckwheat residue (80 g), velvet bean residue (Mucuna pruriens, 56 g), pelleted paper mulch (32 g; Lebanon Seaboard Corporation), brewer`s spent grain (BSG, 64 g; Commonwealth Brewering Company, Virginia Beach, VA, USA), waste coffee grounds (112 g; local Starbucks), and peanut shell (63 g; Wakefield Peanut Co LP, Wakefield, VA, USA). The cover crops were planted in the field on April 2017. Eight weeks following cover crop planting, above-ground cover crop tissue was harvested on June 2017, dried (35°C) and chopped to use as carbon sources for ASD trials. In trial 3, to simulate ethanol fermentation in soil, distiller`s yeast (Distiller’s Active Dry Yeast, Red Star Yeast Co., Milwaukee, WI, USA) was mixed with C sources in appropriate treatments.
The trial 1 had four replicates repeated twice, but in trials 2 and 3, due to limited number of sensors and larger set of treatments, trials had two replications of each treatment in each run and these trials were repeated twice. All of the containers were arranged in trays in a completely random design. The treatments of each trial were as follows:
Trial 1: rice bran (64 g) as positive control, sorghum-sundangrass (67 g), cowpea (74 g), buckwheat (80 g), velvet bean (56 g), paper mulch (32 g), and a non-treated control. This trial was repeated twice from 7 Sep. 2017 to 27 Sep. 2017 and from 13 Oct. 2017 to 2 Nov. 2017.
Trial 2: BSG (64 g), BSG (32 g) + ethanol, paper mulch (32 g), paper mulch (16 g) + ethanol, rice bran (64 g) and rice bran (32 g) + ethanol. A 70% ethanol solution was applied at 50 ml per container, which contained 2 mg C/g soil. To keep the anaerobic condition in bioreactors, the ethanol was sprayed evenly across the soil surface using syringe through six spots on the top and was applied 1 week after ASD was initiated. This trial was repeated, and the trial runs were from 16 Nov 2017 to 7 Dec. 2017 and from 25 Jan. 2018 to 15 Feb. 2018.
Trial 3: Waste coffee grounds (112 g) ± yeast, paper mulch (32 g) ±yeast, BSG (64 g) ± yeast, BSG (32 g) ± yeast, rice bran (64 g) ± yeast, peanut shell (63 g) ± yeast, and non-treated control. Treatments with yeast had 0.06 g of yeast/bioreactor. This trial was repeated twice, once from 1 Mar. 2018 to 22 Mar. 2018 and the second time from 29 Mar. 2018 to 19 Apr. 2018. Results from trial 1 and 2 were considered when making C source choices for trial 3.
Bag Preparation and Sensor Installation
In trials 1 and 2, 100 common chickweed (S. media), 100 redroot pigweed (A. retroflexus), and 10 yellow nutsedge (C. esculentus) were used. All weeds were put in one bag, and the bag was buried in the soil at 2.5 cm above the container bottom. For trial 3, two bags were used. One bag contained 10 yellow nutsedge tubers and 100 common chickweed seeds, and another had 100 white clover (T. repens) seeds and 100 redroot pigweed seeds. Common chickweed, redroot pigweed and white clover were procured from Herbiseed, Twyford, England. Yellow nutsedge tubers were harvested from a local farm in Virginia Beach, VA, USA.
Redox potential (Eh) sensors (ORP2000 Extended Life ORP Sensor, Sensorex, Garden Grove, CA, USA) were installed at a 15 cm depth to evaluate soil anaerobic conditions during the ASD process, and the sensors were connected to an automatic data logging system (CR-1000, Campbell Scientific, Logan, UT, USA). Soil temperature sensors (U12 Deep Ocean Temperature Data Logger, Onset, Bourne, MA, USA) were installed at a 15 cm depth in the containers. Due to the limited sensors available, redox potential was monitored only in half of the replicates per treatment in all trials and soil temperature was measured in three of the four replicates per treatment in trial 1 and two replicates in trials 2 and 3, with the sensor recording readings every 10 minutes.
ASD Treatment Initiation and Post Assessments
At the time of treatment initiation, the top surface of the containers (except the non-treated control) was covered by a piece of black VIF and sealed with duct tape. All of the containers were arranged in trays in a completely randomized design. The trays were filled with tap water and the water level was kept above the bottom of the bioreactors to maintain soil moisture content. Water levels were maintained for the duration of each trial. The non-treated controls were not saturated. Each ASD trial ran 25 days. After ASD, survival of weed seed was determined by a tetrazolium chloride (TZ) assay (Peters, Citation2000), and survival rate of yellow nutsedge tubers was determined by sprouting the tubers in a growth chamber (23°C, 16 h day length).
Statistical Analyses
The data were analyzed by JMP v. 14 (SAS Institute Inc., Cary, NC, USA). The temperature data were averaged for the time of treatment duration, and cumulative redox potential was calculated basing on the hourly average redox potential, and the absolute value of the difference between each hourly average redox potential and calculated critical redox potential (CEh; redox potential value below which is considered anaerobic) was summed up over the whole three-week ASD period. The critical redox potential was calculated by the formula CEh = 595 mV – 60 mV * soil pH (Rabenhorst and Castenson, Citation2005; United States Department of Agriculture-Natural Resources Conservation Service USDA-NRCS, Citation2018). Weed species data were subject to Johnson Su transformation to satisfy assumptions of normality. Thus, the original least-square means are presented in the table, but the separation letters are based on transformed mean values. In trial 1 the C source by run interaction was not significant and data were pooled over runs. Weed seed/tuber viability was analyzed by one-way analysis of variance (ANOVA), comparing the means of the treatments by Fisher’s least significant difference (LSD) at alpha = 0.05. Factor analysis was conducted for trials 2 and 3. Due to lack of a non-treated control with ethanol or yeast, the non-treated control was not involved in factorial analysis. In trial 2, factor analysis among C source types, ethanol and runs were conducted. The three-way or two-way interactions showed that the run had no significant effect (p > .5), and only the main effect of ethanol and C source type was significant. Hence, data from the two runs were pooled (including non-treated control data) to run a one-way ANOVA, and to run LSD at alpha = 0.05 to compare means of the treatments. In trial 3, similar to trial 2, factor analysis was conducted only for all treatments that had C sources among C source type, yeast, and runs. The two-way or three-way interactions showed the effect of runs was not significant. Hence, data from two runs, including non-treated control was pooled and a one-way ANOVA was conducted and mean separation was done using LSD test at alpha = 0.05.
Results
Weed Germination
In trial 1, all ASD treatments suppressed germination of common chickweed, redroot pigweed, and sprouting of yellow nutsedge compared to the non-treated control by at least 50% (). There were no significant differences among C sources, which indicated that for further trials, the consideration of C sources could focus primarily on local availability and cost.
Table 1. Weed germination rates and cumulative soil anaerobic conditions after anaerobic soil disinfestation (ASD) process with several different carbon sources.
In trial 2, interaction analysis, among C source types, ethanol, and runs was conducted (data not presented). For redroot pigweed, only the effect of ethanol was significant on germination (p < .05) when the three-way ANOVA was run, and there was no effect of different trial runs. However, for yellow nutsedge when the three-way ANOVA was run, only the main effect of C source affected weed seed viability (p = .02). For common chickweed, neither C source nor ethanol affected germination rates. Anaerobic soil disinfestation treatments reduced weed germination compared to the non-treated control. Reduced rates of BSG + ethanol application decreased the germination rate of redroot pigweed compared to BSG application at full rate (). Yellow nutsedge emergence was not any different in BSG at full rate and BSG at half rate with ethanol but emergence varied for rice bran and rice bran at full rate was more effective at providing yellow nutsedge control. Overall, these results suggest that a mix of liquid carbon source, i.e., ethanol and solid carbon source can be as effective in weed suppression as solid carbon source at full dose rate.
Table 2. Weed germination rates and cumulative soil anaerobic conditions after anaerobic soil disinfestation (ASD) process with several different carbon sources and ethanol application.
For trial 3, the influence of yeast on weed suppression varied with C sources (). Interaction analysis, among C source, yeast, and runs was conducted for all four weed species. There was no effect of different runs (p > .6), but the main effects of C source and yeast application were significant (p < .01). Moreover, for yellow nutsedge, yeast x C source had a significant effect on the sprouting rate (p < .0001). These results support the hypothesis that yeast application could enhance weed control when certain C sources are used for ASD. Yeast application significantly suppressed the emergence of white clover, common chickweed, and sprouting of yellow nutsedge when the brewer`s spent grain was the C source. For waste coffee grounds, yeast application suppressed the emergence of all weed species except white clover. The addition of yeast improved the effect of rice bran on weed suppression for common chickweed, redroot pigweed, and white clover. All C sources reduced yellow nutsedge emergence compared to the nontreated control.
Table 3. Weed germination rates and cumulative soil anaerobic conditions after anaerobic soil disinfestation (ASD) process with several different carbon sources and yeast amendment.
Cumulative Soil Anaerobic Conditions
There were significant effects of C source on cumulative soil anaerobic conditions for all three trials (, , and ). Overall, the non-treated control had the least anaerobic conditions. Cumulative anaerobic conditions in trial 1 were similar for all C sources, highest for BSG in trial 2 and, in trial 3, cumulative soil anaerobic conditions were the lowest in the non-treated control and not significantly different from paper mulch + yeast and peanut shell treatments.
Temperature
Overall, there was no significant difference in average temperature within the bioreactors/achieved among all treatments during the ASD period. Soil temperatures at a 15 cm depth generally ranged from 9°C to 33°C in trial 1, 9°C to 31°C in trial 2, and 15°C to 39°C in trail 3. There were no significant differences in average temperature among treatments in trial 1. In trial 2, paper mulch with ethanol had the highest average temperature. In trial 3, there were no significant differences in average temperatures among treatments.
Discussion
In these trials, all tested C sources had a significant effect on suppression of chickweed, redroot pigweed, yellow nutsedge, and white clover compared to the non-treated control, and some combinations, such as brewer`s spent grain with yeast inoculation, had comparable effects as rice bran.
Trial 2 was conducted to test the hypothesis that ethanol supplementation to solid carbon sources could improve the weed suppression of ASD, and the addition of ethanol to reduced dosage of solid C sources provided similar weed efficacy as full dose rate of solid C. Ethanol application is not an economically practical application. The current price of ethanol in US is around 0.4 USD/l (United States Grain Council, Citation2019), which indicates the cost of ethanol application for ASD is ~1.15 USD/m2, or 11,500 USD/ha making ethanol unaffordable and impractical for growers. However, there is economic potential to develop the bioethanol fermentation in open-field conditions as an alternative to ethanol application. Trial 3 evaluated the potential of stimulation bioethanol fermentation during the ASD process, using a simple bioethanol production method, which is the distiller`s yeast that are used in craft-brewery to produce alcohol. Although the distiller’s strain of yeast may not suitable for all C sources used in ASD, it has been reported that BSG can generate ethanol following inoculation with yeast (Liguori et al., Citation2015). In trial 3, yeast application only improved weed suppression for certain C sources and weed species and additional research is warranted. Compared to the fumigant cost of 2700 USD/ha (Sydorovych et al., Citation2006), the material cost of BSG is relatively low, which is free () for the amendment and the cost of yeast is approximately 145 USD/ha. However, the cost of labor associated with C sources application could be higher than using fumigant. Additional studies on economical analysis of alternative treatments are warranted.
Table 4. Carbon rate, carbon to nitrogen ratio, and approximate cost of evaluated carbon sources.
The suppression of all four weed species was achieved in the present study, while previous studies indicate that ASD had an effect on suppression of several weed species except redroot pigweed (Amaranthus retroflexus) (Shrestha et al., Citation2016). However, there is no prior study done that uses distiller`s dry yeast in the ASD process for pest management.
Although the meta-analysis study by Shrestha et al. (Citation2016) showed that the effect of ASD on weed suppression required high temperatures (>35°C), Shrestha et al. (Citation2018) showed that under moderate temperatures (mean 25°C) ASD treatment did significantly suppress yellow nutsedge. Moreover, there are several possible reasons why weed control was achieved in our study at low temperatures. Many studies with a higher mean temperature recorded temperature data at 10 cm depth while in this study the temperature was recorded at greater depth of 15 cm. This study maintained the anaerobic conditions by keeping a relatively high water level at around 7–10 cm, outside the container which meant that temperatures may have been moderated by the immediate environment outside the container. The high soil moisture in the soil may prevent the temperature from increasing. Meanwhile, the much higher anaerobicity may promote the weed suppression under low temperature. For example, in a 3-week ASD study on suppression of yellow nutsedge (Butler et al., Citation2012), using molasses as C source, yellow nutsedge was significantly reduced under mean temperature of 25°C. However, cumulative soil anaerobicity in that study was relatively lower as compared to this study (116935 mVh vs 135000 mVh, the mean anaerobicity for all C sources in trial 3). Moreover, compared to some studies that achieved high temperature (>35°C) (Blok et al., Citation2000; Katase et al., Citation2009; Mowlick et al., Citation2013), this study showed relatively higher anaerobicity. In addition, the non-treated control groups were not irrigated to saturation. Thus, there is a need to study the interaction among soil moisture, soil temperature, and weed suppression in ASD, especially using C mixed with yeast for ASD as well as a need to standardize the protocol at which temperature and anaerobicity data are recorded during ASD treatment. The results of this study suggest several locally carbon sources could be used in ASD to control weeds as a pre-plant treatment and indicate the potential to mix certain C sources with brewery yeasts. In order to optimize the ASD method for local strawberry production, the cost of ASD needs to be reduced, and the price of the C source is a major factor in overall cost. Thus, practices such as yeast inoculation or supplementation with reduced rates of liquid C sources should be considered to reduce overall costs, if efficacy can be maintained under field conditions.
Acknowledgments
The authors wish to thank Dr. Sebastian Albu, Ned Jones, Jillian Rajevich, Zachary Landis, Robert (Spencer) Irby and Ethan Murdock for their assistance with this research.
Additional information
Funding
References
- Blok, W.J., J.G. Lamers, A.J. Termorshuizen, and G.J. Bollen. 2000. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology 90(3):253–259. doi: 10.1094/PHYTO.2000.90.3.253.
- Butler, D.M., E.N. Rosskopf, N. Kokalis-Burelle, J.P. Albano, J. Muramoto, and C. Shennan. 2012. Exploring warm-season cover crops as carbon sources for anaerobic soil disinfestation (ASD). Plant Soil 355(1–2):149–165. doi: 10.1007/s11104-011-1088-0.
- Christman, J., and J. Samtani. 2019. A survey of strawberry production practices in Virginia, p. SPES–150P. Blacksburg, VA, USA: Virginia Cooperative Extension, Pub.
- Fennimore, S.A., R. Serohijos, J.B. Samtani, H.A. Ajwa, K.V. Subbarao, F.N. Martin, O. Daugovish, D. Legard, G.T. Browne, J. Muramoto, et al. 2013. TIF film, substrates and nonfumigant soil disinfestation maintain fruit yields. California Agriculture. 67(3):139–146. doi:10.3733/ca.v067n03p139.
- Gao, S., B.D. Hanson, D. Wang, G.T. Browne, R.J. Qin, H. Ajwa, and S.R. Yates. 2011. Methods evaluated to minimize emissions from preplant soil fumigation. California Agric. 65(1):41–46. doi: 10.3733/ca.v065n01p41.
- Honda, H., A. Ohnishi, N. Fujimoto, and M. Suzuki. 2008. Development of a solid-state fermentation system for producing bioethanol from food waste (in Japanese). Environ. Conserv. Eng. 37(3):207–215. doi: 10.5956/jriet.37.207.
- Horita, M., and H.K. Kitamoto. 2015. Biological soil disinfestation using bioethanol fermentation products: Role of residual organic substances J. Gen. Plant Pathol. 81(4):304. doi: 10.1007/s10327-015-0595-x.
- Huang, X., T. Wen, J. Zhang, L. Meng, T. Zhu, and Z. Cai. 2015. Toxic organic acids produced in biological soil disinfestation mainly caused the suppression of Fusarium oxysporum f. sp. cubense. BioControl 60(1):113–124. doi: 10.1007/s10526-014-9623-6.
- Katase, M., C. Kubo, S. Ushio, E. Ootsuka, T. Takeuchi, and T. Mizukubo. 2009. Nematicidal activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Japanese J. Nematol. 39(2):53–62. doi: 10.3725/jjn.39.53.
- Kitamoto, H.K., M. Horita, Y. Cai, Y. Shinozaki, and K. Sakaki. 2011. Silage produces biofuel for local consumption. Biotechnol. Biofuels. 4:46. doi: 10.1186/1754-6834-4-46
- Liguori, R., C.R. Soccol, L. Porto de Souza Vandenberghe, A.L. Woiciechowski, and V. Faraco. 2015. Second generation ethanol production from Brewers’ spent grain. Energies 8(4):2575–2586. doi: 10.3390/en8042575.
- Mazzola, M., J. Muramoto, and C. Shennan. 2018. Anaerobic disinfestation induced changes to the soil microbiome, disease incidence and strawberry fruit yields in California field trials. Appl. Soil Ecol. 127:74–86. doi: 10.1016/j.apsoil.2018.03.009.
- Melanson, R., F. J. Louws, C. Johnson, G. Schnabel, R. Schmidt-Jeffris, H. J. Burrack, D. Pfeiffer, A. Sial, K. Jennings, and P. Brannen. 2019. Southeast regional strawberry integrated pest management guide for plasticulture production. The Southern Region Small Fruit Consortium.
- Messiha, N.S., A. Diepeningen, M. Wenneker, A. Beuningen, J. Janse, T.C. Coenen, A. Termorshuizen, A.C. Bruggen, and W. Blok. 2007. Biological Soil Disinfestation (BSD), a new control method for potato brown rot, caused by Ralstonia solanacearum race 3biovar 2. Eur. J. Plant Pathol. 117:403–415. doi: 10.1007/s10658-007-9109-9.
- Momma, N. 2008. Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Jpn. Agr. Res. Q. 42(1):7–12. doi: 10.6090/jarq.42.7.
- Momma, N., M. Momma, and Y. Kubora. 2010. Biological soil disinfestation using ethanol: effect on Fusarium oxysporum f. sp. lycopersici and soil microorganisms. J. Gen. Plant Pathol. doi: 10.1007/s10327-010-0252-3.
- Mowlick, S., H. Yasukawa, T. Inoue, T. Takehara, N. Kaku, K. Ueki, and A. Ueki. 2013. Suppression of spinach wilt disease by biological soil disinfestation incorporated with Brassica juncea plants in association with changes in soil bacterial communities. Crop Protection. 54:185–193. doi: 10.1016/j.cropro.2013.08.012.
- Nalluri, N., and V.R. Karri. 2018. Use of groundnut shell compost as a natural fertilizer for the cultivation of vegetable plants. Inter. J. Adv. Res. Sci. Eng. 7: 97-104.
- Noling, J.W., and J.O. Becker. 1994. The challenge of research and extension to define and implement alternatives to methyl bromide. J. Nematol. 26(4 Suppl):573–586.
- Peters, J. 2000. Tetrazolium testing handbook. Contribution No. 29 to the handbook on seed testing. Assn. of Official Seed Analysts, Ithaca, NY.
- Rosskopf, E.N., D.O. Chellemi, N. Kokalis-Burelle, and G.T. Church. 2005. Alternatives to methyl bromide: A Florida perspective. Plant Health Prog. 6(1):19. doi: 10.1094/PHP-2005-1027-01-RV.
- Rabenhorst, M.C., and K.L. Castenson. 2005. Temperature effects on iron reduction in ahydric soil. Soil Sci. 170(9):734–742.
- Serrano-Pérez, P., E. Rosskopf, A.D. Santiago, and M.C. Rodríguez-Molina. 2017. Anaerobic soil disinfestation reduces survival and infectivity of Phytophthora nicotianae chlamydospores in pepper. Scientia Hort. 215:38–48. doi: 10.1016/j.scienta.2016.12.003.
- Shennan, C., J. Muramoto, S.T. Koike, and O. Daugovish. 2009. Optimizing anaerobic soil disinfestation for non-fumigated strawberry production in California. In: Ann.Inter. Res. Conf. on Methyl Bormide Alternatives and Emissions Reductions. Methyl Bromide Alternatives Outreach, San Diego, CA.
- Shennan, C.J., J. Muramoto, M.S. Koike, G. Baird, S. Fennimore, J. Samtani, M. Bolda, S. Dara, O. Daugovish, G. Lazarovits, et al. 2018. Anaerobic soil disinfestation is a potential alternative to soil fumigation for control of some soil borne pathogens in strawberry production. Plant Pathol. 67:51–66. doi: 10.1111/ppa.12721.
- Shinmura, A. 2000. Causal agent and control of root rot of welsh onion. PSJ Soilborne Disease Workshop Report 20:133–143.
- Shrestha, U., R.M. Augé, and D.M. Butler. 2016. A meta-analysis of the impact of anaerobic soil disinfestation on pest suppression and yield of horticultural crops. Front. Plant Sci. 7:1254. doi: 10.3389/fpls.2016.01254.
- Shrestha, U., E.N. Rosskopf, D.M. Butler, and G. Fried. 2018. Effect of anaerobic soil disinfestation amendment type and C:N ratio on Cyperus esculentus tuber sprouting, growth and reproduction. Weed Res. 58:379–388. doi: 10.1111/wre.12318.
- Sydorovych, O., C.D. Safley, L.M. Ferguson, E.B. Poling, G.E. Fernandez, P.M. Brannen, D.M. Monks, and F.J. Louws. 2006. Economic evaluation of methyl bromide alternatives for the production of strawberries in the Southeastern United States. HortTechnol. 16(1):118–128. doi: 10.21273/HORTTECH.16.1.0118.
- Uematsu, S., C. Tanaka-Miwa, R. Sato, Y. Kobara, and M. Sato. 2007. Ethyl alcohol as a promising material of reductive soil disinfestation for controlling root knot nematode and soilborne plant diseases. In: G.L. Obenauf (ed) Proc. 2007 Ann. Inter. Res. Conf. Methyl Bromide Alternatives and Emissions Reductions, pp 75.1–75.3. San Diego, CA, USA.
- United States Department of Agriculture, Natural Resources Conservation Service. 2018. Field Indicators of Hydric Soils in the United States, Version 8.2. In: L.M. Vasilas, G.W. Hurt, and J.F. Berkowitz (eds.). USDA, NRCS, incooperation with the National Technical Committee for Hydric Soils.
- United States Grain Council. 2019. Ethanol market and price report-July 9.
- Washington, DC. https://grains.org/ethanol_report/ethanol-market-and-pricing-report-july-9-2019/ac