ABSTRACT
In this study, physicochemical changes, phenolics profile, and antioxidant potential in three grape varieties during berry development were assessed. The results showed a significant difference in stage-wise accumulation of phenolics. The fully matured Bangalore blue grapes showed the high total phenolic content (1484.87 mg GAE/100 g) followed by Krishna Sharad (1361.42 mg GAE/100 g) and Dilkhush variety (1108.61 mg GAE/100 g). The Krishna Sharad grapes contain the high anthocyanin content (1622.7 mg/kg) at full maturity. A similar pattern was observed for flavonoid content. The UPLC-PDA analysis showed that chlorogenic acid, catechin, epicatechin, and hesperidin were present in significant amount in Bangalore blue grape variety. In Krishna Sharad, gallic acid, chlorogenic acid, hesperidin, catechin, hesperetin were significant phenolics. Dilkhush grapes were rich in catechin, chlorogenic acid, and caffeic acid. The Bangalore blue and Krishna Sharad showed high antioxidant potential at full maturity in DPPH and ABTS activity. Collectively, the results suggest that berry development stages have an impact on its antioxidant properties, bioactive content, and berry composition.
Introduction
Table grape is one of the most consumed fruits in the world, is gaining an important place in the export market; the majority of these table grapes used as fresh (Rolle et al., Citation2013). Grapes are rich in polyphenols, anthocyanins, phenolic acids, and ascorbic acid. The phenols represent the third most abundant constituent in grapes and wines after carbohydrates and fruit acids (Alonso Borbalan et al., Citation2003). In grapes, a large number of polyphenols and flavonoids are reported, such as catechin, epicatechin, caffeic acid, p-coumaric acid, cinnamic acid, ferulic acid, vanillic acid, quercetin and proanthocyanidins (Liu et al., Citation2016). Other bioactives include stilbenes, are non-flavonoids phenolic substance available in low quantities in the human diet. Stilbenes are present in both colored table and white grapes and resveratrol (3, 5, ‘4’-trihydroxy-trans-stilbene) is the primary representative. Resveratrol has gained much attention as a functional ingredient and used in medicines and health products (Alberdi et al., Citation2013).
The importance of phenolic compounds in human health-related through their multiple biological effects such as antioxidant activity, antimutagenic and anticarcinogenic activities (Derradji-Benmeziane et al., Citation2014). Baiano and Terracone (Citation2011) reported that phenolic compounds also contribute to the sensory quality of foods (color, astringency, and bitterness). Most of the studies on grapes pertain to grape seeds, skin, red wine, or its components. Ramchandani et al. (Citation2010) studied the bioactivity of crude polyphenolic fractions of a whole, pulp with skin and seeds in four Indian grape varieties. They have quantified the six major phenolic compounds, including catechin, epicatechin, procyanidin, and resveratrol in grape pulp and skin. Further, showed the antioxidants richness in Indian grapes using lipid peroxidation, DPPH assay. Apart from human health benefits, the accumulation of polyphenols plays a vital role in the growth and propagation of grape plants and protects tissue damage. The phenols as pre-infection inhibitors, providing plants an essential resistance against pathogenic microorganisms (Satisha et al., Citation2008). The composition of berries and accumulation of bioactive principles are affected by various factors such as grape cultivar, ripening time, climate, soil, and location of growth. However, they much depend on cultivar (Baiano and Terracone, Citation2011). Therefore, identification of the critical stage of berry maturity for picking that provides significant health benefits could be unusual to reap the vital bioactive principles. Given the above, the present study aims to investigate physicochemical properties, phenolic profile pattern, and antioxidant capacities of three distinctive varieties of Indian grapes (colored and white Indian Grapes (Vitis vinifera L.)). The study also aims to identify the most critical berry maturity stage that provides the highest quantities of these important bioactive principles for significant health benefits.
Material and Methods
Details of the Experimental Site and the Microclimatic Characteristics of the Vineyards
The experimental site of grape vineyards of all three grape(Vitis vinifera L.) varieties, Bangalore blue (black, seeded), Anab-e-Shahi (White Seeded) and Krishna Sharad (Black, Seedless) are located in the mild tropical region of agro-climatic zone, lying between 10 and 15 °N latitude covering Bangalore and Kolar Districts of state Karnataka, India. The maximum temperature in a year seldom 28–32°C, the minimum is 12°C. Cool nights and hot days favorable microclimate during ripening that favors the color development in colored grapes (Bangalore blue (black, seeded), and Krishna Sharad (Black, Seedless). Three Vitis vinifera L. grape varieties, Bangalore blue (seeded colored), Krishna Sharad (selection from Sarad seedless colored), and Dilkhush (white seeded, a clone of Anab-e-shahi) selected in this study. The Bangalore blue and Krishna Sharad grapes were commercially cultivated in the grape vineyards for the principal purpose of making fruit juice beverages and less for table purpose, while Dilkhush grapes commercially grown for table purpose. Currently, Bangalore blue (black, seeded) occupies 15% of the total grape area, followed by Anab-e-Shahi (White Seeded-15%) and Krishna Sharad (Black, Seedless) by 5% of the total grape area in this region.
Details of the Main Agronomical Practices of the Vineyards
The main agronomical practices that were used include propagation by hardwood stem cuttings obtained from October fruit-forward pruning, four noded cuttings from well mature canes on proven vines are made. Dog ridge systems are employed for these varieties as the soil is saline in nature. Field grown vines of these three varieties grafted on the dog ridge rootstock were selected. Harvesting of berries at different stages of cluster development followed manually by using harvesting tools (). Each time of sampling, grapes are harvested during the cool time of the day. Harvested grapes are trimmed graded and packed. The corrugated cardboard boxes along with SO2 grape guard pads were used as an in packing material to check the postharvest diseases and newspapers as cushioning material during transit and storage conditions. These boxes were stored at 0 ± 1°C; 90–95% RH).
Chemicals
The phenolic standards (Gallic acid, protocatechuic acid, vanillic acid, chlorogenic acid, catechin hydrate, syringic acid, procyanidin B2, epicatechin, caffeic acid, ferulic acid, p-coumaric acid, sinapic acid, trans-resveratrol, hesperidin, hesperetin, and quercetin), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid) and 2, 6-dichlorophenolindophenol were purchased from Sigma Aldrich, Bangalore (India). All other reagents -were of the analytical grade for the experiments obtained from SRL Pvt. Ltd (Bangalore, India). Solvents (Ethanol, ethyl acetate, MS grade methanol, and acetonitrile) used for extraction and analysis of samples were procured from Fisher Scientific, Bangalore. Water used was of Millipore quality.
Sampling Procedure
The total soluble solids content, acidity, and visible berry color development considered as reliable maturity indices for harvest. Each time, the grape berry samples (75 kg) from each yvariety were periodically collected during berry development and maturity stages. A randomized complete block (RCB) design with three replications (n = 3) from all the three varieties from Devenhalli grapevine orchards, (Bangalore, India) as per the following schedule: Bangalore Blue (25, 55, 75, 102 days after post-anthesis (DAPA), Krishna Sharad (25, 55, 75, 105 DAPA) and the Dilkhush (20, 48, 69, 98 DAPA). Each block of grape varieties consisting of three vine panels, with five plants in each vine panel consisting of 100 m length. Total of fifteen plants per block of each variety. Each time twenty-five kg from each of three blocks of each variety was analyzed for various physicochemical component composition, bioactive component profiles, and antioxidant properties. Each time, the samples harvested from these grapes were packed in corrugated fiber boxes and immediately transported to the laboratory and were kept in the cold room (4 ± 2°C) for 10–12 h to remove field heat and then, samples were water washed, grounded and packed in low-density polypropylene (LDPE) bags and finally stored at −20°C for further physicochemical analysis.
Total Soluble Solids and Titrable Acidity
The total soluble solids (TSS) of the homogenate sample was measured by using a Hand Refractometer (Model: Erma, Tokyo) and the results expressed in °Brix. The acidity of the homogenate sample was estimated as per the method (AOAC, Citation1995) and reported the values as in % anhydrous tartaric acid.
Ascorbic Acid Content and Total Monomeric Anthocyanin Content
The titrimetric method used for ascorbic acid estimation using 2, 6-dichlorophenolindophenol reagent. The total monomeric anthocyanin content (TAC) of the grape extracts was quantified as per the method (Ranganna, Citation1999).
Preparation of the Grape Extracts
For estimation of bioactive components and antioxidant activities, 25 g of whole grape pulp was homogenized in 100 mL of aqueous ethanol (80%,v/v, 20 h), in a shaking incubator (at 120 rpm) according to the modified method (Shrikant et al., Citation2015). The extracts after filtration (paper filter, Whatman No.1) evaporated to dryness (at 35°C) using a rotary evaporator (Heidolph, Germany) and the residues recovered with distilled water. 20 mL of aqueous extract was taken and further extracted three times, with 20 mL of ethyl acetate at room temperature under darkness. The organic phase was combined, evaporated to dryness at 35°C. The residue was recovered with 10 mL of methanol and used for further analysis.
Total Phenolic Content and Total Flavonoid Content
Total phenolics content (TPC) of grape extracts was determined spectrophotometrically at 765 nm, using UV-1700 (Shimadzu, Japan), by using Folin-Ciocalteau(FC) phenol reagent method using gallic acid as the phenolic standard (Zhou et al., Citation2004). Total flavonoid content (TFC) in the grape extract was determined as per the method (Zhishen et al., Citation1999).
DPPH, ABTS Free Radical Scavenging Activity and Total Antioxidant Activity (FRAP Assay)
Antioxidant activities of the grape extract from fresh berries evaluated for scavenging ability on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, ABTS (2, ‘2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radicals and reducing power by FRAP (Ferric reducing antioxidant power) method. The DPPH radical scavenging antioxidant capacity assay determined by using a previously reported method (Wang et al., Citation2008).
ABTS radical cation decolorization assay was carried out to measure the antioxidant activity of each grape by following a method reported by Nile and Park (Citation2014a). FRAP assay was carried out as per the Nile and Park method reported previously (Nile and Park, Citation2014b).
Estimation of Polyphenols
The separation and quantification of phenolic compounds in grape extracts done as per our previous optimized method (Prakash et al., Citation2019). Briefly, the sample (100 g) was homogenized with 150 mL of ethanol (70%) thrice. The pooled extract was concentrated using a rotary evaporator (Type: Basis Hei-VAP HL, Heidolph Instruments, Germany) and further extracted with ethyl acetate (three times). The ethyl acetate fraction was concentrated and redissolved in HPLC grade methanol. 20 µL of each sample was injected in UPLC-PDA (Waters Acquity UPLC-H Class). The solvent system and program used were the same, as mentioned by Prakash et al. (Citation2019). The chromatographic peaks were identified by comparing the retention time of samples with authentic standards. The quantification based upon peak area for each sample was carried out using standard curves and reported as mg/kg of grapes.
Statistical Analysis
All the results are presented as mean±standard deviations of determinations (n = 5) were carried out, and statistical analysis was performed using the SPSS software (version SPSS16.0). One way ANOVA used for statistical significance testing between the extracts and individual pair difference tested using Duncan’s multiple range tests. Significance of varietal differences determined by analysis of variance. The difference considered statistically significant when the ‘p’ value was <0.05.
Results and Discussion
Chemical Quality of Grapevine Cultivars
TSS Content, Titrable Acidity, and Ascorbic Acid Content
The TSS content in all three grape varieties was found to be increased significantly (p ≤ 0.05) from the immature green stage to a fully mature stage. The Krishna Sharad had the highest TSS content in the 4th stage in comparison to the 3rd stage of maturity than Bangalore blue and Dilkhush (). The increase in TSS of all three grape varieties was observed as an essential natural phenomenon in fruits as growth starts from immature berry to mature ripe berries. The maturity and ripening process involves a combination of both metabolism and catabolism activities or the interconversions of a series of carbohydrates. Therefore, the increase in TSS content attributed due to the enzymatic conversion of higher polysaccharides into simple sugars (Tripathi et al., Citation1992). The titrable acidity was following a (p ≤ 0.05) decreased trend in all three grape varieties as the developed berry advanced toward maturity (). There was a loss of ascorbic acid content that has been noticed as the fruit growth advanced from the green stage to the matured stage ().
Table 1. Nutritional compositional changes, bioactive compounds and various antioxidant activities of colored and white grapes (Vitis vinifera L.) during berry development and maturity
The results indicated that there is considerable variation among the grape varieties examined. The fruit compositional changes () showed that the Krishna Sharad had the (P ≤ 0.05) highest TSS content at full maturity. It could be because of chemical and enzymatic changes of polysaccharides into sugars and degradation of natural acids present in grape (Harbertson et al., Citation2003). There was a loss in ascorbic acid content in all three grape cultivars with the decreased trend in the titrable acidity as the fruit starts developing from the green stage to the matured stage (). The degradation or loss in ascorbic acid content could be because of oxidation due to the presence of oxygen (respiration process), and the formation of dehydroascorbic acid and other factors includes processing and storage temperature (Harbertson et al., Citation2003).
Total Monomeric Anthocyanin Content
Accumulation of anthocyanins in the berry skin, primarily in red grape cultivars at the beginning of veraison, is one of the critical features of berry growth and development (Balik et al., Citation2013). The total anthocyanin content was calculated stage-wise for all three grape varieties. There was (p ≤ 0.05) increase in anthocyanin content, and a dramatic increase observed in the last stage-fully ripened one () than other stages. These results obtained in this study for matured fruits are within the range of earlier reported studies in 19 grape cultivars of Moravia (EU wine-growing, B-zone), Czech Republic (Ghosh et al., Citation2002) and are comparatively less than anthocyanin content of 170.6 mg/100 g in Krishna Sharad grapes as reported earlier (Jeandet et al., Citation1995).
Table 2. Antioxidant activities of colored and white grapes (Vitis vinifera L.) during berry development and maturity
The results show that there is also considerable variation among the grape cultivars examined in the anthocyanin content of the berry skin. The Krishna Sharad showed the (P ≤ 0.05) highest anthocyanin content (1622.6 mg/kg), and Dilkhush showed the least anthocyanin content (6.3 mg/kg) in fully matured stages. In fully developed fruit of Bangalore blue and Krishna Sharad, there was an upsurge in anthocyanin content, and at the same time increase in the pools of non-colored phenolics was observed. It could be due to enzyme involved in anthocyanin biosynthesis, may compete more at later stages of berry development (Lee and Talcott, Citation2004).
Total Phenolic Content
The total phenolics are the principal constituent of fruits and provide antioxidant potential and benefits to human health and considered as one of the essential chemical maturity indices. The genotypes and environmental effects are responsible for the production of secondary metabolites. Therefore, the content of total phenolics could vary between different varieties according to geographical location and harvest time (Nothlings et al., Citation2007). The analysis of total phenolic content showed a (p ≤ 0.05) increment with the advancement in maturity stages. The amount of phenolic content was observed to be highest in Bangalore blue, followed by Krishna Sharad and Dilkhush grape varieties (). The fully matured stage of Bangalore blue and Krishna Sharad showed a significantly (P ≤ 0.05) high amount of phenolic and flavonoids. The results indicate that a fully matured stage is the most crucial stage of berry development and maturity, at which significant (P ≤ 0.05) presence of bioactives has noticed. Our results are in agreement with those earlier reported (Rajha et al., Citation2017) such that grape varieties with higher amounts of phenolics, flavonoids, and anthocyanin compounds exhibit potent antioxidative activities at full maturity of berries under biotic, abiotic stress conditions.
Total Flavonoid Content
The significant difference in flavonoid content also observed during different stages of fruit growth (). Total flavonoid content in Bangalore blue variety varied from 71.01 to 437.3 mg QRE/100 g while in Krishna Sharad (64.28 to 388.1 mg QRE/100 g) and Dilkhush (55.90 to 319.4 mg QRE/100 g) on the fresh weight basis. The fully matured stage of Bangalore blue grape variety contained the highest amount of total flavonoids (437.3 mg QRE/100 g) followed by Krishna Sharad (388.1 mg QRE/100 g) and Dilkhush variety (319.4 mg QRE/100 g).
Antioxidant Activities
The tested in vitro DPPH activity of the partially purified ethanolic extract of selected grape varieties showed that antioxidant activity increases with maturity (). A positive correlation observed between total phenolics, anthocyanin content, and antioxidant activities. The ABTS radical scavenging assay examined in grape varieties at different maturity stages showed a (p ≤ 0.05) in antioxidant activities among the selected three types. The Bangalore blue grapes showed a high level of antioxidant potential at full maturity in ABTS assay with IC50 41.61 µg/mL, followed by Krishna Sharad (59.21 µg/mL) and Dilkhush (62.82 µg/mL) grape varieties. The FRAP assay, commonly used for evaluating the antioxidants in dietary polyphenols. It measures the potential of an antioxidant by its ferric reducing ability. The results of all three grape varieties showed high reducing power, which (p ≤ 0.05) varied among three grape varieties and also in their different developmental stages. The Bangalore blue showed a variation in FRAP activity with IC50 ranges from 11.43 to 38.57 µg/mL during various stages of growth, followed by Krishna Sharad (12.02 to 39.47 µg/mL) and Dilkhush (14.51 to 36.98 µg/mL) grape varieties.
As free radicals can cause damages to biological systems leads to degenerative and chronic diseases. The late maturing grape varieties (Bangalore blue and Krishna Sharad) showed a high level of antioxidant potential at full maturity (102 to 105 DAPA) in DPPH activity as compared to Dilkhush variety. The Bangalore blue grapes showed a high level of antioxidant potential at full maturity of berries in ABTS assay and high reducing power in FRAP assay as compared to Krishna Sharad and Dilkhush grape varieties. It indicates overall Bangalore blue comparatively had the highest antioxidant potential by its ferric reducing ability during berry development and berry maturity stages. However, there was an inclined trend in antioxidant activities observed in all three grape varieties. It may be due to the high accumulation of phenolic acids and various flavonoids at different developmental stages of grape berries (Ignat et al., Citation2011). The results provided evidence that fourth stage fresh berries in the present study could be beneficial in the biomedical applications for reducing oxidative stress.
Polyphenols Estimation
The three varieties of Vitis vinifera L. (Bangalore blue, Krishna Sharad, and Dilkhush) were analyzed for the phenolic content in different stages of growth and maturity (). The phytochemicals have been obtained by solid-liquid extraction with 80% ethanol and partially purified by liquid-liquid extraction with ethyl acetate (six times). The main classes of polyphenol compounds were quantified by UPLC-PDA, according to the method previously developed and validated (Om Prakash et al., Citation2019). The result of the study showed that the content of phenolic compounds was increased significantly (p ≤ 0.05) from early-stage to the fully matured stage in all three grape varieties. In total, 15 phenolic compounds, of which 5 were hydroxybenzoic acids (Gallic acid, protocatechuic acid, vanillic acid, p-coumaric acid, and syringic acid) four hydroxycinnamic acids (Chlorogenic acid, caffeic acid, ferulic acid, and sinapic acid), five flavonoids (Catechin, epicatechin, procyanidin B2, hesperidin, and hesperetin) and one stilbene (Resveratrol) were identified based on retention times of authentic standards. Individual phenolic compounds were quantified by producing calibration curves with available pure standards of respective phenolic compounds () and expressed in mg/kg of grape berries (fresh weight basis). shows the main phenolics in the 4th stage of all three varieties of grapes. The present study showed that phenolic compounds like chlorogenic acid, catechin, epicatechin, and hesperidin were present in good amount in Bangalore blue grape variety. In Krishna Sharad, gallic acid, chlorogenic acid, hesperidin, catechin, hesperetin, and epicatechin were among the major phenolics present. The Dilkhush variety was found to be rich in catechin, chlorogenic acid, caffeic acid, protocatechuic acid, and epicatechin. The phenolic profile showed an increase in bioactives during berry ripening in all three varieties, and their content was dependent on the Vitis genotype. In Bangalore blue, there was a significant (p ≤ 0.05) change in the accumulation of phenolic compounds during all four stages of berry development. Initially, there was a slight increase in phenolics from stage 1 to 2 and then increased rapidly. The significant phenolics like chlorogenic acid, catechin, and epicatechin showed a five, nine, and fivefold change respectively from the first to the fourth stage of berry development and maturity. Overall, six-fold increment (Stage 1–4) in all the estimated phenolic compounds. The resveratrol content in Bangalore blue varied from 0.41 ± 0.09 to 4.73 ± 0.83 mg per kg grapes. The other two varieties (Krishna Sharad and Dilkhush) showed similar trends in total estimated phenolics accumulation () during berry development.
Table 3. Polyphenols (mg/kg) estimation in three Indian grape (Vitis vinifera L.) varieties during berry development and maturity
Figure 2. Major phenolic compounds in three Indian grape(Vitis vinifera L.) varieties ((a): Bangalore blue, (b): Krishna Sharad and (c): Dilkhush)
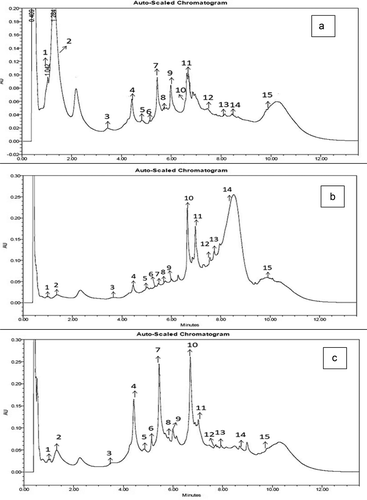
Moreover, there were also differences in compound contents among the cultivars. In comparison, Bangalore blue grapes were found high in catechin, epicatechin, and chlorogenic acid than Krishna Sharad and Dilkhush grapes. The present work is well supported by phenolic profile and antioxidant study of Niu et al. (Citation2016) on white-skinned grapes and their colored genotypes berry development. The phenolics data reveals that even though berries having the near about same TSS content which is a significant maturity index but have high variability in accumulation of individual phenolic content during different stages of growth and berry development which could be due to genetic factors, particular vintage year and variety (Siemann and Creasy, Citation1992).
Conclusion
In conclusion, this research has comprehensively investigated the chemical composition and antioxidant capacity of both colored (Bangalore Blue, Krishna Sharad) and white commercial grape(Vitis vinifera L.) varieties during development and maturity. The results demonstrated the significant (p < .05) differences in the content of total phenolics, flavonoids, and antioxidant capacity during berry maturity and among the grape varieties. The remarkable impact of stages of berry development and maturity was seen on the level of individual phenolic composition and antioxidant capacity, which could help in harvest time and selection of grape variety for significant health benefits. Collectively, picking berries of colored grapes from their native grapevine orchards at complete maturity (102 to 105 DAPA) is the most critical stage and can be utilized in pharmaceutical industries and development of functional food from the significant bioactive compounds present.
Disclosure Of Potential Conflicts Of Interest
The authors declare that they have no conflict of interest, and this work has not been published elsewhere and has not been submitted simultaneously for publication elsewhere.
Acknowledgments
The authors acknowledge the CSIR, New Delhi, for funding this Project.
Additional information
Funding
Literature Cited
- A.O.A.C. 1995. Official methods of analysis, method 967.2110. 16th ed. Washington, DC. Association of Official Analytical Chemists.
- Alberdi, G., V.M. Rodriquez, J. Miranda, M.T. Macarulla, I. Churruca, and M.P. Portillo. 2013. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 141:1530–1535. doi: 10.1016/j.foodchem.2013.03.085.
- Alonso Borbalan, A.M., L. Zorro, D.A. Guillen, and C.G. Barroso. 2003. Study of the polyphenol content of red and white grape varieties by liquid chromatography-mass spectrometry and its relationship to antioxidant power. J Chromatogr A. 1012:31–38. doi: 10.1016/S0021-9673(03)01187-7.
- Baiano, A., and C. Terracone. 2011. Varietal differences among the phenolic profiles and antioxidant activities of seven table grape cultivars grown in the south of Italy based on chemometrics. J Agr Food Chem. 59:9815–9826. doi: 10.1021/jf203003c.
- Balik, J., M. Kumsta, and O. Rop. 2013. Comparison of anthocyanins present in grapes of Vitis vinifera L. varieties and interspecific hybrids grown in the Czech Republic. Chem Pap 67:1285–1292.
- Derradji-Benmeziane, F., R. Djamai, and Y. Cadot. 2014. Antioxidant capacity, total phenolic, carotenoid, and vitamin c contents of five table grape varieties from Algeria and their correlations. J Int Sci Vigne Vin 48:153–162.
- Ghosh, N., V. Malathy, K.K. Shalia, and J.S. Pai. 2002. Anthocyanins from Indian varieties of grapes. J Food Sci Tech 39:353–358.
- Harbertson, J.F., E.A. Picciotto, and D.O. Adams. 2003. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. AJEV 54:301–306.
- Ignat, L., I. Volf, and V.I. Popa. 2011. A critical review of methods for the characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026.
- Jeandet, P., M. Sbaghi, R. Bessis, and P. Meunier. 1995. The potential relationship of stilbene (resveratrol) synthesis to anthocyanin content in grape berry skins. Vitis 34:91–94.
- Lee, J.H., and S.T. Talcott. 2004. Fruit maturity and juice extraction influence ellagic acid derivatives and other antioxidant polyphenolics in muscadine grapes. J Agr Food Chem. 52:361–366. doi: 10.1021/jf034971k.
- Liu, X., J. Li., Y. Tian, M. Liao, and Z. Zhang. 2016. Influence of Berry Heterogeneity on phenolics and antioxidant activity of grapes and wines: A primary study of the new winegrape cultivar Meili (Vitis vinifera L.). PLoS One 11(3):e0151276.
- Nile, S.H., and S.W. Park. 2014a. HPTLC analysis antioxidant, anti-inflammatory, and antiproliferative activities of Arisaema tortuosum tuber extract. Pharm Biol. 52:221–227. doi: 10.3109/13880209.2013.831110.
- Nile, S.H., and S.W. Park. 2014b. Antioxidant: A-glucosidase and xanthine oxidase inhibitory activity of bioactive compounds from maize (Zea mays L.). Chem Biol Drug Des. 83:119–125. doi: 10.1111/cbdd.12205.
- Niu, S., F. Hao, H. Mo, J. Jiang, H. Wang, C. Liu, X. Fan, and Y. Zhang. 2016. Phenol profiles and antioxidant properties of white-skinned grapes and their colored genotypes during growth. Biotechnol Biotechnol Equip 31(1):58–67. doi: 10.1080/13102818.2016.1258329.
- Nothlings, U., S.P. Murphy, L.R. Wilkens, B.E. Henderson, and L.N. Kolonel. 2007. Flavanols and pancreatic cancer risk: The multiethnic cohort study. AJEPID 166:924–931.
- Prakash, O., R. Baskaran, and V.B. Kudachikar. 2019. Characterization, quantification of free, esterified, and bound phenolics in P. pashia (Pyrus pashia Buch.-ham. Ex D. Don) fruit pulp by UPLC-ESI-HRMS/MS and evaluation of their antioxidant activity. Food Chem. 125114. doi:10.1016/j.foodchem.2019.125114.
- Rajha, H.N., N.E. Darra, S.E. Kantar, Z. Hobaika, N. Louka, and R.G. Maroun. 2017. A Comparative study of the phenolics and technological maturities of Red Grapes grown in Lebanon. ANTIGE 6:8.
- Ramchandani, A.G., R.S. Chettiyar, and S.S. Pakhale. 2010. Evaluation of antioxidant and anti-initiating activities of crude polyphenolic extract from seedless and seeded grape varieties. Food Chem. 119:298–305. doi: 10.1016/j.foodchem.2009.06.032.
- Ranganna, S. 1999. Handbook of analysis and quality control for fruits and vegetable products. Ranganna, 3rd ed. Tata McGraw-Hill Publishing Co. Ltd, New Delhi, India. p. 61–129.
- Rolle, L., S. Giacosa, V. Gerbi, M. Bertolino, and V. Novello. 2013. Varietal comparison of the chemical, physical, and mechanical properties of five colored table grapes. Int J Food Prop. 16:598–612. doi: 10.1080/10942912.2011.558231.
- Satisha, J., P. Doshi, and P.G. Adsule. 2008. Influence of rootstocks on changing the pattern of phenolic compounds in Thompson seedless grapes and its relationship to the incidence of powdery mildew. Turk J Agric For 32(1):1–9.
- Shrikant, A., A. Kumar, and V. Govindaswamy. 2015. Resveratrol content and antioxidant properties of underutilized fruits. Int J Food Sci Tech 52:383–390.
- Siemann, E.H., and L.L. Creasy. 1992. The concentration of the phytoalexin resveratrol in wine. AJEV 49:49–52.
- Tripathi, V.K., K. Lyngdoh, and S. Singh. 1992. Studies on blending of pineapple juice with different ratios of guava juice for preparation of RTS beverage. Progr Hortic 24(1–2):60.
- Wang, X., T.H. Beckman, J.C. Morris, F. Chen, and J.D. Gangemi. 2008. Bioactivities of gossypol, 6-methoxygossypol, and 6, ‘6’-dimethoxygossypol. J Agr Food Chem 56:4393–4398.
- Zhishen, J., T. Mengcheng, and W. Jianming. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559. doi: 10.1016/S0308-8146(98)00102-2.
- Zhou, K., L. Su, and L.L. Yu. 2004. Phytochemicals and antioxidant properties in wheat bran. J Agr Food Chem. 52:6108–6114. doi: 10.1021/jf049214g.

