ABSTRACT
Pomegranate (Punica granatum) is one of the most popular fruits which is rich in nutrients and varied in cultivars including Hybrid, Wonderful (a sweet and delicious fruit with big size), and Seedless (sweet taste and very juicy). In this research, micropropagation of hybrid pomegranate cultivar (Wonderful and Seedless) was investigated. For the primary culture, the lateral and apical buds of pomegranate plant were used, and the plant materials were disinfected by Mercuric Chloride in the presence of Ascorbic Acid. Then disinfected explants cultivated to MS culture medium supplemented by 0.2 mg l−1 BAP and IBA 0.01 mgl −1 as an initial culture medium. For elongation and proliferation, BAP at five levels of 0, 0.2, 0.8, 1.4, and 2 mgl −1, and IBA at two levels of 0 and 0.01 mgl−1 were used in woody plant medium (WPM). To determine the most suitable rooting medium, IBA was used at concentrations of 0, 0.5, and 1 mgl−1 in different culture media containg MS, WPM, and DKW. Different soil compositions were also used for the adaptation of plantlets. The results showed that the best concentration for Mercuric Chloride disinfectant was 0.1% and all the disinfection treatment times were appropriate. Moreover, woody plant medium with 1.4 mg l−1 of BAP without IBA was the best culture medium for proliferation. In rooting stage, using woody plant medium was detected as the most suitable too. In the adaptation stage, using Vermicompost, Perlite, and Cocopeat (1: 1: 1) was observed to be the best treatment.
Introduction
Pomegranate tree is one of the most important trees grown in the subtropical regions of the world, belong to the family Punicaceae. The genus Punica is the only genus of this family that has two species, P.granatum and P.protopunica (Soukhak et al., Citation2011). The Pomegranate (Punica granatum) is one of the endemic plants of Iran. Considering appropriate conditions for pomegranate production in Iran, the possibility of its expansion in arid and semi-arid regions, its adaptation to Iran’s climate, and high quality of this product in the country, it is commonly produced in most parts of the country (Moatri, Citation2015). In addition to food consumption, Pomegranate also has medicinal and ornamental consumptions (Maghsoudi, Citation2007). The fruit and extract of pomegranate are good sources of sugars, vitamin C, vitamin B, pantothenic acid, potassium, antioxidant polyphenols, and also iron (Kanwar et al., Citation2010). Pomegranates reproduce in sexual and non-sexual methods. In sexual reproduction, the new plants will not be similar to their parent plant due to the heterozygocity (Saqafi and Shahsavand Herati, Citation2011). In the non-sexual pomegranate propagation can be done using hardwood cuttings, offshoots, layering, and grafting, each of which has its own drawbacks (Hore and Sen, Citation1995).
For example, in the layering method with a high rooting rate, the number of suitable branches is limited and the space around the tree is occupied for one year (Al Wasel et al., Citation1999). In order to raise a rootstock with desirable yield and traits such as resistance to drought, salinity, pests, and diseases, in some cases, nonsexual reproduction methods are needed, among which cutting propagation method is more popular. However, this method has problems such as limited raw materials for preparing cuttings from a selected cultivar. In order for the optimal rooting process, a cutting requires about 12 months (Anonymous, Citation1998), during which there is a low probability of ensuring that the plant is healthy and free from diseases (Kanwar and Rachna, Citation2004).
Micropropagation is a branch of applied science in the plant biotechnology, which recently has undergone a remarkable transformation and plays a decisive role in the propagation and breeding of species of trees, shrubs, and herbal plants. The advantages such as rapid propagation, genetic stability, and no limitation of time and space havemade this trend particularly important (Acikgoz et al., Citation2019; Guney, Citation2019). Today, studies have been done on using in-vitro culture techniques to propagate pomegranate. In this regard, in-vitro reproduction of pomegranate is a reliable and fast method for propagation of pomegranate cultivars. This method not only reproduces healthy and contamination free plants but also can achieve the required number of plants at any time.
Overcoming the problems of propagation via stem cuttings, this method is thus able to produce true to type plants (Moatri, Citation2015).
Most studies on micropropagation of pomegranate are related to the foreign cultivars, and limited research has been done on Iranian pomegranate cultivars. Murashige and Skoog (MS) culture medium was used by most researchers for pomegranate propagation (Patil et al., Citation2011). Woody plant medium (WPM) has been used for proliferation of pomegranate cultivars (Valizadeh et al., Citation2013). Most researchers have used Benzyl Adenine (BA) cytokines for pomegranate proliferation besides Zeatin, Kinetin, and Thiadiazuron (TDZ) (Naik et al., Citation1999). The appropriate amount of growth regulators can vary according to the species and pomegranate cultivar. For example, Singh et al. (Citation2014) research findings showed that the best result for pomegranate regeneration was obtained in MS medium culture containing 0.2–2 mg l−1 BAP and 0.1–1 mg l−1 Naphthalene Acetic Acid (NAA).
Moatri (Citation2015) investigated micropropagation of Robab cultivar of pomegranate under in-vitro conditions and found that the best proliferation rate of 5.66 with the maximum length of 3.83 cm was achieved in the culture medium containing 2 mg l−1 N6-Benzyladenine (BA) and 1 mg l−1 Naphthalene Acetic Acid (NAA). The highest rooting of shoots was observed by using MS medium containing 2 mg l−1 IBA.
Valizadeh et al. (Citation2013) also examined the shoot tip and node explants in two culture media, woody plant medium (WPM) and MS, containing various growth regulators for the in-vitro propagation of the pomegranate Malas-Yazdi. They concluded that the best culture medium was for WPM medium with the hormonal concentration of 9.2 μM Kinetin and 0.54 mg l−1 NAA.
The research conducted by Patil et al. (Citation2011) for investigating the micropropagation of Bhagava pomegranate cultivar resulted in the highest proliferation rate at the MS culture medium composed of 0.1 mg l−1 BA, 0.9 mg l−1 NAA, 1mgl−1 AgNO3, and 30 mg l−1 Adenine sulfate. The maximum leaf number was found in the proliferation medium containing 0.4 mg l−1 BAP and 0.3 mg l−1 NAA.
In this research, due to low propagation and disease development in the pomegranate rootstock and cultivar by asexual methods as well as the increasing demand for it, the micropropagation method was used for propagation of this tree. The best culture medium, the best concentration of vegetative growth regulators in both proliferation and rooting stages, and the most suitable soil for adaptation and acclimatization of pomegranate hybrid cultivar (Wonderful X Seedless), were also investigated.
It should be noted, the ‘Wonderful’ cultivar was discovered in Florida and brought to California in 1896. This is the primary cultivar of commerce in the United States. It is also grown in western Europe, Israel, and Chile (Sepulveda et al., Citation2000). The fruit is very large, dark red, with medium-thick rind; juicy, medium-hard seeds(Karp, Citation2006) and seedless cultivar having spherical fruits, medium fruit size, medium skin thickness and green color of the fruit, sweet taste, and very juicy (Sarkhosh et al., Citation2009)
Materials and Methods
This research was carried out in the tissue culture laboratory of Faculty of Agriculture and Natural Resources, the University of Yazd in the fall of 2018. Plant material were taken from Hybrid, Wonderful, and Seedless pomegranate cultivars, which were grown in the original orchard.
For primary cultivation, the material plant were first washed with urban water to reduce surface contamination, then rinsed under running water for half an hour. After removing the leaves, the branches were cut into pieces including several nodes and then entered the disinfection stage. Five disinfection treatments, including 0.1% Mercuric Chloride, were applied at five different times (3, 3.5, 4, 4.5, and 5 min). The explants were washed three times of 3 minutes with sterile distilled water. The solution of 0.1 g ML−100 Ascorbic Acid was added to all disinfection agents in order to reduce the process of browning.
Disinfected explants were cultured in MS culture medium Murashige and Skooge (Citation1962) with 0.01 mg l−1 IBA and 0.2 mg l−1 BAP for the initial establishment. In this medium, 30 gl−1 Sucrose was used. The culture medium pH was adjusted to 5.7 and then 7 gl−1 Agar was added to the environment. The culture media were autoclaved at a pressure of 1.2–1.3 atm and a temperature of 121°C. After culture medium autoclaving, they were allowed to cool and the mediums were allowed to solidify. Then, the explants were cultured in the media.
After establishment stage (4 weeks after the initial culture), disinfected explants were selected for the proliferation stage. At this stage, WPM culture medium at concentrations of 0 and 0.01 mg L−1 IBA and concentrations of 0, 0.2, 0.8, 1.4, and 2 mg l−1 BAP were used to cultivate the explants. The amount of Agar, Sucrose, and pH were the same as the establishment stage. Then, obtained explant from establishment stage transferred to the proliferation media.
After 4 weeks, the proliferated shoots with more than 2 cm length were taken from the main branch and transferred to the culture media Driver and Kuniyaki Walnut (DKW), MS, and WPM containing 0, 0.5, and 1 mg l−1 IBA for rooting. The Agar, Sucrose, and pH of this stage were similar to the establishment and proliferation media. After transferring plant to rooting media, explants were placed in the dark for one week.
After each stage plants subculture, explants were transferred to the growth chamber with a light intensity of 3000 lux, a photoperiod of 16 h light and 8 h darkness.
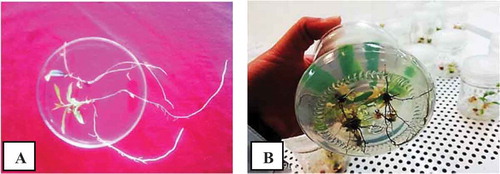
After rooting, plantlets were washed with distilled water and transferred to pots of sterilized soil media containing a mixture of vermicompost, perlite, and cocopeat (1: 1: 1), a mixture of cocopeat and perlite (1: 1), a mixture of Cocopeat and perlite (2: 1), and perlite. All pots were covered with plastic caps afterward. In the first days, irrigation was done in a culture medium at one-third concentration of MS and free of agar, Sucrose and vitamins. This concentration was gradually declined while allowing the plastic cap to be unscrewed from the pots so as to acclimatize the plantlets to the field conditions ().
In this research, the number of contaminated explants, brownish explants, new branches, and produced roots, as well as shoot elongation rate and root length were studied. The length and diameter of the shoot were measured using the ruler and digital caliper. The experiment was carried out in a completely randomized design with 5 replications in the disinfection stage, 12 replications in the proliferation stage, 5 replications in the rooting stage, and 4 replications in the adaptation stage. The obtained data were analyzed via SPSS software, the significance of the treatment effects were examined using the analysis of variance, the means were grouped using Duncan multiple range test at a confidence level of 95%, and the graphs were drawn by the use of Excel software.
Results and Discussion
Results from the Disinfection Stage
The results of variance analysis showed that the time of disinfection with Mercuric Chloride had no significant effect on the survival rate of the explants and the production amount of phenol in Hybrid, Wonderful, and Seedless pomegranate cultivars at 5% significance level. Since all the explants obtained from greenhouse that produced via Tissue culture, due to this only one infected case was observed and all the treated ones were survived. Although ANOVA test resulted in no significant difference between the treatments, the Duncan test classified the means of studied treatments in terms of phenolic production and browning phenomenon. The results of the Duncan test classification indicated that the best disinfection time was 3 min associated with the least browning ().
Figure 1. Mean comparison of the effect of disinfection time with 0.1% mercuric chloride on the browning rate in pomegranate explants of hybrid, wonderful, and seedless cultivars under in-vitro conditions (columns with common letters do not statistically differ from each other at 5% significance level based on the Duncan multiple range test)
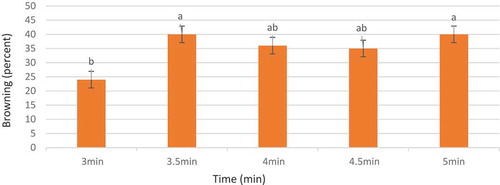
The loss of a considerable number of explants in the early stages of in-vitro culture, especially due to contamination and explants browning, is a problem most likely to occur in wooden cultivars (Green and Muir, Citation1979). In pomegranate cultivar, achieving noncontaminated explants is one of the main problems. Therefore, some infections treated with 0.1% Mercuric Chloride during different infection times were controlled by doing an infection experiment. The best time to reduce contamination and browning of the explants is when the plant is in its full growth since the plant quickly begins to regenerate in the culture medium. It reduces the potential for browning and makes positive cuts in contamination. The results of this study are similar with the results of the study done by Singh et al. (Citation2014)concerning the micropropagation of Ganesh pomegranate cultivar as well as the results of the work of Jafari Mofid Abadi (Citation2001)related to the Spruce explants.
Results from the Proliferation Stage
The results of variance analysis showed that the concentration of BAP growth regulator had a significant effect on the shoot number of Hybrid, Wonderful, and Seedless pomegranate cultivars at 5% significance level; whereas, the effect of IBA on the number of new branches was not significant. Also, there was a significant interactive effect of BAP and IBA on the number of new branches at 5% significance level. The effect of different concentrations of IBA, BAP, and their interactions on elongation was not significant.
The results of mean comparison of the effect of different concentrations of BAP on the shoot number created in in-vitro explants of Hybrid, Wonderful, and Seedless pomegranate cultivars showed that the WPM culture medium with 1.4 mg l−1 BAP and without IBA growth regulator generated the highest number (2.25) of shoots and the control culture medium (without any growth regulator) had the lowest shoot number (). It should be noted that there was no statistically significant difference between the concentrations of 0.2, 1.4, and 2 mg l−1 BAP without IBA and 0.2 mg l−1 BAP with 0.01 mg l−1 IBA ( and ).
Figure 2. Mean comparison of the interactions between the concentration of BAP and IBA growth regulators on the number of new branches created in pomegranate cultivars of hybrid, wonderful, and seedless under in-vitro conditions (columns with common letters do not statistically differ from each other at 5% significance level based on the Duncan multiple range test)
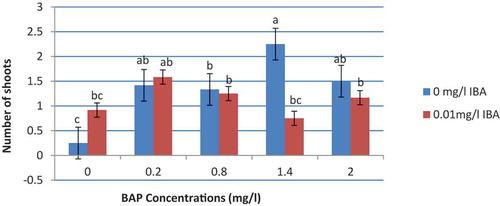
Figure 3. A: proliferation of hybrid, wonderful, and seedless pomegranate cultivars in a culture medium containing 1.4 mg L−1 BAP B: Proliferation treatments of hybrid, wonderful, and seedless pomegranate plants in the growth chamber

Al-Wasel (Citation1999) in his research on in-vitro propagation of Al Balahi pomegranate cultivar and Soni and Kanwar (Citation2016) in their research on the in-vitro propagation of Kandhari Kabuli pomegranate cultivar showed that the highest proliferation rate occurs at 2 mg l−1 concentration of BA growth regulator. It seems that Benzyl Adenine growth regulator plays an important role in increasing buds and consequently proliferation rate. Adding cytokinins to the culture medium is essential for shoot proliferation (Al-Wasel, Citation1999). Hedayat. and Azadi (Citation2002) reported that cytokinins accelerate cell division, shoot formation, and morphogenesis. Cytokinins are also used to overcome apical dominance defects in the buds. The use of auxin as an addition to cytokinin in a culture medium is essential for the shoot proliferation of some Nana cultivars, whereas some other cultivars, such as Ganesh, do not require auxin for proliferation (Naik et al., Citation1999).
The results obtained from our studied cultivars illustrated that the addition of cytokines to the culture medium is necessary for proliferation, which is not similar with the results obtained from a different cultivar studied by Naik et al. (Citation1999). The appropriate amount of growth regulators can vary according to the pomegranate species and cultivars. The findings of Singh et al. (Citation2014) indicated that the best result for pomegranate regeneration was observed for MS culture medium containing 0.2–2 mg l−1 BAP and 0.1–1 mg l−1 NAA, which is similar to our study results.
Results from the Rooting Stage
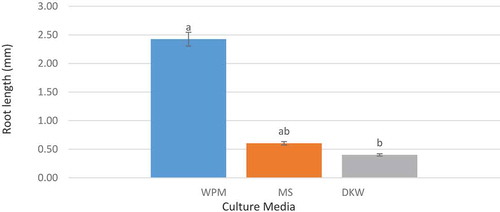
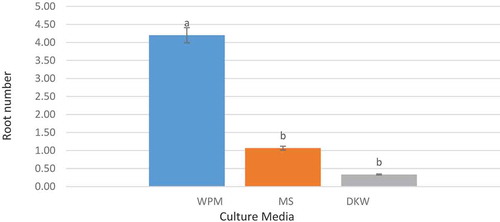
The results of the variance analysis of the effect of different culture media on rooting suggested that this treatment had a significant effect on the root number and root length traits at 5% significance level. On the contrary, the effect of different concentrations of IBA growth regulator and the interactive effects of this regulator concentration and the studied culture media at 5% significance level were not significant. The results of mean comparison of the effect of different culture media on the root number and length, indicated that the most effective medium was WPM culture medium showing a significant difference with other treatments at 5% significance level ( and ). Naphthalene Acetic Acid (NAA) and Indole Butyric Acid (IBA) are the most popular auxins used to induce rooting in in-vitro regenerated shoots of pomegranate.
Figure 4. The effect of culture media on root number (the columns with common letters do not statistically differ from each other at 5% significance level based on the Duncan multiple range test)
Results from the Adaptation Stage
The results of the variance analysis of the effect of different treatments of soil composition showed no significant effect on elongation, leaf number, and stem diameter at 5% significance level in the adaptation stage (). However, observations suggested the soil composition of vermicompost, perlite, and cocopeat as the treatment with the highest elongation, leaf number, and stem diameter and perlite composition as the treatment with the lowest growth (). About four weeks after plant hardening, no problem with survival was observed.
Table 1. Analysis of variance of different soil compositions on the adaptability of pomegranate plantlets (hybrid, wonderful, and seedless)
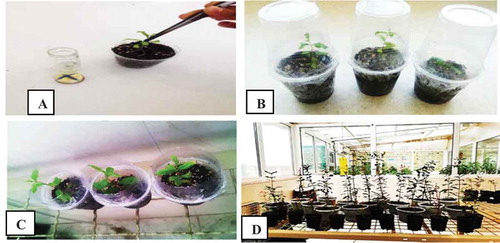
Figure 7. (a) Rooted pomegranate plantlet (hybrid, wonderful, and seedless) transfer into the soil. (b) Adaptation of transferred plants to the soil using plastic caps. (c)Acclimatization of hybrid, wonderful, and seedless pomegranate cultivars into different soil compositions. (d) Hybrid, wonderful, and seedless pomegranate growth and transfer to larger pots
Conclusion
In this research, the results of in-vitro propagation of pomegranate cultivars (Hybrid, Wonderful, and Seedless) through apical and lateral buds indicated that 0.1% Mercuric Chloride disinfectant is effective for the explant disinfection, and the best culture medium for this plant micropropagation was WPM medium with 1.4 mg l−1 BAP and without IBA growth regulators. It can also be discussed that plant response to different concentrations of cytokines and auxins for the proliferation of pomegranate cultivars is highly dependent on the level of internal hormones and cultivar types.
The best rooting was obtained in WPM culture medium that had a significant difference with other treatments. Moreover, the statistical results showed, different soil compositions could be used for the adaptation of this cultivar. However, the most effective soil composition was a mixture of vermicompost, perlite, and cocopeat with (1: 1: 1) ratio.
Additional information
Funding
References
- Acikgoz, M.A., S.M. Kara, A. Aygun, M.M. Ozcan, and E. Bati Ay. 2019. Effects of methyl jasmonate and salicylic acid on the production of camphor and phenolic compounds in cell suspension culture of endemic Turkish yarrow (Achillea gypsicola) species. Turk. J. Agric. For. 43:351–359. doi: 10.3906/tar-1809-54.
- Al-Wasel, A.S. 1999. In vitro clonal propagation of” AL-Belehi” pomegranate (Punica granatum L.). IN vitro cellular and developmental biology animal. 34:1031.
- Anonymous. 1998. The wealth of India, dictionary of Indian raw materials and industrial products. Vol. III. Council of Scientific and Industrial Research(CSIR), New Delhi, India, p. 317–324.
- Bonyanpour, A., and M. Khosh-Khui. 2013. Callus induction and plant regeneration in punica granatum l.? Nana’from leaf explants. J. Central Eur. Agric. 14(3):75–83. doi: 10.5513/JCEA01/14.3.1285.
- El-Agamy, S.Z., R.A. Mostafa, M.M. Shaaban, and M.T. El-Mahdy. 2009. In vitro propagation of Manfalouty and Nab El-gamal pomegranate cultivars. Res. J. Agric. Biol. Sci. 5:1169–1175.
- Green, J.F., and R.M. Muir. 1979. An analysis of the role of potassium in the growth effects of cytokinin, light and abscisic acid on cotyledon expansion. Physiol. Plant. 46(1):19–24. doi: 10.1111/j.1399-3054.1979.tb03179.x.
- Guney, M. 2019. Development of an in vitro micropropagation protocol for Myrobalan 29C rootstock. Turk. J. Agric. For. 43:569–575.
- Hedayat., B., and P. Azadi. 2002. Plant tissue culture: Techniques and experiments (compiled by Roberta H. Smith). First. Mashhad University Press, Iran. p. 154.
- Hore, J.K., and S.K. Sen. 1995. Effect of non-auxinic compounds and indole-3-butyric acid on root formation in stem cuttings of pomegranate. Madras Agric. J. 82:403–405.
- Jafari Mofid Abadi, A and Tabaee Aghdaee, R. 2001, Plant biotechnology (Methods and Applications).Research Institute of Forests and Rangelands.
- Kanwar, K., J. Joseph, and R. Deepika. 2010. Comparison of in vitro regeneration pathways in Punica granatum L. Plant Cell Tissue Organ Cult. (PCTOC) 100(2):199–207. doi: 10.1007/s11240-009-9637-4.
- Kanwar, K., and K.A. Rachna, 2004. In vitro propagation of wild pomegranate (Punica granatum L.). National Seminar on IPR of Horticultural Crops, Solan, India, 12–13 October.
- Karp, D. 2006. The pomegranate: For one and all fruit gardener. CRFG publication, 38:8–12.
- Maghsoudi, S. 2007. pomegranate therapy. Agricultural Science Publishing, Iran, p. 87.
- Moatri, F., 2015. Pomegranate propagation, Third National Conference of Student Societies of Agricultural and Natural Resources, Karaj, Agricultural and Natural Resources Campus of Tehran University, Iran.
- Murashige T and Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures .Physiol. Plant.15:473-97.
- Naik, S.K., S. Pattnaik, and P.K. Chand. 1999. In vitro propagation of pomegranate (Punica granatum L. cv. Ganesh) through axillary shoot proliferation from nodal segments of mature tree. Sci. Hortic. 79(3–4):175–183. doi: 10.1016/S0304-4238(98)00218-0.
- Patil, V.M., G.A. Dhande, D.M. Thigale, and J.C. Rajput. 2011. Micropropagation of pomegranate (Punica granatum L.) ‘Bhagava’cultivar from nodal explant. Afr. J. Biotechnol. 10(79):18130–18136.
- Saqafi, M., and E. Shahsavand Herati, 2011. Study of antioxidant, anti-cancer and anti-inflammatory of pomegranate and properties medicinal compounds, National Congress of Pomegranate, Iran, 227–232.
- Sarkhosh, A., Z. Zamani, R. Fatahi, and H. Ranjbar. 2009. Evaluation of genetic diversity among Iranian soft-seed pomegranate accessions by fruit characteristics and RAPD markers. Sci. Hortic. 121:313–319. doi: 10.1016/j.scienta.2009.02.024.
- Sepulveda, E., Galletti, L., Sanaz, C. & Tapia, M. 2000. Minimal Processing of Pomegranate var.Wonderful Options Mediterraneenns Ser.A 42 237-242
- Singh, J., S. Gupta, and P. Khoshe. 2014. In vitro regenration of pomegranate (Punica granatum L.) from nodal explant. Int. J. Adv. Pharm. Biol. Chem. 3(3):734–736.
- Soni, M., and K. Kanwar. 2016. Rejuvenation influences indirect organogenesis from leaf explants of Pomegranate (Punica granatum L.) ‘Kandhari Kabuli’. J. Hortic. Sci. Biotechnol. 91(1):93–99. doi: 10.1080/14620316.2015.1110998.
- Soukhak, F., A. Khalighi, and S.A. Ghaemmaghami. 2011. Study of direct adventitious shoot regeneration in pomegranate (Punica granatum cv. Malas Saveh) through cotyledonary explants. Int. J. Agric. Sci. Res. 2(3):19–26.
- Valizadeh, B.K., A. Ershadi, and M. Tohidfar. 2013. In vitro propagation of pomegranate (Punica granatum L.) Cv.‘Males Yazdi’. Albanian J. Agric. Sci. 12(1):1–5.