?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Polyphenols are natural antioxidants and play a very vital role in inhibition of oxidative stress as induced by free radicals in the body. Apple and pomegranate peels are significant agro-industrial wastes. The waste could be utilized to extract polyphenols for processing various functional foods and nutraceuticals. An investigation was executed for extraction of polyphenols from apple and pomegranate peels by sonication and maceration. Three different polar solvents: methanol, ethanol, and acetone were used in the study at two different concentrations (50% and 75%). Yield (%), total polyphenolic content (TPC), total flavonoid content (TFC), and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assay were performed. The extracts were then utilized in processing functional date bars with 2% and 3% concentrations. The results from the current study articulated that extraction from sonication yields higher quantity of polyphenols than that of maceration technique. The highest polyphenols were extracted at acetone 75% (44.71 mg GAE/g) in apple peel and at methanol 50% (72.21 mg GAE/g) in pomegranate peel. The results also revealed that pomegranate peel has higher polyphenols and greater radical scavenging activity as compared to apple peel. It was concluded that apple and pomegranate peel polyphenolic extract fortified date bars could be utilized as a preventive therapeutic agent against certain oxidative stress degenerative diseases.
Introduction
It has been reported that a very important role is played by reactive oxygen species in the cell signaling transduction being a middle product of normal metabolism process. When the reactive oxygen species are accumulated excessively, these result in oxidative stress. The oxidative stress is considered to be a root cause of various impairments in the human body, including damage to DNA, cytosolic Ca2+ elevation, and ATP reduction in the body cells (Mao et al., Citation2017). Scientific evidences reveal that oxidative damage is the cause of a substantial number of diseases and disorders either acting as the primary or secondary causative factor (Cutler, Citation2005). Polyphenols are natural antioxidants that play a vital part in inhibition of oxidative stress and hence save from a various numbers of associated diseases (Safdar et al., Citation2017; Santhakumar et al., Citation2014).
Polyphenols are basically natural organic compounds in which one or more number of hydroxyl groups are attached with a phenyl ring. Polyphenols possess a very important role in the defense mechanism of plants against various microorganisms (fungi, viruses, and bacteria) and against herbivores. They are bio-synthesized from tyrosine/phenylalanine (Cui et al., Citation2012; Güllüce et al., Citation2003; Papuc et al., Citation2017).
A diet rich in fruits and vegetable reduces chances of a number of diseases to occur, which include various kinds of cancer and different cardiovascular diseases. Different compounds like carotenoids, phytochemicals, flavonoids, and polyphenols are the key players behind such wonders (Boyer and Liu, Citation2004).
Generally, higher quantity of polyphenols is present in the fruit waste, especially in fruit peel than in fruit pulp. Studies have revealed that fruit peels have more antioxidant potential than that of the fruit pulp. Different phenolic compounds including carotenoids, anthocyanins, flavonoids, and phenolic acids are quite higher in fruit peels than that of pulp (Jabbar et al., Citation2015; Safdar et al., Citation2017).
Apple belongs to the family Rosaceae (Aziz et al., Citation2013; Junjian et al., Citation2013; Misigo, Citation2016). It has been revealed by epidemiological studies that taking apples in diet diminishes the risk of a number of diseases including asthma, diabetes, various cardiovascular diseases, and different kinds of cancer. Apples and more significantly the apple peels are found to be an excellent antioxidant in the laboratory analysis. Besides, consumption of apples is also associated with reduction of lipid oxidation, inhabitation of cancer cell proliferation, and reduction of the cholesterol levels (Boyer and Liu, Citation2004). Kschonsek et al. (Citation2018) articulated that the major polyphenols in apple peels are quercetin glycosides and flavanols in peels are the major contributors to antioxidant activity.
Pomegranates belong to family Punicaceae (Rahimi et al., Citation2012). Pomegranate peel is a major agro-industrial waste but rich in polyphenols, i.e., hydrolyzable ellagitannin; this pomegranate peel serves to be an excellent antioxidant natural source (Kushwaha et al., Citation2015). Pomegranate peel comprises about 30–40% of the total fruit and is a rich source of various bioactive compounds including flavonoids, tannins, phenolic acids, and significantly anthocyanins (Singh et al., Citation2019). Xie et al. (Citation2019) articulated that punicalagin and ellagic acid are major polyphenols in pomegranate peel and punicalagin as a major contributor to antioxidant activity.
Organic polar solvents including ethyl acetate, ethanol, methanol, and acetone are used particularly for extracting polyphenols (Ross et al., Citation2009). Among different techniques used for the purpose of polyphenol extraction, two of the techniques that are used in the current study are maceration, a conventional solid-liquid extraction technique (Garcia-Salas et al., Citation2010), and sonication, ultrasound-assisted extraction (UAE) technique (Jabbar et al., Citation2014). UAE of phenolic compounds is an advanced/non-conventional technique that involves mixing of organic solvent with the sample in a beaker/flask and put in an ultrasonic bath with pre-set temperature and time. It generates sound waves that result in cavitation, breaking sample cell walls, and leads to extraction of polyphenols to the solvent medium from the sample (Da Porto and Decorti, Citation2009). UAE process is very efficient and completes within an hour duration with extraction yield more than 6% to 35% of the traditional extraction methods with more than 12-h extraction time (Vilkhu et al., Citation2008).
Products from natural sources are being used for centuries (Ranjha et al., Citation2020). Due to the current lifestyle of masses, convenient foods such as cereal/dates/fruit bars are becoming very popular among consumers. Nowadays, food consumers demand convenient nutritive foods that are readily available (Orrego et al., Citation2014). Functional foods are processed or natural foods that provide basic nutrition. Also, they have additional physiological benefits in the management, treatment, and prevention of various chronic disorders (Martirosyan, Citation2011).
A research study was planned to compare maceration and UAEtechniques for the extraction of polyphenols from apple and pomegranate peels to estimate their polyphenol content and their antioxidant activity as well as to develop functional date bars.
Materials and Methods
Fully matured and ripened apple and pomegranate fruits of Chak Shazad Fruit Farms, Islamabad, were purchased at early hours from the local fruit market Islamabad-Pakistan and taken to Food Science Research Institute (FSRI), National Agricultural Research Center (NARC), Islamabad-Pakistan. The solvents and reagents used in the study were of analytical grade of Merck, Germany, supplied by Merck Islamabad distributor. Fruits were washed in order to remove any dust, dirt, or residues of any kind of pesticides, weedicides, fungicides, etc. The peels were separated from the pulp of both fruits and cut into small pieces. Then, peels were dried with the assistance of ‘hot air oven’ at 50°C temperature for 48 h. With the assistance of ‘cyclotec sample mill,’ the dried peels, with moisture content less than 10%, were grinded into fine powder. The peel powder was then filled in polyethylene zip bags and it was made sure that the bags were airtight. Then, the bags were kept at refrigeration temperature (4–6°C).
Proximate Analysis
By following the standard methods of AOAC (Citation2016), moisture (%), ash (%), crude protein (%), crude fat (%), crude fiber (%), and NFE (%) were determined for fruit peels as well as raw materials for product development (date bars) and for date bars.
Conventional Extraction (Maceration)
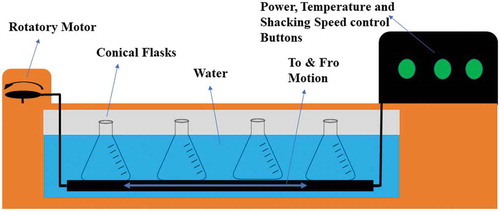
Maceration technique was employed in order to extract polyphenols from pomegranate and apple peels’ powder following the method of Elfalleh et al. (Citation2012) with little amendments. Preliminary studies were conducted to determine optimum solvent concentration (25%, 50%, 75%, and 100%) and sample-to-solvent ratio (1:20, 1:15, and 1:10). After preliminary studies, three different solvents were used including acetone, methanol, and ethanol at two different selected concentrations, i.e., 50% and 75%. The sample-to-solvent ratio was set to 1:15 and temperature for the maceration technique was set to 40°C. Three grams of both the peels were taken in 250 ml of conical flasks separately in triplicate and 45 ml of solvent was introduced at 50% and 75% concentrations. ‘Shaking Water Bath’ (Tecator 1024, FOSS Analytical AB, Pal Anders vag, Hoganas, Sweden) was used in this procedure. The conical flasks were hired in the shaking water bath for about 20 h. After 20 h, all the samples were filtered through Whatmanfilter paper 41 and centrifugation was done for about 10 min at 5,000 rpm (Beckman J2-21, Brea, CA). Then, the solvent was evaporated with the assistance of ‘Rotary Evaporator’ (Buchi Labortechnik, Rotavapor R-300, Meierseggstrasse, Flawil, Switzerland). Evaporation of the solvent was done under vacuum set at 140 mbar and temperature set at 45°C till drying, amber glass bottles were used for collection of extract and placed in refrigerated conditions at 4–6°C.
Ultrasound-Assisted Extraction
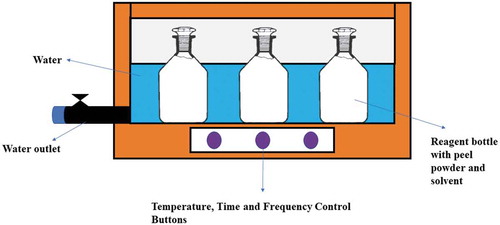
Sonication technique was employed in order to extract polyphenols from pomegranate and apple peels’ powders following the method of Bimakr et al. (Citation2013) with little amendments. Preliminary studies were conducted to determine optimum solvent concentration (25%, 50%, 75%, and 100%), sample-to-solvent ratio (1:20, 1:15, and 1:10), and temperature (35°C, 45°C, and 55°C). After preliminary studies, three different solvents were used including acetone, methanol, and ethanol at two different selected concentrations, i.e., 50% and 75%. Three different solvents were used including acetone, methanol, and ethanol at two different concentrations, i.e., 50% and 75%. The sample-to-solvent ratio was set to 1:20 and temperature for the sonication technique was set to 45°C. About 3 g of peel powder each were taken in 125 ml of reagent bottles separately in triplicate and 60 ml of solvent was added at 50% and 75% concentrations. ‘Sonicator’ (Bandelin RK 510H Sonorex, Heinrichstrabe, Berlin, Germany) was used in this procedure. The reagent bottles were placed in the sonicator bath for 1 h. Filtration of the samples was done with assistance of Whatman filter paper 41 and centrifugation was done for about 10–12 min at 5,000 rpm (Beckman J2-21, Brea, CA). Then, the solvent was evaporated with the assistance of ‘Rotary Evaporator’ (Buchi Labortechnik, Rotavapor R-300, Meierseggstrasse, Flawil, Switzerland). The solvent was evaporated under vacuum set at 140 mbar and temperature set at 45°C till drying. The extracts were collected in amber glass bottles and placed in refrigerated conditions at 4–6°C.
Determination of Yield (%)
The yield (%) of the Pomegranate and Apple peel extracts was determined by the following formula:
Determination of Total Polyphenols
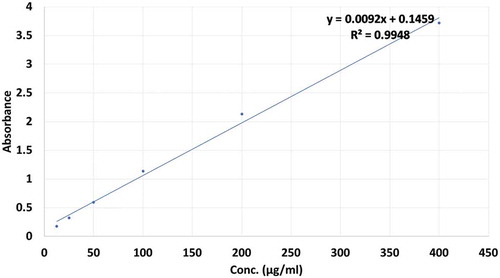
Total polyphenolic content (TPC) of apple and pomegranate peels’ extracts was assessed by Folin Ciocalteau method as described by Singleton et al. (Citation1999). Ethanol, methanol, and acetone solutions of sample extract 10 mg/ml were prepared, respectively. From 50% to 75% concentration of solvents (ethanol, methanol, and acetone), 0.5 ml solvents extract solution was assorted with 2.5 ml of 10% Folin Ciocalteu’s reagent dissolved in water and 2.5 ml of 7.5% sodium carbonate. Blank was concomitantly prepared. Then, these samples were incubated at 25°C for 30 min for blue color development. The absorbance was determined at 765 nm with UV-visible spectrophotometer. Gallic acid standard solution and the calibration curve was prepared from various concentrations (12.5, 25, 50, 100, 200, and 400 µg/ml) of Gallic acid. TPC was represented as mg gallic acid equivalent per gram of extract (mg GAE/g).
Determination of Total Flavonoid (TFC)
TFC of apple and pomegranate peel was determined following to method of Chang et al. (Citation2002) with little amendments. Sodium nitrate (0.5 g) was dissolved in up to 10 ml of distilled water in a 10-ml volumetric flask in order to prepare 5% sodium nitrate solution. One gram of aluminum chloride was mixed in up to 10-ml distilled water in a 10-ml volumetric flask in order to prepare 10% aluminum chloride solution. One gram of NaOH was dissolved in 25-ml distilled water in a 25-ml volumetric flask in order to prepare 1 M NaOH solution. After preparing three different solutions, sample was prepared by taking 0.01 g of extract (pomegranate and apple peels) and then dissolved in 5 mL of respective solvent (methanol, ethanol, and acetone). After that, 1 ml of sample extract solution was taken in a 10-ml volumetric flask and 4 ml of distilled water was added in it. Then, 0.3 ml of 5% sodium nitrite was added. After 5 min, 0.3 ml of 10% aluminum chloride was added. After 6 min, 2 ml of 1 M NaOH was added. Their volume was made up to 10 ml with distilled water. Then, absorbance was checked at 415 nm on UV-visible spectrophotometer. Results were expressed as mg quercetin equivalent per g of extract (mg QE/g of extract).
Evaluation of Antioxidant Activity by DPPH Radical Scavenging Assay
The antioxidant activity of apple and pomegranate peel extracts was determined by DPPH (1,1-diphenyl-2-picryl-hydrazyl) assay following the method of Brand-Williams et al. (Citation1995) with slight amendments. The stock solution was prepared by dissolving 24-mg DPPH with 100-ml methanol and then stored at 20°C until needed. The working solution was obtained by DPPH solution with methanol to obtain an absorbance of about 0.980 at 517 nm using spectrophotometer. Three milliliters of that solution were mixed with 100 µl of the samples at varying concentrations (12.5–400 µg/ml). The solution in the test tubes was shaken well and incubated in dark for 15 min at room temperature. Then, the absorbance was taken at 517 nm. The scavenging activity was estimated based on the percentage of DPPH radical.
Development of Functional Date Bars
Among three solvents used in the study (ethanol, methanol, and acetone), only solvent ethanol is generally regarded as safe for food purposes; therefore, 75% ethanolic extract by UAE technique was utilized for the preparation of functional date bars.
Trial production of functional date bars was carried out at 0%, 1%, 2%, 3%, and 4% peel extract fortification levels. The 2% and 3% peel extract fortification levels have the best results and sensory acceptable more than 1% and 4% peel extract fortification levels. Therefore, 2% and 3% peel extract fortification levels were selected for further research study.
represents the formulation of functional date bars. Date bars (25 g each) were prepared by mixing date paste, roasted gram, and nuts. Apple and pomegranate peel polyphenol extracts of 2% and 3% concentration levels, respectively, were added in different bars along with control bars (without extract). Then, the mixture was transferred to cutting and sheeting table where sheeting was carried out with sheeting roller and cut into date bars of 7 cm length, 2.5 cm width, and 1 cm height. Date bars were placed in hot air oven at 45°C for 25 min. Each bar of approximately 25 g was packed in butter paper along with aluminum foil and stored in refrigerator.
Table 1. Formulation of functional date bar
Physicochemical Analysis
The date paste and the functional date bars were subjected to physicochemical analysis including TSS (total soluble solids), titratable acidity, pH, reducing, non-reducing, and total sugars.
TSS was determined by Atago Hand Refractometer. Four grams of the sample was taken and was diluted five times with distilled water, making the total volume as 20 ml. Few drops of the sample were placed on refractometer prism. Then, TSS of sample was noted from the refractometer brix scale with dark band, multiplied by the dilution factor which was corrected at 20°C from table 900.03 (AOAC, Citation2016).
Percent acidity of date paste and functional date bars was determined according to standard method of AOAC (Citation2016). Five-gram sample was taken and titrated against 0.1 N NaOH using phenolphthalein as indicator till pink endpoint. Percent acidity was calculated by following formula:
Sugars were determined by the Lane Eynon method as reported in AOAC (Citation2016). Ten grams of sample was taken in 1000-ml beaker and 50 ml of distilled water was added. Three drops of phenolphthalein were added and titrated with 0.1 N NaOH till change of color. Then, HCl was added to neutralize the sample solution. Then, distilled water was added to make up the volume to 200 ml, known as solution A. From solution A, 50-ml solution was taken, known as solution B. In the solution B, 5 g of citric acid was added. The beaker was heated on hot plate and allowed to boil for 10 min. The beaker was removed from hot plate and allowed to cool. After cooling, three drops of phenolphthalein were added and titrated with 0.1 N NaOH till change of color. Then, HCl was added to neutralize the sample solution and to retain the original light-yellow color. After neutralization, distilled water was added to make up the volume to 200 ml.
Fehling A solution (5 ml) and Fehling B solution (5 ml) were taken in a conical flask. The flask was heated till boiling without agitation or shaking for about 2 min. Three drops of methylene blue indicator were added. Then, it was titrated with sample solution ‘A’ from the burette drop by drop while boiling the solution without shaking the flask. It was titrated until the blue color changed into brick red color.
For total sugars, 5 ml of sample solution ‘B’ was taken and proceeded similarly as the titration procedure carried out for sample solution ‘A’
Following formulae were used for determination of sugars;
Sensory Evaluation
Sensory evaluation was conducted in Food Science Research Institute at the National Agricultural Research Center, Islamabad. Sensory evaluation Performa (9-point hedonic scale) was used to determine the preferences in color, taste, flavor, and texture according to the procedure described by Stone and Sidel (Citation1998). The judges were given sensory evaluation performa and given proper instructions. The judges were advised to examine each sample carefully. Sensory evaluation was performed in a testing room facilitated with normal lights.
Statistical Analysis
Data were statistically analyzed by applying analysis of variance (ANOVA), post hoc multiple comparison, Fisher least significant difference test (LSD), and descriptive statistics. Then, the results were converted into charts and tables (as appropriate) for better interpretation. Minitab 18.1 was used for analysis.
Results and Discussions
Weight of Peels
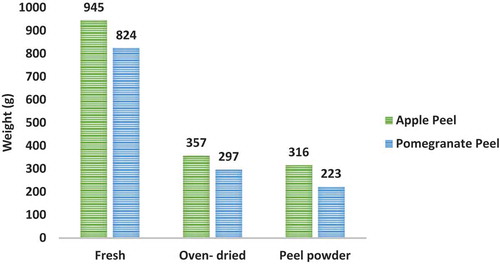
illustrates the weight of apple and pomegranate peels at different stages from fresh to oven-dried and powder. A substantial reduction in the weight was observed during drying and grinding of fresh peels to powder form. The reduction in weight of the peel could be because of moisture loss and loss of sample while grinding dried peels into powder form.
Proximate Analysis
Estimation of proximate composition is significant for assessment of the quality of raw material (Safdar et al., Citation2017). illustrates the proximate composition of apple and pomegranate peels. It can be clearly seen that the pomegranate peel possesses higher moisture, ash, crude fat, crude fiber, and crude protein content but a less content of NFE. Henríquez et al. (Citation2010) reported that apple peels contain ash (2.4%), crude fat (2.7%), crude fiber (19.6%), crude protein (2.7%), and NFE (72.7%). Sharma and Es (Citation2018) in their study on composition of various parts of pomegranate fruit reported that pomegranate peels contain ash (5.6%), fat (1.2%), protein (7.8%), crude fiber (19%), and NFE (64.84%). The variation in the proximate composition of apple and pomegranate peel powder in different studies could be because of different varieties, climatic conditions, topographic locations, and different agronomic practices.
Table 2. Proximate composition (%) of apple and pomegranate peels
Yield (%)
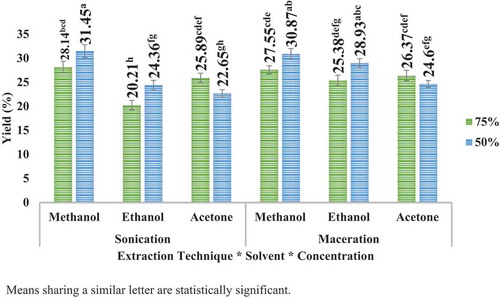
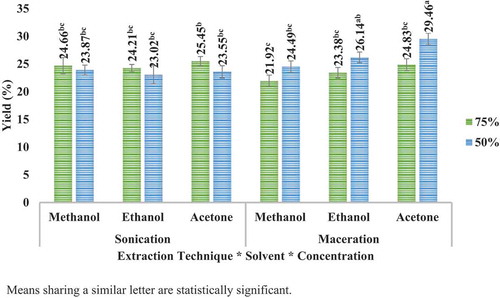
illustrates the yield (%) of apple peel obtained by two different techniques (sonication and maceration) with three different solvents (acetone, methanol, and ethanol) at two different concentrations (50% and 75%). Similarly, illustrates the yield (%) of pomegranate peel obtained following the same procedure. In case of apple peel, highest yield was determined at acetone 50% (29.46%) in maceration technique, while in case of pomegranate peel, highest yield was determined at methanol 50% (31.45%) in UAE technique. Further, it was noticed that apple peel extracted more yield in maceration technique with 50% concentration while with 75% concentrations more yield was obtained in sonication. In case of pomegranate peel extracts, more yield was extracted in maceration with any concentration of ethanol and acetone, but with methanol, more yield was extracted in sonication. The variations in yield extractions using different solvents at different concentrations could be because of dissimilar polarities of the solvents used. Tow et al. (Citation2011) reported that using 50% ethanol was more effective method for yield extraction (48.34%) than using 80% methanol (30.17%) or 80% ethanol in combination with 0.18 N HCl (30.73) from apple peel.
Figure 4. Yield (%) of apple peel extracts by sonication and maceration
Total Polyphenolic Content
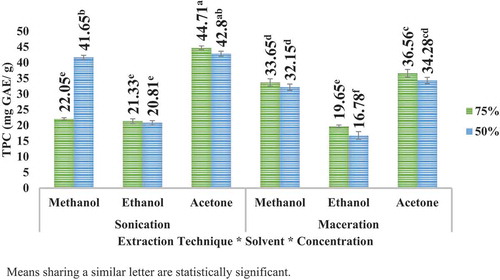
Highest TPC was observed to be at acetone 75% in case of apple peel in sonication and at methanol 50% in case of pomegranate peel. The results further articulated that besides greater amount of yield in maceration (for apple peel), the TPC was higher in sonication for both the peels. The results can be illustrated in (apple peel) and (pomegranate peel). It is further articulated that the TPC of pomegranate peel is much higher than that of TPC of apple peel.
Figure 8. TPC of pomegranate peels’ extracts
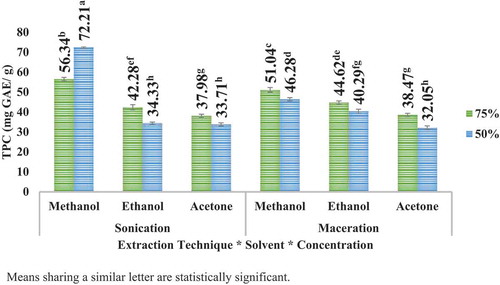
UAE (sonication) technique led to higher TPC than maceration technique for the solvents employed. However, comparatively higher TPC was observed in case of methanolic extract samples, especially for pomegranate peel extracts. UAE (sonication) technique has the advantage over maceration technique that it is comparatively more efficient and far less time-consuming as compared to conventional maceration technique. Tow et al. (Citation2011) testified the yield of about 229 mg GAE/g polyphenols using 50% ethanol from freeze-dried apple waste as compared to 77.26 mg GAE/g using 80% methanol and 141 mg GAE/g 80% ethanol in combination with 0.18 N HCl. Gözlekçi et al. (Citation2011) articulated that TPC in pomegranate peels fluctuated from 1775.4 to 3547.8 mg GAE/L among different cultivars.
Total Flavonoid Content (TFC)
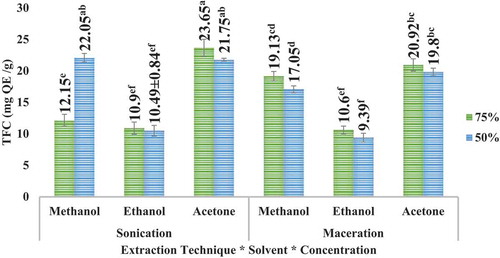
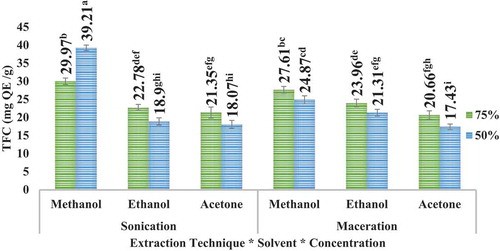
Highest TFC was observed to be at acetone 75% in case of apple peel in sonication and at methanol 50% in case of pomegranate peel. The results further articulated that besides greater amount of yield in maceration (for apple peel), the TFC was higher in sonication for both the peels. The results can be illustrated in (apple peel) and (pomegranate peel). It is further articulated that TFC of pomegranate peel is higher than that of TFC of apple peel.
UAE (sonication) technique led to higher TFC than maceration technique for the solvents employed. However, comparatively higher TFC was observed in case of methanolic extract samples, especially for pomegranate peel extracts. UAE (sonication) technique has the advantage over maceration technique that it is comparatively more efficient and far less time-consuming as compared to conventional maceration technique. Zulkifli et al. (Citation2012) testified that apple peels contain total flavonoids about 177.86 ± 8.51 mg QE/g; the variety used in this study was M. sylvestris. Derakhshan et al. (Citation2018) reported that the total flavonoid in the pomegranate peel among different varieties fluctuated from 36 to 54 mg rutin/g.
DPPH Radical Scavenging Assay
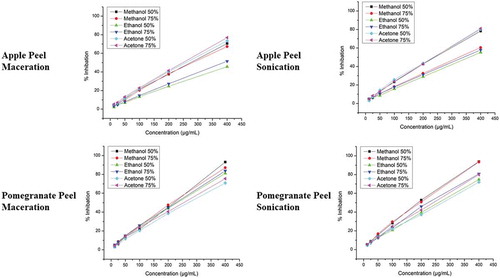
It was shown in the current study that % inhibition of apple and pomegranate peel extract of ultra-sound extraction sample exhibited higher radical scavenging than maceration technique as evaluated by DPPH radical scavenging assay. The % inhibition of ultra-sound extraction sample was increased as concentration increased from 12.5 to 400 µg/ml. illustrates DPPH radical scavenging activity for apple peel maceration, apple peel sonication, pomegranate peel maceration, and pomegranate sonication, respectively. Highest % inhibition of the apple peel extracts was found 81.05% with acetone 75% in sonication and the lowest activity was measured 45.62% with ethanol 50% in the maceration technique. Overall, the sonication extracts exhibited higher antioxidant activity than that of maceration extracts. In case of the pomegranate peel, the highest % inhibition was measured 93.84% with methanol 50% in sonication while the minimum activity was measured 70.84% with acetone 50% in the maceration technique. Overall, the sonication extracts exhibited % inhibition than that of maceration extracts. It was also articulated in the current study that pomegranate peels exhibit greater % inhibition than that of apple peel extracts. There is a positive correlation between TPC and % inhibition as determined by DPPH radical scavenging assay. Generally, higher the polyphenol content, higher will be the % inhibition of polyphenolic extracts. The findings of research study also confirmed that the pomegranate peel extract samples had higher antioxidant activity/percent inhibition owing to their high polyphenol content as compared to apple peel extracts.
Sara et al. (Citation2015) testified the % inhibition of apple peel and it was found to be 63.92%. Kushwaha et al. (Citation2015) in their study articulated the DPPH radical scavenging activity of pomegranate peels about 63.74%. Derakhshan et al. (Citation2018) stated that the pomegranate peel extract possesses higher antioxidant activity in comparison to seeds and juice.
Product Development and Evaluation
Proximate Composition of Roasted Gram Flour
illustrates the proximate analysis of roasted gram flour. It can be articulated from the table that roasted gram flour is a very good source of protein and minerals. Proximate analysis showed that flour selected for preparation of date bars was fresh, had low moisture content, and nitrogen-free extract. During a related study, Rehman et al. (Citation2012) reported that roasted gram flour contains moisture content about 4.1%, ash 2.72%, crude fat 1.35%, crude fiber 4.9%, crude protein 24.19%, and NFE 62.11.
Table 3. Proximate analysis of roasted gram flour (%)
Physicochemical Composition of Date Paste
illustrates the physicochemical analysis of date paste. It can be articulated from the table that date paste possesses a significant amount of sugars. Physicochemical analysis of date paste indicated that dates were fully matured and ripened with total soluble solids and sugar content, whereas the acidity was low with conversely high pH.
Table 4. Physicochemical analysis of date paste
Proximate Composition of Date Bars
illustrates the proximate analysis of different date bars from control bar to bars fortified with extracts. Control bar and 2% and 3% apple and pomegranate peel extract date bars were prepared and subjected to proximate analysis. All date bars had optimum moisture content as recommended for semi-dehydrated food products. The elevated protein content and nitrogen-free extract of date bars showed the higher nutritional value of bars. Fortification of peel extract to date bars had non-significant effect on proximate composition of date bars. In a previous study by Rehman et al. (Citation2012), apricot-date bars were processed and it was reported that the bars contain moisture content about 17.14–19.21%, ash 4.05–4.20%, crude fat 7.30–7.32%, crude fiber 5.66–6.14%, and crude protein 10.17–10.88%. The variation in the proximate composition could be because of using altered raw materials in the bars. Zeeshan et al. (Citation2017) reported moisture content in Dhakki date candies ranging 14.16–16.77% among various treatments.
Table 5. Proximate analysis of date bars
Physicochemical Composition of Date Bars
illustrates the physicochemical analysis of date bars. As evident from the table, control as well as peel extract fortified date bars had high total soluble solids, sugars, and low acidity. The TSS, acidity, and sugars of date bars increased with the increase in fortification level and were maximum at 3% peel extract fortified date bars. In a parallel study by Zeeshan et al. (Citation2017), pH and TSS were assessed among different treatments of date candies. The pH among the various treatments in the study ranged from 5.4 to 5.96 and the TSS ranged from 45.14 to 73.12.
Table 6. Physicochemical analysis of date bars
Table 7. Sensory evaluation of date bars
Total Phenolic Content of Date Bars
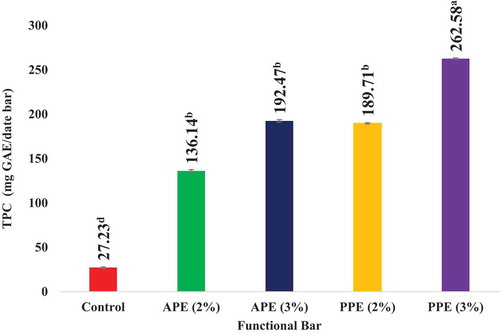
As evident from , the TPC of date bars elevated with the elevation in fortification level of peel extract. Date bars fortified with 3% pomegranate peel extract exhibited the maximum polyphenol content followed by date bars fortified by 3% apple peel extract. Date bars fortified with 2% pomegranate peel extract exhibited almost equal TPC as exhibited by date bars fortified with 3% apple peel extract. It can also be observed that control date bars without peel extract had the minimum polyphenol content. Apple and pomegranate peel polyphenolic extracts were fortified into date bars at 2% and 3% fortification levels revealed that the date bars fortified with pomegranate peel polyphenolic extracts had comparatively high polyphenol content than apple peel polyphenolic extract date bars. Also, 3% fortification level exhibited more functional properties and had more polyphenols than 2% fortification level. Rehman et al. (Citation2012) reported similar findings in apricot-date bars; the TPC ranged from 225 to 263 mg/100 g among the various treatments in the study.
Sensory Evaluation
Sensory evaluation of date bar revealed that fruit bar fortified with 2% pomegranate peel extract scored the highest and preferred than other date bars while the control bars got the lowest score. However, all the bars were accepted by panelists. The color score varied from 6.3 to 7.7 among the different bars, the lowest for the control bars, and highest score was for the bars fortified with 2% pomegranate peel extract. The taste score was also found to be highest in the case of 2% fortified pomegranate extract bars (7.8) and lowest for the control bars (6.9). The results were also similar in case of flavor and texture; the highest scores were given to 2% pomegranate peel extract fortified bars (7.53 and 7.73, respectively) and lowest for the control bars. Zeeshan et al. (Citation2017) evaluated date candies with physicochemical and sensory parameters. The color scores for the date candies ranged from 8.0 to 9.0 for different treatments at zero days, the flavor score ranged from 6.5 to 9.0, the texture score confined 9.0 for all the treatments, and the overall acceptability ranged from 6.0 to 9.0 for different treatments (Zeeshan et al. Citation2017).
Conclusion
Apple and pomegranate peels are excellent sources of phenolic compounds and possess a strong antioxidant potential. Pomegranate peels’ extracts have a higher phenolic content, flavonoid content, and antioxidant potential than that of apple peels. Higher polyphenols could be extracted by using UAE than that of maceration technique. Among three solvents used in the study (ethanol, methanol, and acetone), only solvent ethanol is generally regarded as safe for food purposes. Among the extracts utilized for processing of functional date bars, the bars fortified with 3% pomegranate peel extract had supreme TPC followed by 3% apple peels’ extract, 2% pomegranate peels’ extract, 2% apple peels’ extract, and control date bars. It was suggested that both pomegranate and apple peels could be utilized as a functional ingredient for the preparation of different functional foods. It was concluded that apple and pomegranate peel polyphenolic extract fortified date bars could be utilized as preventive therapeutic agents against certain oxidative stress degenerative diseases.
Disclosure Statement
We have no conflict of interest to disclose.
Literature Cited
- AOAC. 2016. Official methods of analysis of AOAC international. 20th ed. Association of the Official Analytical Chemists, Gaithersburg.
- Aziz, M., M. Anwar, Z. Uddin, H. Amanat, H. Ayub, and S. Jadoon. 2013. Nutrition comparison between genus of apple (Malus sylvestris and Malus domestica) to show which cultivars is best for the province of Balochistan. J. Asian Sci. Res. 3(4):417–424.
- Bimakr, M., R. Abdul Rahman, F. Taip, N. Mohd Adzahan, Z. Sarker, and A. Ganjloo. 2013. Ultrasound-assisted extraction of valuable compounds from winter melon (Benincasa hispida) seeds. Int. Food Res. J. 20:331–338.
- Boyer, J., and R.H. Liu. 2004. Apple phytochemicals and their health benefits. Nutr. J. 3(1):5. doi: 10.1186/1475-2891-3-5.
- Brand-Williams, W., M.E. Cuvelier, and C. Berset. 1995. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci. Technol. 28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5.
- Chang, C., M. Yang, H. Wen, and J. Chern. 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10(3):178–182.
- Cui, Y., Y.J. Oh, J. Lim, M. Youn, I. Lee, H.K. Pak, and S. Park. 2012. AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against gram-positive and gram-negative bacteria. Food Microbiol. 29:80–87. doi:10.1016/j.fm.2011.08.019.
- Cutler, R.G. 2005. Oxidative stress profiling: Part I. Its potential importance in the optimization of human health. Ann. N. Y. Acad. Sci. 1055:93–135. doi:10.1196/annals.1323.027.
- Da Porto, C., and D. Decorti. 2009. Ultrasound-assisted extraction coupled with under vacuum distillation of flavour compounds from spearmint (carvone-rich) plants: comparison with conventional hydrodistillation. Ultrason. Sonochemist. 16(6):795–799. doi: 10.1016/j.ultsonch.2009.03.010.
- Deng, G.F., C. Shen, X.R. Xu, R.D. Kuang, Y.J. Guo, L.S. Zeng, and H. Li. 2012. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 13(7):8308-8323.
- Derakhshan, Z., M. Ferrante, M. Tadi, F. Ansari, A. Heydari, M.S. Hosseini, and E.K. Sadrabad. 2018. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 114:108–111. doi:10.1016/j.fct.2018.02.023.
- Elfalleh, W., H. Hannachi, N. Tilili, Y. Yahia, N. Nasri, and A. Ferchichi. 2012. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6(32):4724–4730. doi: 10.5897/JMPR11.995.
- Garcia-Salas, P., A. Morales-Soto, A. Segura-Carretero, and A. Fernández-Gutiérrez. 2010. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 15:8813–8826. doi:10.3390/molecules15128813.
- Goulas, V., and G.A. Manganaris. 2012. Exploring the phytochemical content and the antioxidant potential of citrus fruits grown in Cyprus. Food Chem. 131:39–47. doi:10.1016/j.foodchem.2011.08.007.
- Gözlekçi, Ş., O. Saraçoǧlu, E. Onursal, and M. Özgen. 2011. Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacogn. Mag. 7(26):161–164. doi: 10.4103/0973-1296.80681.
- Güllüce, M., M. Sökmen, D. Daferera, G. Aǧar, H. Özkan, N. Kartal, and F. Şahin. 2003. In vitro antibacterial, antifungal, and antioxidant activities of the essential oil and methanol extracts of herbal parts and callus cultures of Satureja hortensis L. J. Agric. Food Chem. 51(14):3958-3965.
- Henríquez, C., H. Speisky, I. Chiffelle, T. Valenzuela, M. Araya, R. Simpson, and S. Almonacid. 2010. Development of an ingredient containing apple peel, as a source of polyphenols and dietary fiber. J. Food Sci. 75:6. doi:10.1111/j.1750-3841.2010.01700.x.
- Jabbar, S., M. Abid, T. Wu, M. Muhammad Hashim, B. Hu, S. Lei, and X. Zeng. 2014. Study on combined effects of blanching and sonication on different quality parameters of carrot juice. Int. J. Food Sci. Nutr. 65:28–33. doi:10.3109/09637486.2013.836735.
- Jabbar, S., M. Abid, T. Wu, M.M. Hashim, M. Saeeduddin, B. Hu, and X. Zeng. 2015. Ultrasound-assisted extraction of bioactive compounds and antioxidants from carrot pomace: A response surface approach. J. Food Process. Preserv. 39:1878–1888. doi:10.1111/jfpp.12425.
- Junjian, R., F. Mingtao, L. Yahui, L. Guowei, Z. Zhengyang, and L. Jun. 2013. Optimisation of ultrasonic-assisted extraction of polyphenols from apple peel employing cellulase enzymolysis. Int. J. Food Sci. Technol. 48(5):910–917. doi: 10.1111/ijfs.12041.
- Kschonsek, J., T. Wolfram, A. Stöckl, and V. Böhm. 2018. Polyphenolic compounds analysis of old and new apple cultivars and contribution of polyphenolic profile to the in vitro antioxidant capacity. Antioxidants (Basel, Switzerland) 7(1):20.
- Kushwaha, S.C., M.B. Bera, and P. Kumar. 2015. Extraction of polyphenols from fresh pomegranate peel using response surface methodology. Asian J. Chem. 27(12):4320–4326. doi: 10.14233/ajchem.2015.19051.
- Mao, X., C. Gu, D. Chen, B. Yu, and J. He. 2017. Oxidative stress-induced diseases and tea polyphenols. Oncotarget. 8(46):81649–81661. doi: 10.18632/oncotarget.20887.
- Martirosyan, D.M., Ed. 2011. Functional foods and chronic diseases: Science and practice. Food Science Publisher, USA.
- Misigo, R. 2016. Classification of selected apple fruit varieties using naive bayes. Univ. Nairobi 3(1):56.
- Orrego, B.E., N. Salgado, and C.A. Botero. 2014. Developments and trends in fruit bar production and characterization. Critic. Rev. Food Sci. Nutr. 54:84–97. doi:10.1080/10408398.2011.571798.
- Papuc, C., G.V. Goran, C.N. Predescu, V. Nicorescu, and G. Stefan. 2017. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 16(6):1243–1268. doi: 10.1111/1541-4337.12298.
- Rahimi, H.R., M. Arastoo, and S.N. Ostad. 2012. A comprehensive review of Punica granatum (Pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran. J. Pharm. Res. 11(2):385–400.
- Ranjha, M.M.A.N., S. Irfan, M. Nadeem, and S. Mahmood. 2020. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev. Int. 1–27. doi:10.1080/87559129.2020.1725890.
- Rehman, S.M., M.H. Nadeem, and J.A. Awan. 2012. Development and physico-chemical characterization of apricot-date bars. J. Agric. Res. 50(3):409-421.
- Ross, K.A., T. Beta, and S.D. Arntfield. 2009. A comparative study on the phenolic acids identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chem. 113(1):336–344. doi: 10.1016/j.foodchem.2008.07.064.
- Safdar, M.N., T. Kausar, and M. Nadeem. 2017. Comparison of ultrasound and maceration techniques for the extraction of polyphenols from the mango peel. J. Food Process. Preserv. 41:4. doi:10.1111/jfpp.13028.
- Santhakumar, A.B., A.C. Bulmer, and I. Singh. 2014. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 27(1):1–21. doi: 10.1111/jhn.12177.
- Sara, J., E. Abdolrasoul, K. Ahmad, and B. Hossain. 2015. Evaluation of antioxidant activity of Malus domestica fruit extract from Kashan area. Afr. J. Agric. Res. 10(20):2136–2140. doi: 10.5897/AJAR12.164.
- Sharma, K., and C. Es. 2018. Comparative studies of proximate, mineral and phytochemical compositions of pomegranate (Punica granatum) in peel, seed and whole fruit powder. Int. J. Food Sci. Nutr. 3(2):192–196.
- Singh, B., J.P. Singh, A. Kaur, and N. Singh. 2019. Antimicrobial potential of pomegranate peel: A review. Int. J. Food Sci. Technol. 54(4):959–965. doi: 10.1111/ijfs.13964.
- Singleton, V., R. Orthofer, and R. Lamuela-Raventos. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 299:152–178.
- Stone, H., & Sidel, J. L. (1998). Quantitative descriptive analysis: developments, applications, and the future. Food technology (Chicago), 52(8),48-52.
- Tow, W.W., R. Premier, H. Jing, and S. Ajlouni. 2011. Antioxidant and antiproliferation effects of extractable and nonextractable polyphenols isolated from apple waste using different extraction methods. J. Food Sci. 76:7. doi:10.1111/j.1750-3841.2011.02314.x.
- Vilkhu, K., R. Mawson, L. Simons, and D. Bates. 2008. Applications and opportunities for ultrasound assisted extraction in the food industry. Innov. Food Sci. Emerg. 9:161–169. doi:10.1016/j.ifset.2007.04.014.
- Xie, Z., X. Li, R. Tang, G. Wang, Y. Lu, X. Li, K. Cheng, L. Li, and Q. He. 2019. Reactions of polyphenols in pomegranate peel with nitrite under simulated stomach conditions. Food Sci. Nutr. 7(9):3103–3109. doi: 10.1002/fsn3.1173.
- Zeeshan, M., S. Sa, M. Ayub, M. Shah, and Z. Jan. 2017. Physicochemical and sensory evaluation of Dhakki dates candy. J. Food Process. Technol. 8(3):8–11.
- Zulkifli, K.S., N. Abdullah, N. Aziman, W.S. Kamarudin, and A. Abdullah. 2012. Phytochemical screening and activities of hydrophilic and lipophilic antioxidant of some fruit peels. Malaysian J. Anal. Sci. 16(3):309–317.