ABSTRACT
The aim of this research was to determine the changes in the organic acids and phenolic compounds in fruits of red currant due to maturity. It was harvested two cultivars (Red Lake and Rovada cv.) during four different maturity stages (green color, veraison, pink color, and red color) in red currant orchard in Bolu province. In red currant fruits 12 phenolic compounds (gallic acid, protocatechuic acid, catechin, chlorogenic acid, vanillic acid, syringic acid, p-coumaric acid, ferulic acid, o-coumaric, rutin, phloridzin, and quercetin), 6 organic acids (malic, succinic, citric, fumaric, tartaric, and oxalic acid), and vitamin C were determined depending on maturity. All ripening times were found to be rich in catechin and rutin. It was determined that the contents of vanillic acid, ferulic acid, routine, phloridzin, and quercetin increased with maturation. In the study, the highest organic acids contents were determined at green stages. The content of vitamin C varied depending on the ripening. The results suggest that red currant fruits were richest in organic acids and phenolic compounds in red maturity period.
Introduction
Red currant (Ribes rubrum), which is found in the natural flora of Turkey, is a species of fruit that has been in demand in recent years due to its particularly beneficial for health. In general, fruits of red currant are processed into jam, syrup, and fruit juice as well as fresh consumption in Turkey. This fruit type is consumed more in European countries than in other countries. Red currant fruits are rich in organic acids, phenolic acids, and flavonoids (Okatan et al., Citation2017). It is known to have antidiabetic, antiviral, anti-inflammatory, and anti-cancer effects due to its rich biochemicals (Berk and Tuna, Citation2017).
Organic acids, vitamins, and phenolics are important biochemical compounds with antioxidant properties. Therefore, they play an important role in human health and life. The fruits contain a lot of different phenolics and organic acids which their levels vary depending on the cultivars (Dragovic-Uzelac et al., Citation2007; Eyduran et al., Citation2015; Gündoğdu, Citation2019; Ozturka et al., Citation2019). Ersoy et al. (Citation2018) reported that fruits of red currants are rich in catechin, rutin, gallic acid from phenolic compounds, and citric acid from organic acids. In order to determine the best harvest time in fruits, periods in which the levels of phenolic compounds and organic acids that are important for human health are most important. With ripening, there are significant changes in the physical and biochemical structures of the fruits (Elmastaş et al., Citation2017; Güneş et al., Citation2016). For this reason, the amount of phenolic compounds are abundant in fruits varies (Çoklar and Akbulut, Citation2016; İsbilir et al., Citation2012). Pedisic et al. (Citation2007) in cherry fruit, and Liu et al. (Citation2019) in peach fruits stated that phenolic compounds decreased with ripening, while Usenik et al. (Citation2008) in their study of plum fruit stated that phenolic compounds increased. Also, Mikulic-Petkovsek et al. (Citation2015) reported that organic acid contents which are important criteria in terms of fruit taste are reduced by ripening.
During the maturity of red currant fruits, there are many changes in the chemical composition of the fruit and these changes affect fruit taste importantly. Studies on the change of phenolic compounds in red currant are mostly performed in ripe fruits and include group compounds but do not include individual phenolic compounds. Therefore, in this study, changes in the amount of phenolic compounds, organic acids, and vitamin C were investigated during the maturity of red currant fruits.
Material and Methods
Plant Material
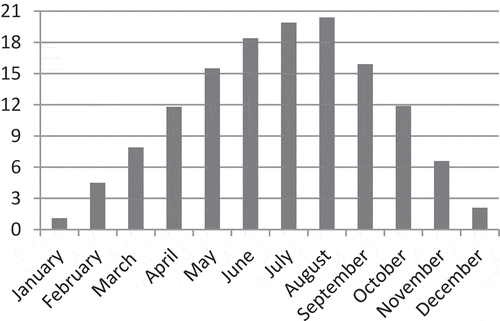
This research was conducted in 2017–2018 in red currant orchard at Mudurnu Süreyya Astarcı Vocational School located in Mudurnu district of Bolu province. The trial included two red currant cultivars: Red Lake and Rovada. Cultivars were planted at 4 × 4 m row spacing and intrarow spacing in single-crop orchards. Drip irrigation with 12-day intervals was employed for irrigated farming conditions. Experiments were conducted in randomized blocks design with four replications with five plants in each replication. Mudurnu is a region with a transitional climate between the marine and the continental climate. In 2018, the highest rainfall recorded in June, March, and May, respectively (). The average temperature increased in May–June, which is the fruit development period. The temperature increased from 15.5°C in May to 18.4°C, 19.9°C, and the hottest average of the year in August to 20.4°C ().
Figure 1. The distribution of the total monthly rainfall during the year in Mudurnu district (means of 2017 and 2018 years)
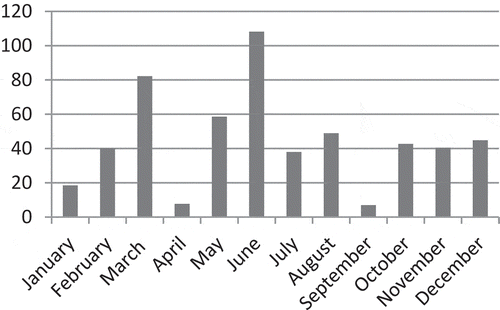
Figure 2. The average monthly temperature distribution during the year in Mudurnu district (means of 2017 and 2018 years)
Red currant fruits were hand-harvested in four maturity stages for cultivars; 1: fruits were green color, 2: fruits were veraison, 3: fruits were pink color, 4: fruits were red color, and 5: ripe. Then, the fruits were packed in plastic box, and kept at −20°C until extraction. In red currant fruits were determined 12 phenolic compounds (gallic acid, protocatechuic acid, catechin, chlorogenic acid, vanillic acid, syringic acid, p-coumaric acid, ferulic acid, o-coumaric, rutin, phloridzin, and quercetin), 6 organic acids (malic, succinic, citric, fumaric, tartaric, and oxalic acid), and vitamin C.
Analysis of Phenolic Compounds
The method of Rodriguez-Delgado et al. (Citation2001) was developed and used for the separation of phenolic compounds with HPLC (High-Performancee Liquid Chromotography) in red currant. The fruits collected were distilled with distilled water at the ratio of 1:1. They were centrifuged at 15000 rpm for 15 min., the supernatant was filtered with 0.45 µm millipore filters and then injected to HPLC. The chromatographic separation was conducted by using a DAD detector (Agilent. USA) and 250*4.6 mm, 4 µm ODS colon (HiChrom, USA) in Agilent 1100 (Agilent) HPLC system. Solvent A Methanol-acidic acid-water (10:2:88) and Solvent B Methanol-acidic acid-water (90:2:8) were used as the mobile phase. The separation was conducted at 254 and 280 nm and the flow rate was determined as 1 mL/min., and the injection volume was determined as 20 µL.
Analysis of Organic Acids
The organic acid composition of the fruits was determined according to Bevilacqua and Califano (Citation1989). 20 g of each sample was mixed with 80 ml of 0.009 N H2SO4 (Heidolph Silent Crusher M, Germany), then homogenized with a shaker for 1 h (Heidolph Unimax 1010, Germany). The mixture was centrifuged at 15000 rpm for 15 min and the supernatants were filtered twice through a 0.45 um membrane filter followed by filtration through a coarse filter (Millipore Millex-HV Hydrophilic PVDF, Millipore, USA) and passed through a SEP-PAK C18 cartridge. Organic acid readings were performed by HPLC using the Aminex column (HPX-87 H, 300 mm x 7.8 mm, Bio-Rad Laboratories, Richmond, CA, USA) at 214 and 280 nm wavelengths in the Agilent package program (Agilent, USA).
Analysis of Vitamin C
Vitamin C content was detected based on a modified HPLC procedure suggested by Cemeroglu (Citation2007). 5 ml of the fruit extracts were supplemented with 2.5% (w/v) metaphosphoric acid (Sigma, M6285, 33.5%), then centrifuged at 6500 rpm for 10 min at 4°C. 0.5 ml of the mixture was brought to a final volume of 10 ml with 2.5% (w/v) metaphosphoric acid. Supernatants were filtered with a 0.45 µm PTFE syringe filter (Phenomenex, UK). C18 column (Phenomenex Luna C18, 250° 4.60 mm, 5 µ) was used for the identification of ascorbic acid at 25°C. Ultra distilled water with 1 ml/min flow rate and pH of 2.2 (acidified with H2SO4) was used as a mobile phase. Spectral measurements were made at 254 nm wavelength by using a DAD detector. Different standards of L-ascorbic acid (Sigma A5960) (50, 100, 500, 1000, and 2000 ppm) were used for the quantification of ascorbic acid readings.
Statistical Analysis
In the determination of significant differences, Duncan’s multiple range test was used. All the statistical evaluations were performed through IBM SPSS 23 package program. The principal component analysis (PCA) was used to determine the correlation between ripening times. XLSTAT 2016 (Addinsoft, New York, USA) was used to perform statistical analyses.
Results and Discussion
In this research, 12 phenolic compounds, 6 organic acid, and vitamin C were determined in red currant at different maturity stages and shown in . The differences between maturity stages were statistically significant (P < .05). Phenolic compounds content was increased, while acidity decreased in the ripening of red currant fruits. In the green and veraison stages, it is determined that both Red Lake and Rovada cultivars of fruits contain higher catechin, rutin, and chlorogenic acid, respectively (). Also, pink and red stages were found to be rich in catechin, rutin, and vanillic acid, respectively ( and ). The highest gallic acid, protocatechuic acid, chlorogenic acid, syringic acid, p-coumaric acid, and o-coumaric acid values were recorded in the green period and decreased with ripening. On the other hand, the highest catechin, vanillic acid, ferulic acid, rutin, phloridzin, and quercetin amount were recorded in the red maturation in both cultivars and these phenolic compounds increased with maturation ( and and ).
Table 1. Gallic acid, protocatechuic acid, catechin, chlorogenic acid, vanillic acid, and syringic acid levels of red currant fruits (Red Lake) harvested at four maturity stages (mg 100 g−1)
Table 2. Gallic acid, protocatechuic acid, catechin, chlorogenic acid, vanillic acid, and syringic acid levels of red currant fruits (Red Lake) harvested at four maturity stages (mg 100 g−1)
Table 3. P-Coumaric acid, ferulic acid, o-Coumaric, rutin, phloridzin, and quercetin levels of red currant fruits (Red Lake) harvested at four maturity stages (mg 100 g−1)
Table 4. P-Coumaric acid, ferulic acid, o-Coumaric, rutin, phloridzin, and quercetin levels of red currant fruits (Red Lake) harvested at four maturity stages (mg 100 g−1)
Table 5. Oxalic, citric, tartaric, malic, succinic, fumaric acid (g kg−1), and ascorbic acid (mg 100 g−1) levels of red currant fruits (Red Lake) harvested at four maturity stages
Table 6. Oxalic, citric, tartaric, malic, succinic, fumaric acid (g kg−1), and ascorbic acid (mg 100 g−1) levels of red currant fruits (Red Lake) harvested at four maturity stages
Figure 3. Correlation between different ripening periods and phenolic compounds distributions of Redlake fruits
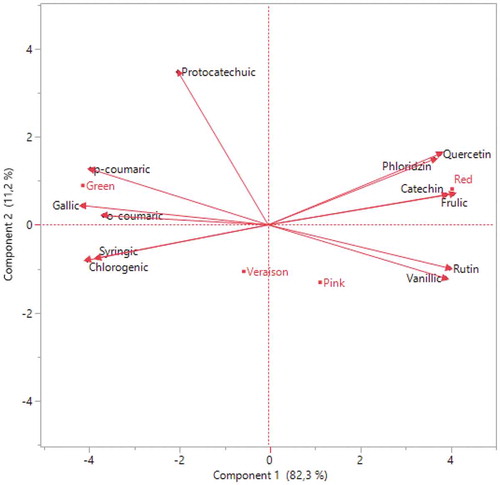
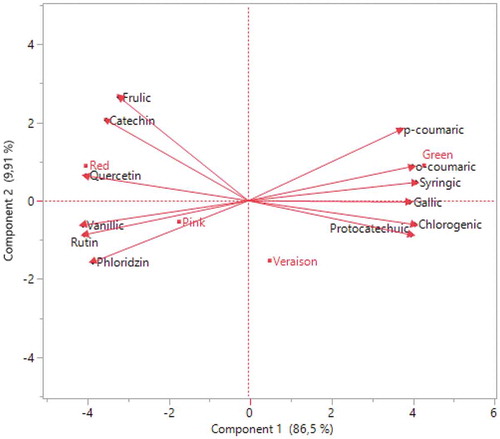
Figure 4. Correlation between different ripening periods and phenolic compounds distributions of Rovada fruits
In the present study, gallic acid content decreased with the maturation of fruits, from 1.04 mg 100 g−1 to 0.80 mg 100 g−1 in Red Lake and from 2.24 mg 100 g−1 to 1.27 mg 100 g−1 in Rovada ( and ). Protocatechuic acid values were measured at least in the veraison (0.34 mg 100 g−1 in Red Lake) and red (0.28 mg 100 g−1 in Rovada) periods. The highest catechin value was measured in Red Lake cultivar as 13.27 mg 100 g−1 in the red period (). Chlorogenic acid increased in Rovada cultivar from 2.94 mg 100 g−1 in the red period to 4.56 mg 100 g−1 in the green period. Vanillic acid amount was recorded at green maturation of 2.30 mg 100 g−1 in Red Lake and of 2.47 mg 100 g−1 in Rovada. It was observed that this content increased to 2.98 mg 100 g−1 (Red Lake) and 3.64 mg 100 g−1 (Rovada) with maturation ( and ). In Rovada cultivar, syringic acid decreased twice (0.87–0.45 mg 100 g−1) in the red period compared to the green period. This phenolic acid was recorded as the highest 0.68 mg 100 g−1 at green maturation in Red Lake cultivar (, ).
As shown in and , the highest p-coumaric (0.79 mg 100 g−1) and o-coumaric acid (0.07 mg 100 g−1) were determined in green maturation (Red Lake cultivar). However, the lowest ferulic acid was measured as 0.70 mg 100 g−1 (Red Lake) in green and 0.92 mg 100 g−1 (Rovada) in this stage. Rutin value increased from 5.21 mg 100 g−1 (green) to 9.93 mg 100 g−1 (red) in Red Lake and from 4.71 mg 100 g−1 (green) to 7.48 mg 100 g−1 (red) in Rovada cultivar. The content of phloridzin was 0.555 mg 100 g−1 in pink maturation (Red Lake) and 0.570 mg 100 g−1 in veraison and pink maturation (Rovada). In this study, the amount of quercetin increased approximately treble from the green maturation to the red maturation in both cultivars ( and ). Andreotti et al. (Citation2008) and Voća et al. (Citation2014) recorded that the phenolic compound of fruit decreased depending on ripening. Schulz et al. (Citation2015) concluded that rutin, p-coumaric acid, and quercetin value of juçara (Euterpe edulis Martius) fruit’s increased with maturation. Our results present that p-coumaric acid decreased with ripening in red currant. In another study, the researchers found that the highest phenolic compound was catechin (42.43 mg 100 g−1) in the red maturation period of red currant (Adina et al., Citation2017). Okatan et al. (Citation2017) conducted a study in Van and obtained the highest phenolic compound in the fruits of ripe-red currant as rutin. Authors measured rutin content of 18.52 µg g−1 in Red Lake and 7.23 µg g−1 in Rovada cultivar and protocatechuic acid of 2.84 µg g−1 in both cultivars.
Generally, that phenolic acids except vanillic and ferulic acid were higher in the green stage, while flavonoids were higher in red ripening (– and and ). Therefore, it can be said that phenolic compounds of red currant vary with maturation. This condition was confirmed by İsbilir et al. (Citation2012) and Elmastaş et al. (Citation2017) in their study on Arbutus and Rosa species. Similarly, Mikulic-Petkovsek et al. (Citation2016) found a decline in hydroxycinnamic acids value of red currant. However, while the amount of flavonoids increased with ripening in our study, they reported that the amount of flavonol decreased with ripening. In a study conducted in Slovenia, researchers found that the amount of polyphenol decreased in the last 3 weekss of ripening in red, pink, and white currant. Authors reported that the relative concentrations of hydroxycinnamic acids and flavonols increased in white and pink currants (Zorenc et al., Citation2017). Also in another studies recorded that phenolic acids decreased from 51.18 mg kg−1 (light-redd maturation) to 42.97 mg kg−1 (red maturation) in Rovada cultivar. They reported that flavanol value increased during ripening while flavonol value decreased in red currant (Mikulic-Petkovsek et al., Citation2015). Additionally, studies show that darker fruit color increases the amount of phenolic compounds (Gundogdu et al., Citation2017). In this study, the amount of phenolic compounds increased due to the coloration and ripening of the fruit with increasing air temperature ().
When organic acids were examined, it was found that acidity decreased with ripening except ascorbic acid in both cultivars. Ascorbic acid value increased in veraison but later decreased in pink and red maturation. In the green maturation, it was determined that both Red Lake and Rovada cultivars of fruits contain higher citric, ascorbic, and malic acid, respectivelyy. Also, veraison, pink and red maturations involved higher vitamin C, citric, and malic acid, respectivelyy ( and ; and ).
Figure 5. Correlation between different ripening periods and organic acid distributions of Redlake fruits
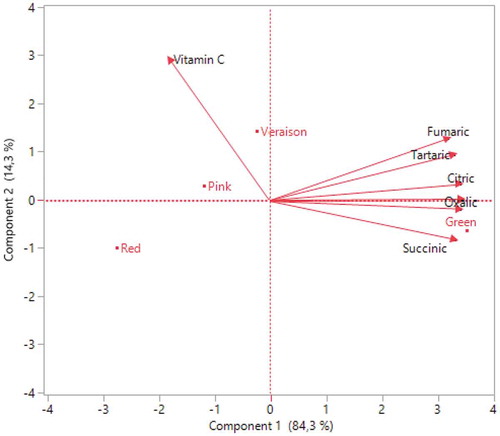
Figure 6. Correlation between different ripening periods and organic acid distributions of Rovada fruits
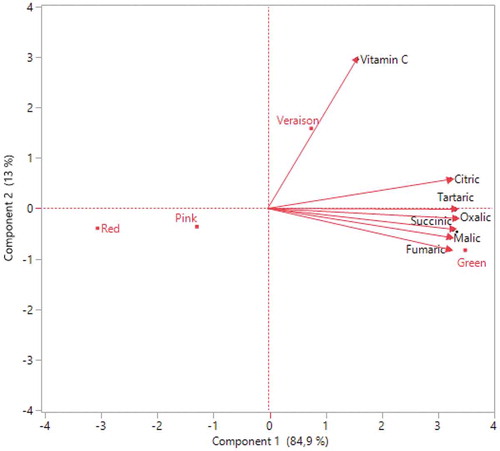
As shown in, it was determined that oxalic acid was 3.16 g kg−1 in green, 0.54 g kg−1 in pink, and 0.40 g kg−1 in red maturation in Red Lake cultivar. This acid was measured in pink maturation of 1.09 g kg−1 and decreased to 0.35 g kg−1 in Rovada (). In both cultivars, citric and tartaric acid diminished approximately 2 times. The highest citric acid was recorded 32.28 g kg−1 in green maturation in Red Lake cultivar () and 39.24 g kg−1 in Rovada cultivar (). The highest tartaric acid contents were obtained in Red Lake (1.52 g kg−1) and Rovada (1.39 g kg−1) cultivars during the green stage ( and ). The highest malic acid was recorded in green maturation of 10.56 g kg−1 in Red Lake and 9.38 g kg−1 in Rovada. In the pink period, succinic acid and fumaric acid were recorded as 3.56 g kg−1 and 0.29 g kg−1 in Rovada cultivar (), respectively. The highest vitamin C value in Red Lake cultivar, found in veraison maturation of 39.070 mg 100 g-1 (), while the lowest value was recorded in green maturation (24.075 mg 100 g-1) (). In Rovada cultivar, vitamin C had the highest values of 50.895 mg 100 g-1 in the veraison period () and the lowest of 38.045 mg 100 g-1 during red maturation (). There are studies showing that the amount of organic acid changes with ripening. Contrary to our findings, Glew et al. (Citation2003) reported that while malic acid value increased, ascorbic acid content decreased with ripening in medlar. However, Sun et al. (Citation2018) conducted a study in China and determined that the amount of vitamin C increased by 7 times in blueberry during maturation. Similarly, Rubinskienė et al. (Citation2008) reported that ascorbic acid content increased in black currant depend on ripening. Also, Nour et al. (Citation2011) indicated that vitamin C content varied between 23.23 and 44.62 mg 100 g−1 in ripe fruit of red currant. Ersoy et al. (Citation2018) reported that citric acid level measured as 15.07- mg 100 g−1 in Red Lake and 20.20 mg 100 g−1 in Rovada cv. In addition to the ripening, ecological factors affect the acidity of fruits. While the increase of air temperature causes to decrease the acidity rapidly, it is known that this decrease is slow at low temperatures (Güleryüz et al., Citation2001). It can be seen as the reason of this difference between other studies and our findings. Therefore, it was determined that a decrease of fruit acidity toward the harvest period depends on the increase in air temperatures () and the decrease in precipitation ().
Conclusion
In this study, it was found that the currant berries have richer biochemical compounds at the full maturation stage. When looking at the organic acid contents, it was found that citric acid was higher than other organic acids. The highest organic acid contents were determined in the green stage of fruits. Depending on maturation in both cultivars, reduction in organic acid content was determined. In particular, these reductions in oxalic acid and fumaric acid amounts were more clearly found. In the study, it was determined that the phenolic acids generally decreased depending on maturation, while the other phenolic compounds increased. Catechin and routine contents of Rovada and Red Lake fruits were determined as the highest phenolic compounds. The highest vitamin C content of both cultivars was found in the veraison stage.
References
- Adina, F., G. Cecilia, G. Felicia, D. Carmen, and T. Ovidiu. 2017. Identification and quantification of phenolic compounds from red currant (Ribes rubrum L.) and raspberries (Rubus idaeus L.). Int. J. Pharmacol. Phytochem. Ethnomed. 6:30–37.
- Andreotti, C., D. Ravaglia, A. Ragaini, and G. Costa. 2008. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 153(1):11–23. doi: 10.1111/j.1744-7348.2008.00234.x.
- Berk, S., and S. Tuna. 2017. Frenk Üzümünün (Ribes sp.) Biyolojik aktivitesi ve insan sağlığı üzerine etkileri. Bahçe 46:113–118.
- Bevilacqua, A.E., and A.N. Califano. 1989. Determination of organic acids in dairy products by high performance liquid chromatography. J. Food Sci. 54:1076–1079. doi: 10.1111/j.1365-2621.1989.tb07948.x.
- Cemeroglu, B. 2007. Food analysis. Food Technology Society Publication. No. 34, Ankara, Turkey, 168–171.
- Çoklar, H., and M. Akbulut. 2016. Olgunlaşma ile Alıç (Crataegus orientalis) Meyvesinin Antioksidan Aktivite, Toplam Fenolik Madde ve Fenolik Profilindeki Değişim. Meyve Bilimi 3(2):30–37.
- Dragovic-Uzelac, V., B. Levaj, V. Mrkic, D. Bursac, and M. Boras. 2007. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 102:966–975. doi: 10.1016/j.foodchem.2006.04.001.
- Elmastaş, M., A. Demir, N. Genç, Ü. Dölek, and M. Güneş. 2017. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 235:154–159. doi: 10.1016/j.foodchem.2017.05.004.
- Ersoy, N., M. Kupe, M. Gundogdu, G. Ilhan, and S. Ercisli. 2018. Phytochemical and antioxidant diversity in fruits of currant (Ribes spp.). Not. Bot. Hort. Agrobot. Cluj. 46(2):381–387. doi: 10.15835/nbha46211103.
- Eyduran, S.P., M. Akin, S. Ercisli, E. Eyduran, and D. Maghradze. 2015. Sugars, organic acids, and phenolic compounds of ancient grape cultivars (Vitis vinifera L.) from Igdir province of Eastern Turkey. Biol. Res. 48:1–8. doi: 10.1186/0717-6287-48-2.
- Glew, R.H., F.A. Ayaz, C. Sanz, D.J. VanderJagt, H.S. Huang, L.T. Chuang, and M. Strnad. 2003. Changes in sugars, organic acids and amino acids in medlar (Mespilus germanica L.) during fruit development and maturation. Food Chem. 83(3):363–369. doi: 10.1016/S0308-8146(03)00097-9.
- Güleryüz, M., S. Ercişli, and E. Erkan. 2001. Erzincan ovasında yetiştirilen bazı elma çeşitlerinin meyve gelişimi dönemlerinde meydana gelen fiziksel ve kimyasal değişimler ile bunlar arasındaki ilişkiler. Atatürk Üniversitesi Ziraat Fakültesi Dergisi 32(1):51–59.
- Gündoğdu, M. 2019. Effect of rootstocks on phytochemical properties of apricot fruit. Turk. J. Agric. For. 43:1–10. doi: 10.3906/tar-1803-99.
- Gundogdu, M., S. Ercisli, S. Berk, T. Kan, I. Canan, and M.K. Gecer. 2017. Diversity on color and phenolic compounds in apricot fruits. J.Food Meas. Charac. 11(4):2087–2093. doi: 10.1007/s11694-017-9592-4.
- Güneş, M., Ü. Dölek, and M. Elmastaş. 2016. Pomological changes in some rosehip species during ripening. J. Agric. Fac. Gaziosmanpasa Uni 33(3):214–222. doi: 10.13002/jafag1139.
- İsbilir, S.S., H.H. Orak, H. Yagar, and N. Ekinci. 2012. Determination of antioxidant activities of strawberry tree (Arbutus unedo L.) Flowers and fruits at different ripening stages. Acta Sci. Pol. Hortorum Cultus 11(3):223–237.
- Liu, H., W. Jiang, J. Cao, and Y. Li. 2019. Changes in extractable and non-extractable polyphenols and their antioxidant properties during fruit on-tree ripening in five peach cultivars. Hortic. Plant J. 5(4):137–144. doi: 10.1016/j.hpj.2019.04.005.
- Mikulic-Petkovsek, M., J. Rescic, V. Schmitzer, F. Stampar, A. Slatnar, D. Koron, and R. Veberic. 2015. Changes in fruit quality parameters of four Ribes species during ripening. Food Chem. 173:363–374. doi: 10.1016/j.foodchem.2014.10.011.
- Mikulic-Petkovsek, M., R. Veberič, F. Stampar, and D. Koron. 2016. Quality parameters of black and red currants during ripening. Acta Hortic. 1139:651–656. doi: 10.17660/ActaHortic.2016.1139.112.
- Nour, V., I. Trandafir, and M.E. Ionica. 2011. Ascorbic acid, anthocyanins, organic acids and mineral content of some black and red currant cultivars. Fruits 66(5):353–362. doi: 10.1051/fruits/2011049.
- Okatan, V., M. Gündoğdu, S.F. Güçlü, A. Çelikay, A.M.Ç. Özaydın, N. Korkmaz, and M.A. Aşkın. 2017. Phenolic profiles of currant (Ribes spp.) cultivars. YYU J. Agric. Sci. 27(2):192–196.
- Ozturka, A., Y. Kenan, B. Ozturk, O. Karakaya, S. Gun, S. Uzun, and M. Gundogdu. 2019. Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT - Food Scie. Tech. 111:117–124. doi: 10.1016/j.lwt.2019.05.033.
- Pedisic, S., B. Levaj, V. Dragovic-Uzelac, and K. Kos. 2007. Physicochemical composition, Phenolic Content and Antioxidant Activity of Sour Cherry Cv. Marasca during Ripening. Agriculturae Conspectus Scienticus. 72(4):295–300.
- Rodriguez-Delgado, M.A., S. Malovana, J.P. Perez, T. Borges, and F.J. Garcia-Montelongo. 2001. Separation pf phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. 912:249–257. doi: 10.1016/S0021-9673(01)00598-2.
- Rubinskienė, M., P. Viškelis, V. Stanys, T. Šikšnianas, and A. Sasnauskas. 2008. Quality changes in black currant berries during ripening. Sodininkyste Ir Daržininkyste 27(2):235–243.
- Schulz, M., G.D.S.C. Borges, L.V. Gonzaga, S.K.T. Seraglio, I.S. Olivo, M.S. Azevedo, and D.A. Spudeit. 2015. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res. Int. 77:125–131. doi: 10.1016/j.foodres.2015.08.006.
- Sun, Y., M. Li, S. Mitra, R. Hafiz Muhammad, B. Debnath, X. Lu, and D. Qiu. 2018. Comparative phytochemical profiles and antioxidant enzyme activity analyses of the southern highbush blueberry (Vaccinium corymbosum) at different developmental stages. Molecules 23(9):2209. doi: 10.3390/molecules23092209.
- Usenik, V., D. Kastelec, R. Veberič, and F. Štampar. 2008. Quality changes during ripening of plums (Prunus domestica L.). Food Chem. 111(4):830–836. doi: 10.1016/j.foodchem.2008.04.057.
- Voća, S., J. Žlabur, N. Dobričević, L. Jakobek, M. Šeruga, A. Galić, and S. Pliestić. 2014. Variation in the bioactive compound content at three ripening stages of strawberry fruit. Molecules 19(7):10370–10385. doi: 10.3390/molecules190710370.
- Zorenc, Z., R. Veberic, D. Koron, S. Miosic, O.S. Hutabarat, H. Halbwirth, and M. Mikulic-Petkovsek. 2017. Polyphenol metabolism in differently colored cultivars of red currant (Ribes rubrum L.) through fruit ripening. Planta 246(2):217–222. doi: 10.1007/s00425-017-2670-3.
