ABSTRACT
The effect of Citrus stubborn disease (CSD), caused by Spiroplasma citri, on chemical composition and antimicrobial activities of the essential oils of (Citrus sinensis (L) Osbeck) was studied. The results showed that the essential oil extracted from both healthy and Citrus fruit infected by the stubborn disease did not have the same physicochemical characteristics, but have identical chemical composition, with octanal, β-phellandrene, d-limonene, 1 r-α pinene, and d-limonene as major compounds. The infected fruits contained new toxic compounds (2-cyclohexen-1-ol, 2-methyl-5-(1-methylethenyl)-cis, decanal, trans-p-mentha-2, 8-dienol, 2-cyclohexen-1-ol, and, 1-methyl-4-[1-methy-4-(1-methyletheyl)]-cis), at low concentrations (<1%). Both essential oils showed very interesting antibacterial activity against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853. However, they did not exert any antifungal activity against Botrytis cinerea. The minimum inhibitory concentration (MIC) for the bacterial strains, which were sensitive to the essential oil extracted from both infected and uninfected C. sinensis, was of 4 µL and 32 µL/mL, respectively.
Introduction
Citrus fruits peel is one of the parts of Citrus plant rich in secondary metabolites, such as essential oils (Dhanavade et al., Citation2011; Kamal et al., Citation2011). Citrus peel essential oil has become in recent years a matter of considerable economic importance and it is used in many countries for pharmaceuticals, cosmetics (perfumes), food, or agricultural uses (Bakkali et al., Citation2008; Ouis, Citation2015; Sqalli et al., Citation2009; Tajkarimi et al., Citation2010).
Chemically, essential oils of Citrus fruits contain a large amount of aromatic compounds, such as terpenes, phenols, methyl ethers, oxides, esters, and ketones (Bousbia, Citation2011; Isman, Citation2005). Various epidemiological studies have demonstrated a beneficial therapeutic effect of essential oils of Citrus fruits with reduced risk of some major diseases such as some types of cancer (Bousbia, Citation2011) and prevention against predators, insects, fungi, and pollinator attraction (Pichersky and Gershenzon, Citation2002; Unsicker et al., Citation2009). The chemical compositions of essential oils of Citrus fruits are influenced by both genetic factors such as species and cultivars, and other factors such as maturity at harvest, geographical area of harvest, storage conditions (Baltazari et al., Citation2019; Colombo et al., Citation2013; Oussou, Citation2009), virus, and phytoplasma (Möller, Citation2008; Salle, Citation2004).
Spiroplasma citri is a member of the class Mollicutes which are, phylogenetically related to positive Gram bacteria (Mycoplasmatales: Spiroplasmataceae). S. citri is a wall-less prokaryote with helical morphology. It is an important pathogen worldwide, causing CSD (Markham et al., Citation1974). It also infects many plant species other than Citrus fruits. S. citri affect the appearance and texture of infected fruits decreasing their acceptability to the consumer (Baltazari et al., Citation2019; Breton, Citation2009). CSD is characterized by deformed fruits and abnormally colored with aborted seeds (Breton, Citation2009; Bové and Garnier, Citation2000). The reduction in fruit weight, size, and number in severely symptomatic trees validates the concern that CSD is a significant constraint to production and marketability (Mello et al., Citation2010). Citrus fruits peels can be used in the extraction of several types of sugars, acids, and essential oils that find many applications (food industry, cosmetics, nutraceutical, biofuel, and material production industries) in the industrial field (Ledesma-Escobar and Luque de Castro, Citation2014).
The main objective of the current study was to determine the chemical quality and antimicrobial activity of Citrus fruits essential oil extracted from Citrus fruits affected by S. citri.
Materials and Methods
Sweet orange var: Washington navel Citrus sinensis (L) Osbeck was harvested from orchard of Baghlia, village of Boumerdes region, located at a latitude of 36.8169 and longitude of 3.8572 (GPS coordinates: 36°48ʹ56ʺ North, 3°51ʹ40ʺ East) Algeria. The experiment was conducted from May 2017 to June 2018. During sample collection, two types of orange fruit samples (healthy and infected with S. citri () were harvested when fully mature (big round oranges ready to fall off from tree). All fruit samples were cleaned and they were cut to remove seeds and pulp. The collected peels were cut into 3 mm thick pieces and then dried at 35 ± 2°C. The fully dried peel sample materials with 6–8% moisture content were stored under ambient conditions (28 ± 2°C). The dried Citrus fruit peels were subjected to hydrodistillation for 4 hours using a Clevenger apparatus (Zheljazkov et al., Citation2014).
Figure 1. Cross of orange Var. Washington navel (Citrus sinensis) with: (a) a normal columella, (b) a curved columella
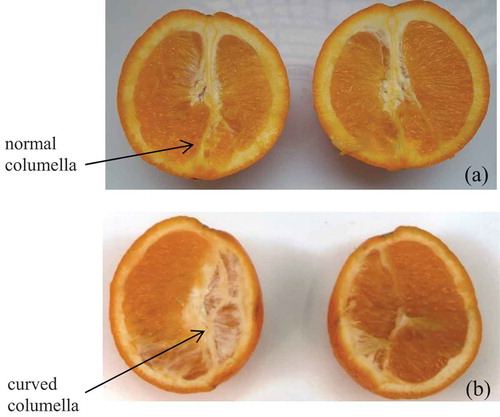
Figure 2. GC-MS chromatogram of Citrus essential oils. (a) EO extracted from healthy orange fruit, (b) EO extracted from orange fruits infected by the stubborn agent
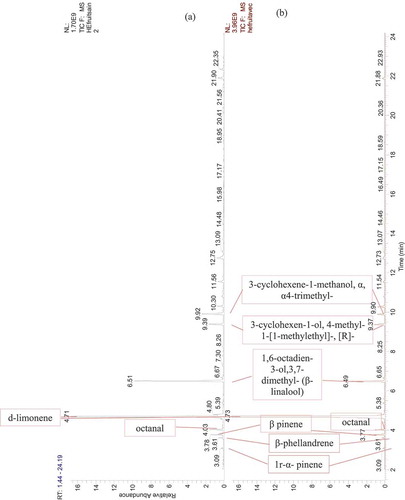
Figure 3. GC-MS chromatogram of Citrus essential oils. (a) EO extracted from healthy orange fruits, (b) EO extracted from orange fruits infected by the stubborn agent
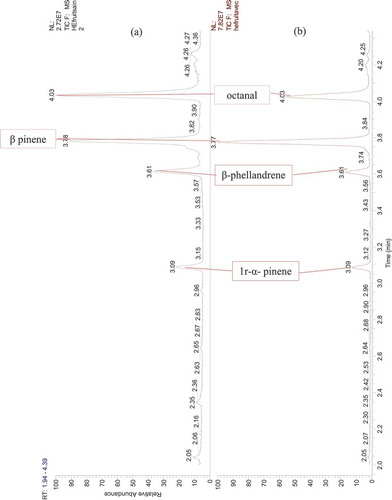
Figure 4. GC-MS chromatogram of Citrus essential oils. (a) EO extracted from healthy orange fruits, (b) EO extracted from orange fruits infected by the stubborn agent
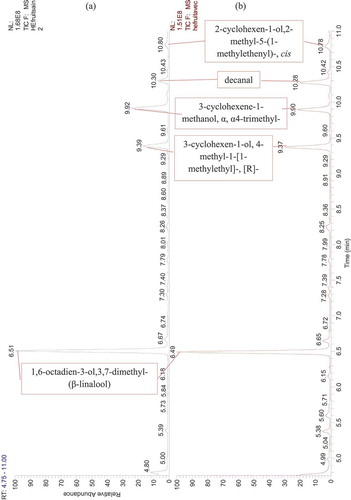
Figure 5. GC-MS chromatogram of Citrus essential oils. (a) EO extracted from healthy orange fruits, (b) EO extracted from orange fruits infected by the stubborn agent
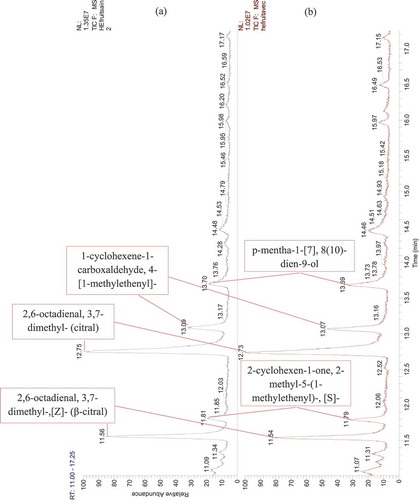
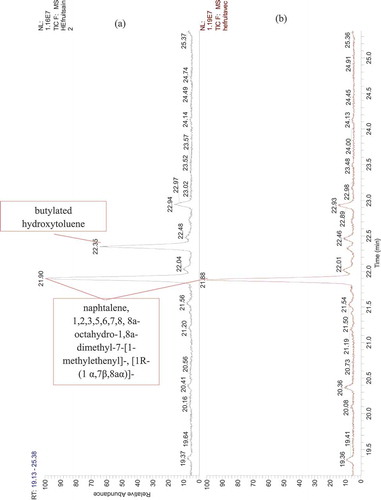
Figure 6. GC-MS chromatogram of Citrus essential oils. (a) EO extracted from healthy orange fruits, (b) EO extracted from orange fruits infected by the stubborn agent
The collected oil was dried over anhydrous sodium sulfate (Na2SO4) and stored in glass vials at 4°C until analysis.
Chemical analyzes were conducted to determine various chemical parameters namely density at 20°C, refractive index, acid index, ester index, and saponification index. Density at 20°C was carried out by means of a pycnometer, where an aliquot (1 mL) was taken for analysis in accordance with standards (NF T 75–111). Refractive index was measured by including air in the essential oil and then stored it at a constant temperature of 20°C (AFNOR, Citation1999, Citation2000). Acid index was determined using titrimetric analysis, where an aliquot 1 g was used in titration by the mixed solution of 0.1 M of potassium hydroxide (KOH) and ethanol which was prepared respecting (10/10 V/V) proportion (NF ISO 1242: 1999 (T 75–103); AFNOR, Citation2000). Ester index was determined using titrimetric analysis using 0.5 M of hydrochloric acid (HCl) (AFNOR, Citation2000). Saponification index was also determined using titrimetric analysis with potassium hydroxide (KOH).
Essential Oil Composition
Gas Chromatography-Mass Spectrometry (GC-MS) analyses were performed using an Agilent 6890 with selective mass detector 6890. A capillary column (30 m × 0.25 mm, 0.25 μm film thickness) was used. The analysis was carried out at 280°C, the oven at initial 50°C for 5 minutes. The injector temperature was set to 250°C. Helium was used as a carrier gas at a flow rate of 1.0 mL/minute.
Samples were prepared with ionization at 200°C and an electronic impact at 70 eV. Individual GC peaks and mass spectra were identified by database of mass spectra matching with standard available in the libraries like NIST 98 and by comparison of their mass spectrum.
Antimicrobial Activity
Antimicrobial activities of hydrodistilled essential oils, obtained from dried peels of both healthy and infected orange fruit, were evaluated against three bacterial strains (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923), and one fungi strain Botritys cinerea by agar disc diffusion method (Rahal, Citation2005). A suspension containing 108 CFU/mL of bacteria was spread on Mueller-Hinton agar and 104 CFU/mL of fungus was spread on Sabouraud agar. Sterile 6 mm paper disks were impregnated with 10 µL of a volatile oil in dimethylsulfoxide (DMSO 5% (V/V)) (essential oils obtained from dried peels of both healthy and infected orange fruit) and were deposited in agar plates. Negative controls were prepared with DMSO. Amoxicillin (AX, 25 µg), and cefoxitin (FOX, 30 μg) were used as positive standards to determine the sensibility of each bacterial strain tested. Amphotericin B was used as a positive standard to determine the sensibility of fungal strain tested. Antimicrobial activity was evaluated by measuring inhibition diameters against the tested strains. Tests were done in triplicate.
Determination of Minimum Inhibitory Concentration (MIC)
A broth microdilution assay was performed using the method of Oussou et al. (Citation2004) for the determination of the MIC.
Geometric dilutions ranging from 0.0625 to 32 µL/mL of the essential oils in DMSO were prepared in a (96-well) microliter plate. Then, 95 µL of BHIB and 100 µL of each dilution were added onto microplates. Finally, 5 µL of standardized microorganism suspensions were inoculated onto microplates. The same tests were performed simultaneously for the positive and negative control. The positive control is composed of 190 µL of BHIB supplemented with 5 µL of the antibiotic Gentamicin and 5 µL of the bacterial inoculum. The negative control is composed of 185 µL of BHIB supplemented with 10 µL of DMSO and 5 µL of the bacterial inoculum. The microplates were incubated at 37°C for 24 h. The MIC was calculated as the highest dilution showing complete inhibition of the tested strains.
Determination of Minimum Bactericidal Concentration (MBC)
5 µL of each dilution were taken from the wells showing complete absence of growth and were plotted onto agar plates (BHIB), and incubated at previously mentioned times and temperatures. The MBC was the concentration at which there was no microbial growth.
Results
The yield of essential oil from dried peels of orange fruits infected by stubborn was 0.65% w/v through hydrodistillation. This yield was higher than those obtained from healthy Citrus fruits 0.53% w/v. On the other hand, we could not observe any difference in color between the essential oils obtained from healthy trees and those obtained from infected trees; the two essential oils had the same golden yellow color.
The most interesting physico-chemical properties of both essential oils issued from infected and healthy orange fruits are presented in . These oils presented properties similar to those required by AFNOR (the French national organization for standardization and its International Organization for Standardization member body) standards. It was also noticed that the orange fruits infected with stubborn disease had a lower density of essential oils and the refractive index was 1.365, which was much lower than the reference refractive index which usually ranged from 1.471 to 1.474 ().
Table 1. Physico-chemical properties of Citrus sinensis essential oils
Instrumental Analysis
Twenty-three compounds were detected by GC-MS analysis from dried peel orange (-), but out of these which, six compounds could not be identified as their mass fragmentation showed low similarity (). The d-limonene was the major compound accounting for 95%. Some of the total compounds recorded. Some of the minor components included α-pinene, 1 r-α pinene and β-pinene, aldehydes (octanal, decanal), oxygenated monoterpenes (β-citronellal) in addition to cyclohexenes and β-phellandrene. All other compounds were found to be present in traces ().
Table 2. Chemical compounds in the percentage of both essential oils extracted from orange fruits (healthy and infected with the stubborn)
The present study has highlighted the production of some compounds (trans-p-mentha-2,8-dienol and 2-cyclohexen-1-ol,1-methyl-4-[1-methy-4-(1-methylethyl)]-cis) by infected oranges with stubborn (healthy oranges do not produce them).
According to the results of this study, essential oil extracted from healthy dried peel orange showed the highest activity especially on S. aureus ATCC 25923 (25.16 ± 2.75 mm) (). It was found that this activity was higher compared to that of the essential oil issued from infected oranges with S. citri (16.00 ± 2.64 mm). While essential oil extracted from infected oranges with S. citri showed the lowest activity on E. coli ATCC 25922 (7.00 ± 0.00 mm) and P. aeruginosa ATCC 27853 (6.00 ± 0.00 mm). However, no antifungal activity was observed; both essential oils did not show any effect on B. cinerea.
Table 3. Inhibition diameters (mm) observed under the effect of both extracted orange essential oils (n = 3)
For S. aureus ATCC 25923, the minimum inhibitory concentrations (MIC) of essential oil extracted from healthy fruits was about 4 µL/mL and minimum bactericidal concentration (MBC) of essential oil extracted from infected oranges with S. citri fruits was about 32 µL/mL
Discussion
The variability of the physico-chemical properties of the essential oil assessed can be attributed to the effect of the pathogen on photosynthesis, Such as fructose utilization by the spiroplasmas was postulated to deprive companion cells of fructose, thereby impairing sucrose loading in the sieve elements. Alternatively, preferential use of fructose was hypothesized to increase invertase activity, leading to glucose accumulation, and photosynthesis inhibition. Both model mechanisms may contribute to S. citri pathogenicity (Renaudin, Citation2006), and consequently, on the chemical composition of the essential oil and on their density.
Recently, a study analyzed the chemical composition of essential oils from some Citrus varieties. They found that the major constituents were α-pinene, sabinene, β-pinene, β-myrcene, d-limonene, linalool, m-cymene and 4-terpineol. Also, d-limonene was the most abundant compound (Bozkurt et al., Citation2017).
Our results were different from those of Bousbia (Citation2011), who had analyzed the essential oil composition by GC-MS of the same variety Washington navel and Tangelo, extracted by Microwave-Assisted Diffusion and Gravity, Hydrodistillation, and Cold Expression. Most studies had emphasized that the ratio of limonene is high in Citrus oils (Bozkurt et al., Citation2017; Hosni et al., Citation2010). Some variations were observed in the composition and percentage of constituents in the essential oils when compared to previous studies. These variations were probably due to climate change, soil, altitude, health status of the plant and the infected organ, and other conditions (M’hiri, Citation2015; Möller, Citation2008).
The current study showed that the stubborn agent induced the decrease of many compounds and allowed the availability of toxic compounds such as the decanal, which is an important organic compound of buckwheat odor. It is also considered as a uremic toxin that tends to accumulate in the blood either through dietary excess or through poor filtration by the kidneys. Most uremic toxins are metabolic waste products and are normally excreted in the urine or feces. But, chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney diseases, and cardiovascular diseases (Schulz et al., Citation2014; Young and Wu, Citation2012).
According to toxicity studies of cyclohexane in mice and rats exposed to 7000 ppm, clinical signs of hyperactivity and central nervous system stimulation were observed. The clinical signs of toxicity included: hyperactivity, circling, jumping/hopping, excessive grooming, kicking of rear legs, standing on hind legs, and occasional flipping behavior (Malley et al., Citation2000). Yasugi et al. (Citation1994) found that workers that were exposed to even low levels of cyclohexane via inhalation for 6 hours, have developed dimmed vision. The same study, carried out by Yuasa et al. (Citation1996) showed other effects such as sleepiness, dizziness, limb weakness, sensorial disturbances (hypoesthesia and paresthesia), and motor dysfunction of the median, ulnar, and peroneal nerves.
The obtained results confirmed that the low sensitivity of gram-negative bacteria was in relation to the presence of a lipopolysaccharidic (LPS) membrane that plays the role of a barrier against essential oils. Indeed, the LPS of the wall of gram-negative bacteria given them a hydrophilic character which makes their outer membrane impervious to most hydrophobic constituents of essential oils (Saei-Dehkordi et al., Citation2010).
Our results were in agreement with other researchers results, which had confirmed that the antimicrobial activity of certain essential oils could be attributed to the presence of minor compounds (terpenic alcohols (linalool, etc.)) present at low levels. According to El Malki et al. (Citation2018) and Aberchane et al. (Citation2003), the antimicrobial effectiveness of an essential oil was mainly due to the contents and the nature of the constituents that can act separately or in synergy.
The antibacterial effects of Citrus essential oil were explained mainly by the presence of terpenes C10 and C15, since hydroxyls of phenolic groupings were able to bind to the active sites of the target enzymes by hydrogen bonds. Terpenic alcohols (borneol, linalool, p-cymene-8-ol, α-terpineol, etc.) are known for their strong antimicrobial capacity and their solubility in water, which confers them with a high ability to penetrate the walls of the bacterial cells (Belletti et al., Citation2004). In our case, the inhibitory potential of our essential oils is mainly due to the presence of linalool and its derivatives known for their effectiveness against microbial agents according to Satrani et al. (Citation2004). A bactericidal action with a value of MIC = CMB = 4 µL/mL was recorded for the essential oil of the healthy sample. Our results coincided with those obtained by Oussalah et al. (Citation2007) who founded by working on 28 essential oils of which 24 were able to inhibit S. aureus . Among the 28 tested at concentrations ≤4 µL/mL, and that the latter was the most sensitive bacteria to the activity of essential oil. Also, Bozkurt et al. (Citation2017) investigated the antimicrobial activities of eight essential oils of some Citrus varieties on the six bacteria strains (E. coli, B. cereus, S. aureus, S. typhimurium, L. monocytogenes, and E. faecalis). All eight essential oils had inhibitory effects on the six bacteria tested, and Moro blood orange essential oils were most effective against E. coli, and S. typhimurium. It was noted that the development of an antifungal molecule is dependant on the ultra-structure of the fungal cell which presented three barriers: the chitinous cellular wall, the ergo-sterol membrane, and the core eucaryote, and on the antifungal molecules themselves, which can naturally generate resistance (Prasad and Kapoor, Citation2004). Generally, the chemical composition of essential oils must be taken in its entirety. Indeed, we must not neglect the synergistic effect between different constituents, including the minority constituents.
Orange peels are a rich source of nutritional ingredients (water, proteins, sugars, and minerals) and functional ingredients (essential oils, fibers, carotenoids, vitamin C, and phenolic compounds) (Singh et al., Citation2010). Orange peels can be used for the production of biofuels (ethanol), biogas (Lohrasbi et al., Citation2010), and biodegradable plastic (Byrne et al., Citation2004). These compounds can be extracted by the processing industries not only from the good oranges but also from the infected and unfit for consumption oranges.
The extraction of essential oils from orange peel generates interesting by-products such as concentrated and deterpenated oils, formulations of alcohol, and d-limonene (Kesterson and Braddock, Citation1976). This last can be used in a wide variety of applications as a component of synthetic resins, a solvent as a substitute of mineral solvents, and as a base for the synthesis of aromatic compounds (Di Giacomo et al., Citation1992).
Conclusion
In this study, essential oils from orange fruits infected and uninfected with the stubborn (Spiroplasma citri) were extracted by hydrodistillation and analyzed using GC-MS. The major constituents of orange essential oils were d-limonene, α-pinene, 1 r-α-pinene and β-pinene. The d-limonene was the most abundant compound. The yield of essential oil from dried peels of orange fruits infected by stubborn was higher than those obtained from healthy Citrus fruits. Also, the present study has highlighted the production of some compounds (trans-p-mentha-2,8-dienol and 2-cyclohexen-1-ol,1-methyl-4-[1-methy-4-(1-methylethyl)]-cis) by infected oranges with stubborn (healthy oranges do not produce them). Besides, healthy and infected orange essential oils had a potent inhibitory effect on S. aureus ATCC 25923. Nevertheless, both essential oils did not show any effect on B. cinerea. On the basis of our findings, it can be concluded that the essential oil extracted from fruits healthy and/or infected by the stubborn agent may be can be evaluated for other biological activities.
Acknowledgments
The authors wish to express their gratitude to Professor MAHMOUD Rachid from polytechnic school of Bordj El-Bahri (Algiers) for his help in the characterization of the essential oils.
References
- Aberchane, M., B. Satrani, M. Fechtal, and A. Chaouch. 2003. Effect of infection of Atlas cedar wood by Trametes pini and Ungulina officinalis on the chemical composition, antibacterial and antifungal activity of essential oils. Acta Bot. Gallica 150(2):223–229. doi: 10.1080/12538078.2003.10515420.
- AFNOR NF ISO 1242 (T 75-103). 1999. Essential oils: Determination of essential oils - Determination of the acid index - Reference method (approved September 5, 1994). Official Journal of February 23, 1999, N° 147: Notice relating to the approval and cancellation of standards. NOR: ECOI9910009V.
- AFNOR. 2000. Compendium of standards: Essential oils, p. 661–663. Vol. 2. Monographs relating to essential oils, Paris.
- Bakkali, F., S. Averbeck, D. Averbeck, and M. Idaomar. 2008. Biological effects of essential oils. Rev. Food. Chem. Toxicol. 46(2):446–475. doi: 10.1016/j.fct.2007.09.106.
- Baltazari, A., H.D. Mtui, M.W. Mwatawala, L.M. Chove, T. Msogoya, J. Samwel, and J. Subramanian. 2019. Effects of storage conditions, storage duration and post-harvest treatments on nutritional and sensory quality of orange (Citrus sinensis (L) Osbeck) fruits. Int. J. Fruit Sci. 14. doi: 10.1080/15538362.2019.1673278.
- Belletti, N., M. Nidagijimana, C. Sistro, M.E. Guerzoni, R. Lanciotti, and F. Gardini. 2004. Evaluation of the antimicrobial activity of Citrus essences on Saccharomyces cerevisiae. J. Agric. Food Chem. 52(23):6932–6938. doi: 10.1021/jf049444v.
- Bové, J. M., and M. Garnier 2000. Stubborn. pp. 48-50 in: Compendium of Citrus Diseases. L. W. Timmer, S. M. Garnsey, and J. H. Graham, (eds.) American Phytopathological Society Press, St. Paul, MN.
- Bousbia, N. 2011. Extraction of essential oils rich in antioxidants from natural products and agro-food co-products. Algiers, Process Science, Food Science. National School of Agronomy, PhD diss. p. 175.
- Bozkurt, T., O. Gülnaz, and Y.A. Kaçar. 2017. Chemical composition of the essential oils from some Citrus species and evaluation of the antimicrobial activity. IOSR J. Environ. Sci. Toxicol. Food Technol. 05. doi: 10.9790/2402-1110XXXXX.
- Breton, M. 2009. The plasmids pSci of Spiroplasma citri GII3: Characterization functional and role in transmission by the vector insect. Science, technology, health. N° 1673, Victor Segalen, University of Bordeaux II. PhD. diss. p. 174.
- Byrne, C.M., S.D. Allen, E.B. Lobkovsky, and G.W. Coates. 2004. Alternating copolymerization of limonene oxide and carbon dioxide. J. Am. Chem. Soc. 126:11404–11405. doi: 10.1021/ja0472580.
- Colombo, R.P., A.E. Martineza, A.F. Di Pardo, L.F. Bidondo, C. Van Baren, P. Di Leo Lira, and A.M. Godeas. 2013. Differential effects of two strains of Rhizophagus intraradices on dry biomass and essential oil yield and composition in Calamintha nepeta. Rev. Argent. Microbiol. 45:114–118. doi: 10.1016/s0325-7541(13)70010-5.
- Dhanavade, M.J., C.B. Jalkute, J.S. Ghosh, and K.D. Sonawane. 2011. Study antimicrobial activity of lemon (Citrus lemon L.) peel extract. Br. J. Pharmacol. Toxicol. 2(3):119–122.
- Di Giacomo, A., P. Rapisarda, and G. Safina. 1992. The Citrus derivatives industry, experimental station for Citrus fruit essences industry, p. 71. Reggio Calabria, Italy.
- El Malki, F., K. Eddaraji, R. Alloudane, H. Greche, H. Essalmani, and S. Barrijal. 2018. Antimicrobial activity of essentials oils extracted from leaves of native Moroccan plants against clinical bacterial isolates. Int. Arab. J. Antimicrob. Agents 8,1(4):2174–9094. doi: 10.3823/819.
- Franchomme, P., and D. Penoël. 2001. Aromatherapy exactly: Encyclopedia of the therapeutic use of aromatic extracts, (eds.) Roger, J., ISSBN: 2-87819-001-7, France, p. 512. .
- Garnero, J. 1996. Essential oils, engineering techniques K 345: Physico-chemical constants, Vol. N°: K2. France, 45: 10.
- Hosni, K., N. Zahed, R. Chrif, I. Abid, W. Medfei, M. Kallel, N.B. Brahim, and H. Sebei. 2010. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 123:1098–1104. doi: 10.1016/j.foodchem.2010.05.068.
- Isman, M.B. 2005. Problems and opportunities for the commercialization of botanical insecticides, p. 283–291. In: C. Regnault-Roger, B.J.R. Philogene, and C. Vincent (eds.). Biopesticides of plant origin. Lavoisier, Paris.
- Kamal, G.M., F. Anwar, A.I. Hussain, N. Sarri, and M.Y. Ashraf. 2011. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Food Res. Int. 18(4):1275–1282.
- Kesterson, J.W., and R.J. Braddock. 1976. By-products and specialty products of Florida Citrus. Fla. Agr. Exp. Sta. Tech. Bull. N° 784, p. 4–25. University of Florida, Gainesville.
- Ledesma-Escobar, C.A., and M.D. Luque de Castro. 2014. Towards a comprehensive exploitation of Citrus. Trends Food Sci. Technol. 39:63–75. doi: 10.1016/j.tifs.2014.07.002.
- Lohrasbi, M., M. Pourbafrani, C. Niklasson, and M.J. Taherzadeh. 2010. Process design and economic analysis of a Citrus waste biorefinery with biofuels and limonene as products. Bioresour. Technol. 101:7382–7388. doi: 10.1016/j.biortech.2010.04.078.
- M’hiri, N. 2015. Comparative study of the effect of extraction methods on phenols and the antioxidant activity of orange peel extracts «Half-blooded Maltese» and exploration of the corrosion inhibiting effect of carbon steel. I. N.A. Tunis, PhD. diss. p. 204.
- Malley, L.A., J.R. Bamberger, J.C. Stadler, G.S. Elliott, J.F. Hansen, T. Chiu, J.S. Grabowski, and K.L. Pavkov. 2000. Subchronic toxicity of cyclohexane in rats and mice by inhalation exposure. Drug. Chem. Toxicol. 23(4):513–537. doi: 10.1081/DCT-100101969.
- Markham, P.G., R. Townsend, M. Bar-Joseph, M.J. Daniels, and B.M. Meddins. 1974. Spiroplasmas are the causal agents of Citrus little leaf disease. Ann. Appl. Biol. 78:49–57. doi: 10.1111/j.1744-7348.1974.tb01484.x.
- Mello, A.F.S., M.E. Payton, R. Yokomi, and J. Fletcher. 2010. Effect of Citrus stubborn disease on navel orange production in a commercial orchard in California. J. Plant Pathol. 92(2):429–438.
- Möller, K. 2008. Still distillation, an art for everyone. (ed.), Editorial UNICO. ISBN-13: 978-8493055806, France, p. 152.
- Ouis, N. 2015. Chemical and biological study of coriander essential oils, fennel and parsley. Algeria, University of Oran, PhD. diss. p. 239.
- Oussalah, M., S. Caillet, L. Saucier, and M. Lacroix. 2007. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 18:414–420. doi: 10.1016/j.foodcont.2005.11.009.
- Oussou, K.R. 2009. Chemical study and biological activity of the essential oils of seven aromatic plants of the Ivorian pharmacopoeia. Abidjan, University of Cocody, PhD. diss. p. 241.
- Oussou, K.R., K. Coffi, G. Nathalie, S.K. Gerard, D. Mireille, T.N. Yao, F. Gilles, and C.H. Jean-Claude. 2004. Antibacterial activities of essential oils of three aromatic plants of Ivory Coast. Chem. Rep. 7:1081–1086.
- Pichersky, E., and J. Gershenzon. 2002. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 5:237–243. doi: 10.1016/S1369-5266(02)00251-0.
- Prasad, R., and K. Kapoor. 2004. Multi drug resistance in yeast Candida. Int. Rev. Cytol. 242:215–248.
- Rahal, K. 2005. Standardization of the antibiogramme in human medicine on a national scale according to recommendations of the World Health Organization (WHO). 4th ed. Ministry for Health, the Population and the Hospital Reform, Algeria, p. 95.
- Renaudin, J. 2006. Sugar metabolism and pathogenicity of Spiroplasma citri. INRA- University of Bordeaux 2, I.B.V.M. J. Plant Pathol. 88(2):129–139. (ed.). ETS Pisa.
- Saei-Dehkordi, S.S., H. Tajik, M. Moradi, and F. Khalighi-Sigaroodi. 2010. Chemical composition of essential oils in Zataria multiflora Boiss from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 48:1562–1567. doi: 10.1016/j.fct.2010.03.025.
- Salle, J.L. 2004. Essential oils, aromatherapy synthesis and introduction to sympathicotherapy, (eds.), Frison-Roche, 2nd. France, p. 220.
- Satrani, B., A. Farah, M. Fechtal, M. Talbi, and M.L. Bouamrani. 2004. Chemical composition and antibacterial and antifungal activity of the essential oil of Ammi visnaga (L.) Lam. from Morocco. Acta Bot. Gallica 151(1):65–71. doi: 10.1080/12538078.2004.10516021.
- Schulz, A.M., C. Terne, V. Jankowski, G. Cohen, M. Schaefer, F. Boehringer, M. Tepel, D. Kunkel, W. Zidek, and J. Jankowski. 2014. Modulation of NADPH oxidase activity by known uraemic retention solutes. Eur. J. Clin. Invest. 44(8):802–811. doi: 10.1111/eci.12297.
- Singh, A., V.K. Singh, and M.A. Quraishi. 2010. Effect of fruit extracts of some environmentally benign green corrosion inhibitors on corrosion of mild steel in hydrochloric acid solution. J. Mater. Environ. Sci. 1:162–174.
- Sqalli, H., A. El Ouarti, A. Farah, A. Ennabili, A. Haggoud, S. Ibnsouda, A. Houari, and M.H. Iraqui. 2009. Antibacterial activity of Thymus pallidus Batt. and determination of the chemical composition of its essential oil. Acta Bot. Gallica 156(2):303–310. doi: 10.1080/12538078.2009.10516160.
- Tajkarimi, M.M., S.A. Ibrahim, and D.O. Cliver. 2010. Antimicrobial herb and spice compound in food. Food Control 21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003.
- Unsicker, S.B., G. Kunert, and J. Gershenzon. 2009. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 12:479–485. doi: 10.1016/j.pbi.2009.04.001.
- Yasugi, T., T. Kawai, K. Mizunuma, R. Kishi, I. Harabuchi, J. Yuasa, T. Eguchi, R. Sugimoto, K. Seiji, and M. Ikeda. 1994. Exposure monitoring and health effect studies of workers occupationally exposed to cyclohexane vapor. Int. Arch. Occup. Environ. Health 65(5):343–350. doi: 10.1007/BF00405700.
- Young, G.H., and V.C. Wu. 2012. KLOTHO methylation is linked to uremic toxins and chronic kidney disease. Kidney Int. 81(7):611–612. doi: 10.1038/ki.2011.461.
- Yuasa, J., R. Kishi, T. Eguchi, I. Harabuchi, T. Kawai, M. Ikeda, R. Sugimoto, H. Matsumoto, and H. Miyake. 1996. Investigation on neurotoxicity of occupational exposure to cyclohexane: A neurophysiological study. Occup. Environ. Med. 53:174–179. doi: 10.1136/oem.53.3.174.
- Zheljazkov, V.D., T. Astatkie, and V. Schlegel. 2014. Hydrodistillation extraction time effect on essential oil yield, composition, and bioactivity of coriander oil. J. Oleo Sci. 63(9):857–865. doi: 10.5650/jos.ess14014.
