ABSTRACT
The research was conducted in the 2017 and 2019 seasons in two different orchards (Bouka and Sarabion), province of Latakia, Syria, in order to identify the optimal pollinizer for ‘Dermlali’ olive cultivar. Fruit set with/without emasculation of flowers after self-, open-, and cross-pollination was studied. ‘Khoderi,’ ‘Picholine Languedoc,’, and ‘Frantoio’ were used as pollinizers in Bouka, while ‘Picholine Languedoc’ and ‘Khoderi’ were used in Sarabion. Pistil abortion and the average number of flowers per inflorescence were also studied. Results showed that ‘Dermlali’ was characterized by a low number of flowers per inflorescence (12.6–11.4 in Bouka and 11.1–9.4 flower/inflorescence in Sarabion in 2017 and 2019, respectively), and low pistil abortion percentage (10.3–19.6% as an average of the 2017 and 2019 seasons in Bouka, and Sarabion, respectively). ‘Dermlali’ was highly self-incompatible in Bouka (ISI = 0–0.06), but partially self-incompatible in Sarabion (ISI = 0.42–0.50). In both study orchards, the highest final fruit set of ‘Dermlali’ was observed after cross-pollination with ‘Picholine Languedoc.’ Significantly lower final fruit sets of ‘Dermlali’ were observed after cross-pollination with ‘Frantoio’ in both orchards. Based on the recent-discovered diallelic self-incompatibility system in olive and compatibility relationships of ‘Dermlali’ (♀) with ‘Picholine Languedoc’ and ‘Frantoio,’ the S-genotype of ‘Dermlali’ would be S1S1. Overall results suggest that planting ‘Picholine Languedoc’ as pollinizer in ‘Dermlali’ olive orchards could significantly improve fruit set and yield.
Introduction
Syria is an important olive producer with total production reached about 0.85 MT in 2017 (Ministry of Agriculture and Agrarian Reform (MOAAR), Citation2017). Olive cultivation is closely related to Syrian culture and traditions, so efforts were made to improve yields. Reproductive biology is a complex process that significantly affecting olive tree yield. Self-incompatibility (SI) is one of the most important factors involved in olive reproductive behavior. This phenomenon leads to the rejection of pollen grain sharing the same S-alleles with the receptor plant/genotype (Takayama and Isogai, Citation2005).
The gametophytic versus sporophytic nature of self-incompatibility in olive has been questioned for a long time in the literature (Saumitou-Laprade et al., Citation2017). Self-incompatibility in olive has been classified as gametophytic (GSI) for a long time based on some characteristics like wet stigma, bi-nucleate pollen, and inhibition of pollen tube growth shortly after germination and penetrating the stigma surface after self-pollination (Selak et al., Citation2014; Serrano et al., Citation2008). Based on success or failure of fruit set after reciprocal pollination among tens of cultivars, Breton and Berville´ (Citation2012) developed the first model for SI in olive. The model was based on sporophytic self-incompatibility. They proposed the existence of six self-incompatibility alleles in olive (R1 to R6), attributed to different olive cultivars (Breton et al., Citation2016, Citation2014; Farinelli et al., Citation2015). Based on this model, Koubouris et al. (Citation2014) found that ‘Koroneiki’ is carrying/R4R6/and ‘Mastoides’ is/R1R2/.
In 2017, a new model for SI in olive was published by Saumitou-Laprade et al. (Citation2017). The authors reported that the SI in olive is diallelic self-incompatibility (DSI) as the system identified in Phillyrea angustifolia and Fraxinus ornus other members of Oleaceae family, and there are two SI groups (G1) with/S1S2/genotype and (G2) with/S1S1/genotype. In further studies, results on Olea europaea subsp. laperrinei and cultivated olive supporting the DSI hypothesis were reported (Aslmoshtaghi et al., Citation2019; Besnard et al., Citation2020; Mariotti et al., Citation2020).
Many studies have been conducted worldwide to identify the optimal pollinizers for olive cultivars using fruit set resulting from self-, open-, and cross-pollination. Ghrisi et al. (Citation1999) reported that the highest fruit set rates were obtained when ‘Picholine Languedoc’ was pollinated with ‘Souri’; and that ‘Picholine Languedoc’ (♀) was compatible with ‘Sourani’ (♂). El-Hady et al. (Citation2007) studied different types of pollination and found that ‘Koroneiki’ was a good pollinizer for ‘Arbequina.’ Farinelli and Tombesi (Citation2006) studied self-sterility and the significance of cross-pollination of 23 Italian and foreign olive cultivar depending on fruit sets after self-, cross-, and open-pollination; and they reported the suitable pollinizers for the studied cultivars. Selak et al. (Citation2011) found that ‘Oblica’ olive is partially self-incompatible but cross-compatible with ‘Leccino’ and ‘Levantinka.’ In Italy, the best pollinizer for ‘Ascolana Tenera’ was ‘Carolea’ followed by ‘Picholine Languedoc,’ by the same way ‘Carolea’ was the optimal pollinizer for ‘Leccino’ (Farinelli et al., Citation2012). In Iran, ‘Fishomi-Roudbar’ and ‘Dezfoul’ were suitable pollinizers for ‘Roghani’ and ‘Shiraz’ (Taslimpour and Aslmoshtaghi, Citation2013). In Spain, Sánchez-Estrada and Cuevas (Citation2018) found that ‘Manzanillo’ and ‘Picual’ are efficient pollinizers for ‘Arbequina.’ Other studies used Paternity tests on embryos resulting from open-pollinated olive cultivars. Results of paternity tests of ‘Istrska Belica’ showed that ‘Leccino’ and ‘Leccio del corno’ were the most frequent fathers (Arbeiter et al., Citation2014).
‘Dermlali’ (also known as ‘Doebli’ or ‘Tamrani’) is an autochthonous Syrian olive cultivar. This cultivar is characterized by high fruit weight, high productivity, with a biennial bearing habit, and appears to be resistant to olive knot and verticillium wilt. This cultivar is a dual purpose with medium oil content and adapts well to damp areas. The main distribution area of this cultivar is the Syrian coastal region (Latakia and Tartus) and Tel-Kalakh (Navero et al., Citation2000). Moutier (Citation2002) mentioned that olive yield could be enhanced by adding pollinizers; but the yield depends on the identity of the pollinizers. According to this, a two years experiment was conducted to investigate the self-compatibility of ‘Dermlali’ in order to identify the optimal pollinizer for this cultivar and to predict the SI alleles in ‘Dermlali’ based on the recent models of SI in olive.
Materials and Methods
Study Site
The study was carried out in the 2017 and 2019 seasons in two experimental orchards:
(1) The first experimental orchard was located in Bouka (latitude 35º32ʹ North, 35º48ʹ East), province of Latakia, 40 m above sea level, characterized by clay calcareous soil. This orchard is an olive collection with about 44 autochthonous and introduced olive cultivars. ‘Homisi,’ ‘Tanche,’ ‘Chemlal de Kabylie,’ ‘Musaabi,’ ‘Jlot,’, ‘Gordal,’, and ‘Coratina’ are the closest to ‘Dermlali’ trees. The temperature and precipitation are presented in () and (). It is worth mentioning that January and February of 2017 were colder than in 2019, but April and May were warmer. In 2017, precipitation (mm) stopped in February and then continued in March. Precipitations (mm) were higher in the 2019 season, but it stopped in May resulting in dry weather in May and June. Orchard management included spring tillage before flowering, spraying fungicides to control olive peacock eye disease, second tillage after fruit set, third tillage before harvesting, and tree pruning at the end of fall. No fertilization was applied.
Table 1. Average monthly minimum (min.) and maximum (max.) temperatures (ºC) for the periods from January to June in the 2017 and 2019 seasons in Bouka; and the average (aver.) temperature for the period from 2010 to 2016 in Sarabion site
Figure 1. Average monthly precipitation (mm) for the periods from January to June in the 2017 and 2019 seasons in Bouka; and average monthly precipitation (mm) for the period from 2010 to 2016 in Sarabion
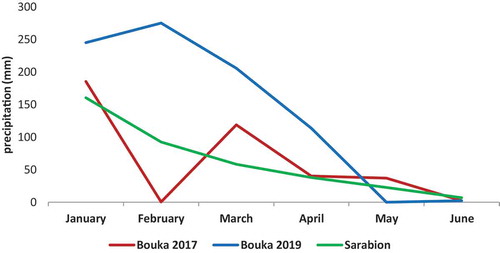
(2) The second experimental orchard was located in Sarabion (35º13ʹ North, 36º01 East), province of Latakia, 407 m above sea level, characterized by Basalt soil. ‘Dermlali’ is the main olive cultivar (more than 98% of the trees), with some trees of ‘Khoderi’ randomly distributed in the orchard. We could not obtain meteorological data for the study seasons but average temperature and precipitation for the period from 2010 to 2016 are presented in and . The average temperature for the period from 2010 to 2016 was approximately one degree lower than in the Bouka site; precipitation in Sarabion was also lower than in Bouka as an average. Orchard management included herbicide spraying prior to flowering, insecticide spraying at the end of June, and tree pruning at the end of fall. No fertilization was applied.
Plant Material
Autochthonous olive cultivar ‘Dermlali’ was used as a pollen receptor. The trees used in the experiments are about 30 years old in Bouka, and between 20 and 30 years in Sarabion.
The cultivars used as pollinizers were ‘Picholine Languedoc,’ ‘Frantoio,’ and the autochthonous cultivar ‘Khoderi.’ All studied cultivars in both orchards were grown under rainfed conditions and in a planting distance of 7 × 7 m. Those cultivars are characterized by regular bearing habit, adaptability, and good yield under local conditions.
Methods
1-Experimental Orchard of Bouka
Three trees of ‘Dermlali’ were chosen. Samples of 50 inflorescences from each tree were taken randomly at the white bud stage in order to estimate the average number of flowers per inflorescence and pistil abortion incidence (%).
Pollination Treatments
2017 Season
Five 1-year-old shoots were tagged in every side of the trees (20 in each tree); emasculation of the flowers on the shoots (four out of the five shoots in every side of the trees) was performed at the white bud stage using hand forceps. Flowers at another stage were eliminated. After emasculation shoots were enclosed inside white paper bags. Pollination was done the next day of emasculation by opening the bags and brushing the stigmas of ‘Dermlali’ flowers with the stamens of fully opened flowers on inflorescences were taken the same day from the pollinizers (‘Khoderi,’ ‘Picholine Languedoc,’ ‘Frantoio’ for cross-pollination, and ‘Dermlali’ for self-pollination; i.e. Self-pollination was treated by the same method used for cross-pollination but with pollen grains from the same cultivar), and then additional inflorescences with fully opened flowers of pollinizers were transferred inside the bags. The bags were closed and shaken.
The fifth shoot in every side of the trees was used for open pollination (four for each tree) and the number of inflorescences on each shoot was regulated to the same number (12 inflorescence) and kept without emasculation.
Twenty days after full bloom, bags were removed. Then, the initial fruit set (%) and the final fruit set (%) were estimated 30 and 65 days after pollination as the percentage of the number of fruits/the number of emasculated flowers. In open-pollination treatment, since no emasculation was done, the fruit set was estimated as the percentage of the number of fruits/the number of hermaphrodite flowers.
2019 Season
Pollination was performed without emasculation by tagging shoots in the white bud stage, after unifying the number of inflorescences on each shoot (15 inflorescences). Bagging with white paper bags was then done. Pollination was performed by opening the bags and immediately transferring flowering shoots with opened flowers and dehiscent stamens of the pollinizer into the bags at full flowering (stage 65) according to (Sanz-Cortéz et al., Citation2002) of ‘Dermlali’ and reclose the bags, then shaking the bags daily for 3 days to ensure pollen dispersal inside the bags. Initial and final fruit sets (%) were calculated as (the number of fruits/total number of flowers)×100. Pollination without emasculation enabled us to simulate field conditions.
Index of self-incompatibility was calculated as fruit set obtained by self-pollination/fruit set obtained by open- or cross-pollination. ISI evaluated as follows: ISI = 0: completley self-incompatible, ISI = 0.1–0.2: highly self-incompatibile, ISI = 0.2–0.9: partially self-incompatibile, ISI≥1: completely self-compatibile (Zapata and Arroyo, Citation1978). In order to obtain the most efficient evaluation of the pollinizers, the highest fruit set resulted from cross-pollination was used instead of free pollination in ISI calculations.
2-Experimental Orchard of Sarabion
Six uniform trees of ‘Dermlali’ were chosen in 2017 and four in 2019. On them, the average number of flowers per inflorescence and pistil abortion (%) were determined as mentioned previously. Pollination treatments were self- and open-pollination in 2017. In 2019, pollination treatments were:
-Self-pollination: shoots were enclosed at the white bud stage with white paper bags.
-Cross-pollination: shoots of ‘Dermlali’ were enclosed at the white bud stage. At full flowering (stage 65) according to (Sanz-Cortéz et al., Citation2002), pollination was done as mentioned previously in Bouka orchard in the 2019 season by opening the bags and immediately transferring flowering shoots with open flowers and dehiscent stamens of the pollinizer into the bags and reclose it and shaking the bags to ensure pollen dispersal. Flowering shoots of the pollinizer (‘Picholine Languedoc’) were taken from Bouka experimental orchard, while ‘Khoderi’ shoots were taken from the same orchard.
-Open-pollination: shoots were kept unclosed for open (free) pollination. No emasculation was done in any pollination treatment. Every treatment was performed in four 1-year-old shoots distributed around the canopy. The number of inflorescences was 15 for each shoot (60 inflorescences for each tree/replicate). Any additional inflorescence was removed.
The final fruit set (%) was estimated based on the number of hermaphrodite flowers. The 2018 season was excluded because it was an “Off–Year” of ‘Dermlali’ in both experimental orchards.
Average pollen germination (%) of the studied cultivars in the 2017 and 2019 seasons were 35.5, 30.25, 42.8, and 54.25% for ‘Dermlali,’ ‘Picholine Languedoc,’ ‘Khoderi,’ and ‘Frantoio,’ respectively in Bouka orchard (Douay et al., Citation2020) and 52.9% for ‘Dermlali’ in Sarabion orchard.
Experimental Design and Statistical Analysis
Completely randomized blocks design was used with three replications (trees) in Bouka and four to six trees in Sarabion. Data were arcsine transferred and subjected to ANOVA. Means were separated using Duncan multiple range test (P≤ 0.05). In pistil abortion and the average number of flowers per inflorescence study, a t-student test was used to analyze the differences between the 2017 and 2019 seasons. CoStat version 6.400 Copyright(c) 1998–2008 CoHort software, CA, USA was used for data analysis.
Results
Pistil Abortion and Average Number of Flowers per Inflorescence
shows that the number of flowers/inflorescence was low in both study sites. It was 12.6–11.4 flower/inflorescence in Bouka and 11.1–9.4 in Sarabion in 2017 and 2019, respectively. No significant differences were observed among seasons in any orchard.
Figure 2. Average number of flowers per inflorescence (Flowers/inf.) and Pistil abortion (%) for ‘Dermlali’ olive cultivar in Bouka (A) and Sarabion (B) in the 2017 and 2019 seasons. Columns sharing the same letters have no significant differences (P ≥ 0.05) using t-student test
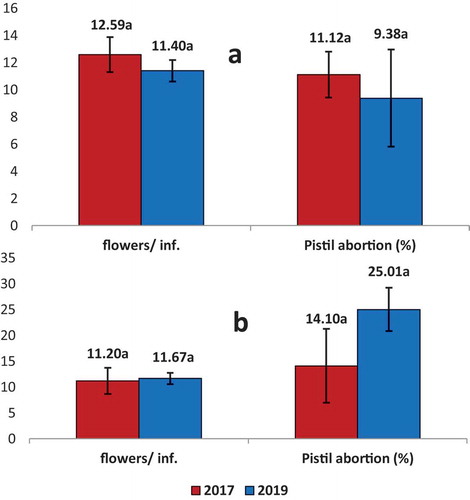
Pistil abortion of ‘Dermlali’ was low (10.3%) as an average of 2017 and 2019 in Bouka and 19.6% in Sarabion (). No significant differences were observed among seasons in Bouka or Sarabion.
Fruit Set
Bouka Orchard
Initial Fruit Set
shows that the highest initial fruit set in 2017 was for pollination with ‘Picholine Languedoc’ and the lowest was for open-pollination followed by self-pollination. In 2019, no significant differences were observed among treatments.
Table 2. Initial fruit set (IFS %), final fruit set (FFS %), and self-incompatibility index (ISI) of ‘Dermlali’ olive cultivar as affected by different types of pollination (Self-, Cross-, and open-pollination) in the 2017 and 2019 seasons in Bouka orchard
Final Fruit Set
shows that the highest final fruit set was for cross-pollination with ‘Picholine Languedoc’ in both seasons. Significant differences were observed among this treatment and the other pollination types in 2017. In 2019, ‘Picholine Languedoc’ gave the highest fruit set, but the differences between ‘Picholine Languedoc,’ ‘Khoderi,’ and open-pollination treatments were not significant. In 2019, massive fruit abscission was observed in all treatments, i.e. 100% of the fruits were abscised in the case of self-pollination and cross-pollination with ‘Frantoio,’ also 76.6% of the fruits resulting from cross-pollination with ‘Picholine Languedoc’ abscised. This abscission might be due to lack of fertilization, competition among fruits, and drought in May and June of 2019 ().
Self-incompatibility Index
Depending on the final fruit set, shows that ‘Dermlali’ was highly self-incompatible in 2017, while it was completely self-incompatible in the 2019 season since no fruit was obtained by self-pollination.
Sarabion Orchard
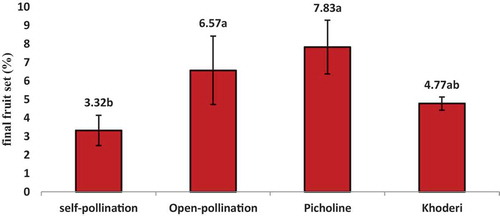
The highest final fruit set (%) was for cross-pollination with ‘Picholine Languedoc’ followed by open pollination and cross-pollination with ‘Khoderi’ (). The lowest fruit set was obtained for self-pollination. This behavior is similar to Bouka but the difference was on ISI. ISI of ‘Dermlali’ was 0.5–0.42 in the 2017 and 2019 seasons, respectively. These results categorize ‘Dermlali’ as partially self-incompatible.
Figure 3. Final fruit set (%) of ‘Dermlali’ olive cultivar as affected by three types of pollination: Self-, open- and cross-pollination with ‘Picholine Languedoc’ and ‘Khoderi’ in Sarabion orchard in the 2019 season. Columns sharing the same letters have no significant differences (P ≥ 0.05) using Duncan’s test
Discussion
In this paper, flower characteristics and sexual compatibility of ‘Dermlali’ an autochthonous olive cultivar in Syria were studied. ‘Dermlali’ showed a low number of flowers per inflorescence and low pistil abortion (%) in both orchards. Dway and Makhoul (Citation2004) studied the adaptation of some local and imported olive cultivars in Bouka orchard for three consecutive years (2000 to 2002) and they found that ‘Dermlali’ had the lowest number of flowers per inflorescence (10.97 flower/inflorescence) and a low percentage of staminate flowers (6.34%) which corresponds to the present results. Non-significant differences between seasons indicate the major role of genetic background as the main factor controlling these two characters. Lavee et al. (Citation1996) reported the role of genetic factor in pistil abortion; also Alagna et al. (Citation2016) identified a set of genes that is putatively involved in pistil abortion. The genes were related to starch and sucrose metabolism, polyamine biosynthesis, cell wall metabolism, programmed cell-death (PCD), regulation of flavonoid biosynthesis, and MYB and MADS-box transcription factors.
Most studies dealing with self- and cross-compatibility depend on fruit set estimation. The final fruit set is often a better indicator rather than the number of retained fruits at harvest, mostly because of fruit abscission in the summer due to conditions not related to pollination like drought, high temperature, and pests (Selak et al., Citation2018).
Self-incompatibility index (ISI) differed between sites but not between seasons, ‘Dermlali’ was high to partially self-incompatible depending on the study site. This behavior could be due to different environmental conditions in the two study sites. Unfortunately, we could not obtain meteorological data from the Sarabion site, but the average monthly temperature and precipitation for the period from 2010 to 2016 showed some differences from Bouka, also differences in soil type are clear between the two sites. Many studies reported differences in self-incompatibility for the same cultivar but in different regions, Gonzalez and Cuevas (Citation2012) found a difference in ISI for ‘Arbequina’ olive in different areas, ISI was 0.03 in La Cañada de San Urbano and 1.51 in Tabernas, by the same way Farinelli et al. (Citation2008) found that ‘Frantoio’ is self-compatible in Italy, but this cultivar behaves as self-incompatible in Syrian coast (Mhnna et al., Citation2015). By the same way, Selak et al. (Citation2011) found that ISI changed considerably depending on site and year for ‘Drobnica,’ ‘Leccino,’ and ‘Oblica’ olive cultivars.
Recent studies mentioned that all olive cultivars are partially self-incompatible, but in certain conditions some are behaving like self-compatible, a phenomenon named “pseudo-self-compatibility” and consequently could overcome self-incompatibility and produce seeds after self-pollination (Breton et al., Citation2016; Marchese et al., Citation2016; Samitu-Laprade et al., Citation2017). Our results showed that ‘Dermlali’ could overcome self-incompatibility in certain conditions like Sarabion orchard, but this needs further studies to confirm this phenomenon.
Self-incompatibility in olive was reported to be gametophytic (Selak et al., Citation2014; Serrano et al., Citation2008), but recent studies were not in agreement with the GSI system. Collani et al. (Citation2011) reported cytological and molecular evidences supporting the sporophytic system in olive. Breton and Berville´ (Citation2012) developed a model of self-incompatibility in olive based on direct and reverse pollination of olive cultivars; the model reported sporophytic SI with six S-alleles in olive/R1, R2 … R6/with dominance relationships among them. S-Alleles attributed to ‘Picholine Languedoc’ was R1R3 with co-dominance between R1 and R3, ‘Frantoio’ was R4R5 with R5 dominant over R4 (Breton and Berville, Citation2014). The most recent model for SI in olive is based on di-allelic sporophytic SI was published by Saumitou-Laprade et al. (Citation2017). Based on this model, two SI groups are found in olive G1 with S1S2 genotype (S2 is dominant over S1) and G2 with S1S1 genotype. ‘Picholine Languedoc’ was reported to be S1S2 and ‘Frantoio’ is S1S1. Paternity tests performed on Olea europaea subsp. laperrinei were also supporting diallelic self-incompatibility system (Besnard et al., Citation2020).
In this study the cross-pollination between ‘Dermlali’ (♀) and ‘Picholine Languedoc’ was compatible, but was not compatible with ‘Frantoio,’ so according to the model of (Breton and Berville, Citation2012), ‘Dermlali’ could not have neither R1 nor R3, and the potential alleles present could be: R2, R4, R5, and R6. Incompatibility with ‘Frantoio’ suggested the existence of R5 in ‘Dermlali.’ According to this, the predictable SI genotype would be one of the following: R2R5, R4R5, or R5R6. Any wa the accurate SI-genotype of ‘Dermlali’ based on this model needs reverse pollination with the pollinizers used and crossing with other cultivars carrying R2 and/or R6 alleles. On other hand, results showed that ‘Dermlali’ was cross-compatible with ‘Picholine Languedoc’ but cross-incompatible with ‘Frantoio,’ so based on the DSI model of (Saumitou-Laprade et al., Citation2017), the SI-genotype of ‘Dermlali’ would be S1S1 and this cultivar is referred to G2 SI group.
Anyway, conducting two experiments in two study sites was aimed to evaluate the stability of the results in different environments, so the conclusions could be more reliable. This was mostly accomplished; i.e., the optimal pollinizers in the two sites were the same. Currently, studies are being conducted to investigate reverse compatibility between ‘Dermlali’ (♂) and ‘Picholine’, ‘Frantoio,’, and ‘Khoderi.’ It is worth mentioning that all studied cultivars overlapped in flowering in the same orchard (Mhanna et al., Citation2019a, Citation2019b).
Conclusions
‘Picholine Languedoc’ was the optimal pollinizer for ‘Dermlali’ followed by ‘Khoderi’ in all study seasons and sites, so our results suggest that planting those two cultivars especially ‘Picholine Languedoc’ in ‘Dermlali’ olive orchards would improve fruit set and consequently tree yield.
Authors’ contribution
All the authors contributed equally to this study. M. A. Mhanna wrote the manuscript.
Declaration of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the General commission for scientific agricultural research (GCSAR), Damascus, Syria under Grant number 2495/O CAR/18, 7, 2016.
Additional information
Funding
References
- Alagna, F., M. Cirilli, G. Galla, F. Carbone, L. Daddiego, P. Facella, L. Lopez, C. Colao, R. Mariotti, N. Cultrera, et al. 2016. Transcript analysis and regulative events during flower development in olive (Olea europaea L.). PLoS One 11(4):e0152943. doi: 10.1371/journal.pone.0152943.
- Arbeiter, A.B., J. Jakše, and D. Bandelj. 2014. Paternity analysis of the olive variety ‘istrska belica’ and identification of pollen donors by microsatellite markers. Sci World J Article ID 208590. 6. doi: 10.1155/2014/208590.
- Aslmoshtaghi, E., A.R. Shahsavar, M. Talebi, and A. Dazeh. 2019. Comparison of different classical and molecular methods for identifying self-incompatibility in two olive cultivars. Spanish J Agric Res 17(3):e0804. doi: 10.5424/sjar/2019173-14761.
- Besnard, G., P.O. Cheptou, M. Debbaoui, P. Lafont, B. Hugueny, J. Dupin, and D. Baali-Cherif. 2020. Paternity tests support a diallelic self-incompatibility system in a wild olive (Olea europaea subsp. laperrinei, Oleaceae). Ecol Evol 10(4):1876–1888. doi: 10.1002/ece3.5993.
- Breton, C.M., and A. Berville´. 2012. New hypothesis elucidates self-incompatibility in the olive tree regarding S-alleles dominance relationships as in the sporophytic model. Comptes Rendus Biologies 335(9):563–572. doi: 10.1016/j.crvi.2012.07.006.
- Breton, C.M., D. Farinelli, G. Koubouris, and A. Bervillé. 2016. A model based on S-allele dominance relationships to explain pseudo self-fertility of varieties in the olive tree. Euphytica 210(1):105–117. doi: 10.1007/s10681-016-1708-0.
- Breton, C.M., D. Farinelli, S. Shafiq, J. Heslop-Harrison, M. Sedgley, and A.G. Bervillé. 2014. The self-incompatibility mating system of the olive (Olea europaea L.) functions with dominance between S-alleles. Tree Genet Genomes 10(4):1055–1067. doi: 10.1007/s11295-014-0742-0.
- Collani, S., G. Galla, A. Ramina, G. Barcaccia, F. Alagna, E.M. Càceres, G. Barcaccia, L. Baldoni, R. Muleo, and G. Perrotta. 2012. Self-incompatibility in olive: A new hypothesis on the S-locus genes controlling pollen-pistil interaction. Acta Hortic 967(967):133–140. doi: 10.17660/ActaHortic.2012.967.15.
- Douay, F., M. Rajab, and M. Mhanna. 2020. Efficiency of pollination with some pollinizers in improving fruit set of syrian olive cultivar “Khoderi”. Tishreen Univ J Studies Sci Res Biolo Sci Series 1(24):119–133.
- Dway, F., and G. Makhoul. 2004. Study of the possible adaptation of some local and foreign olive varieties under Syrian coast conditions. Tishreen Univ J Studies Sci Res Biolo Sci Series 26(1):39–51.
- El-Hady, E., L. Haggag, M. Abd El-Migeed, and I. Desouky. 2007. Studies on sex compatibility of some olive cultivars. Res J Agric Biol Sci 3(5):504–509.
- Farinelli, D., and A. Tombesi. 2006. Results of four years of observations on self-sterility behavior of several olive cultivars and significance of cross-pollination. Olivebioteq 1:275–282.
- Farinelli, D., A. Tombesi, and D. Hassani. 2008. Self-sterility and cross-pollination responses of nine olive cultivars in Central Italy. Acta Hortic 791(791):127–136. doi: 10.17660/ActaHortic.2008.791.16.
- Farinelli, D., C.M. Breton, F. Famiani, and A.J. Berville´. 2015. Specific features in the model of olive self-incompatibility system: Method to decipher S-allele pairs for varieties spread worldwide. Sci. Hortic. 181:62–75. doi: 10.1016/j.scienta.2014.10.056.
- Farinelli, D., P. Pierantozzi, and A.M. Palese. 2012. Pollenizer and cultivar influence seed number and fruit characteristics in Olea europaea L. HortScience 47(10):1430–1437. doi: 10.21273/HORTSCI.47.10.1430.
- Ghrisi, N., B. Boulouha, M. Benichou, and S. Hilali. 1999. Agro-physiological evaluation of phenomenon of pollen compatibility in olive. Case of the Mediterranean collection at the Menara station,”Marrakech”. OLIVAE 79:51–59.
- Gonzalez, M. and J. Cuevas. 2012. Cross-pollination response in ‘Arbequina’ olive. Acta Hortic., 949:99–104.
- Koubouris, G.C., C.M. Breton, I.T. Metzidakis, and M.D. Vasilakakis. 2014. Self-incompatibility and pollination relationships for four Greek olive cultivars. Sci. Hortic. 176:91–96. doi: 10.1016/j.scienta.2014.06.043.
- Lavee, S., L. Rallo, H.F. Rapoport, and A. Troncoso. 1996. The floral biology of the olive: Effect of flower number, type and distribution on fruit set. Sci. Hortic. 66(3–4):149–158. doi: 10.1016/S0304-4238(96)00941-7.
- Mariotti, R., A. Fornasiero, S. Mousavi, N. Cultrera, F. Brizioli, S. Pandolfi, V. Passeri, M. Rossi, G. Magris, S. Scalabrin, et al. 2020. Genetic mapping of the incompatibility locus in olive and development of a linked sequence-tagged site marker. Front. Plant Sci. 10:1760. doi: 10.3389/fpls.2019.01760.
- Marchese, a., F.B. Mara, F. Costa, A. Quartararo, S.Fretto, and T. Caruso. 2016. An investigation of the self-and inter-incompatiblity of olive cultivars ‘Arbequina’ and ‘Koroneiki’ in the Mediterranean climate of Sicily. Australian journal of crop science, 10(1):88–93.
- Mhanna, M., F. Douay, and M. Rajab. 2019a. ‘Khoderi’ olive cultivar as an efficient polliniser for some French and Italian olive cultivars. Agric Forestry 65(4):211–220. doi: 10.17707/AgricultForest.65.4.19.
- Mhanna, M., F. Douay, and M. Rajab. 2019b. Flower characteristics and sexual compatibility of Italian olive cultivar Coratina under Syrian coast conditions. Agric Sci Technol 11(4):346–351. doi: 10.15547/ast.2019.04.059.
- Mhnna, M., F. Douay, and F. Al-Qaiem. 2015. Self-sterility in some olive cultivars and its influence on parthenocarpic fruits “shot berries” formation. SJAR 1(2):117–128.
- Ministry of Agriculture and Agrarian Reform (MOAAR). 2017. Statistic bureau. Damascus, Syria.
- Moutier, N. 2002. Self-fertility and inter-compatibilities of sixteen olive varieties. Acta Hortic., 586:209–212.
- Navero, D.B., A. Cimato, P. Fiorino, L.R. Romero, A. Touzani, C. Castaneda, F. Serafini, and I.T. Navas. 2000. World Catalogue of olive varieties. International OLive Oil Council, First ed. Spain. p. 360.
- Sánchez-Estrada, A., and J. Cuevas. 2018. ‘Arbequina’ olive is self-incompatible. Sci. Hortic. 230:50–55. doi: 10.1016/j.scienta.2017.11.018.
- Sanz-Cortéz, F., J. Martinez-Calvo, M.L. Badenes, H. Bleiholder, H. Hack, G. Llacer, and U. Meier. 2002. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 140(2):151–157. doi: 10.1111/j.1744-7348.2002.tb00167.x.
- Saumitou-Laprade, P., P. Vernet, X. Vekemans, S. Billiard, S. Gallina, L. Essalouh, A. Mhaïs, A. Moukhli, A. El Bakkali, G. Barcaccia, et al. 2017. Elucidation of the genetic architecture of self-incompatibility in olive: Evolutionary consequences and perspectives for orchard management. Evol Appl 10(9):867–880. doi: 10.1111/eva.12457.
- Selak, G.V., J. Cuevas, S. Goreta Ban, and S. Perica. 2018. Determination of compatibility relationships between olive cultivars: An overview of available methods. Acta Hortic (1199):115–120. doi: 10.17660/ActaHortic.2018.1199.19.
- Selak, G.V., J. Cuevas, S.G. Ban, and S. Perica. 2014. Pollen tube performance in assessment of compatibility in olive (Olea europaea L.) cultivars. Sci. Hortic. 165:36–43. doi: 10.1016/j.scienta.2013.10.041.
- Selak, G.V., S. Perica, S. Ban, and M. Radunic. 2011. Reproductive success after self-pollination and cross-pollination of olive cultivars in Croatia. Hortscience 46(2):186–191. doi: 10.21273/HORTSCI.46.2.186.
- Serrano, I., C. Suárez, A. Olmedilla, H.F. Rapoport, and M.I. Rodríguez-García. 2008. Structural organization and cytochemical features of the pistil in Olive (Olea europaea L.) cv. Picual at anthesis. Sex Plant Reprod 21(2):99–111. doi: 10.1007/s00497-008-0075-y.
- Takayama, S., and A. Isogai. 2005. Self-incompatibility in plants. Annu. Rev. Plant Biol. 56(1):467–489. doi: 10.1146/annurev.arplant.56.032604.144249.
- Taslimpour, M.R., and E. Aslmoshtaghi. 2013. Study of self-incompatibility in some Iranian olive cultivars. Crop Breeding J 3(2):123–127.
- Zapata, T.R., and M.T.K. Arroyo. 1978. Plant productive ecology of secondary tropical forest in Venezuela. Biotropica 10(3):221–230. doi: 10.2307/2387907.
