?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In Morocco, walnut tree (Juglans regia L.) cultivation is ancestral with complicated misidentification and unknown local genetic diversity. Atlas Mountains host a rich walnut tree germplasm, which has arisen from seedlings. The use of molecular markers makes it possible to overcomes confusion usually encountered in phenotypic characterization, thereby elucidating the real genetic diversity magnitude. This study aimed to investigate the genetic relationships among 33 local walnut trees grown in two contrasted agroecosystems, while comparing them with 8 Bulgarian varieties belonging to an ex-situ collection, using ISSR markers. The use of 13 primers generated 120 reproducible ISSR markers, with 7 to 13 bands and an average of nine bands per primer. This number reflected the high level of polymorphism between genotypes revealed by the selected primers. The average polymorphism rate was 75.2%, with the polymorphic information content varying from 0.212 to 0.370 and with an average of 0.32. Genetic distances and the pairwise comparison showed that all pairs of genotypes are distinct using more than 10 markers with a maximum of 50 bands. The dendrogram showed an obvious phylo-geographic structuring and the differentiation of genotypes by origin area. The Bulgarian cultivars form distinct groups that confirms the specificity of the Moroccan walnut gene pool. However, the dendrogram obtained showed that the Moroccan genotypes are genetically close to a certain number of Bulgarian genotypes. This indicates that some Moroccan genotypes and the Bulgarian varieties could have a common ancestor.
Introduction
Genetic resources assessment and conservation of agrodiversity have a major role to ensure food security for forthcoming generations, through a continuous supply of new genitors and improved cultivars (Doğan et al., Citation2014). Morocco is one of the most important hotspot diversities in the Mediterranean basin, particularly for tree species including walnut, of which an important genetic diversity is naturally preserved in traditional agroecosystems. Species such as olive and the fig which coexist with the walnut in same areas and which have almost the same modes of multiplication show significant levels of diversity (Charafi et al., Citation2008; Hssaini et al., Citation2020; Khadari et al., Citation2008). Most of these species have not been subjected to intensive plant breeding programs and, thus, many trees populations exhibit a rich genetic diversity, which can only be fully exploited once it is accurately recognized and assessed. Walnut trees cover an area of 7600 ha in Morocco, and are considered a forestry fruit tree by local farmers and populations. The most important populations were identified in the high and medium Atlas Mountains. More than half of the trees are seedlings resulting from the prevailing way of propagation known by farmers, since grafting is unknown (Kodad et al., Citation2016). The trees can be found in humid and warm conditions, in the Rif and Atlas Mountains (High and Middle Atlas) and in arid regions in Southeastern Morocco (Kabiri et al., Citation2018).
Despite its ancient culture, walnut breeding started in the twentieth century (Bernard et al., Citation2018b). In the meantime, these species (Juglans spp) have undergone a great evolution because of its breeding characteristics, and thus formed large genetic diversities through a long-term cultivation under several contrasted environments (Wu et al., Citation2000; Yang, Citation2005). Nevertheless, the actual diversity within walnut germplasm throughout the world has not been deciphered yet to its full extent (Shah et al., Citation2018a). Phylogenetic associations and genetic variability among well-known varieties and unidentified clones in mountains and traditional orchards need to be ascertained through latest tools so that the huge potential of walnut diversity can be revealed and exploited (Bernard et al., Citation2018b; Torokeldiev et al., Citation2019). Molecular characterization of walnut germplasm is complementary to morphological characterization. Therefore, is mandatory requirement for establishing breeding purposes and establishment of proprietary rights (Shah et al., Citation2018a). Unfortunately, some approaches used for genetic diversity assessment in perennial fruit crop species, such as morphological, physiological, and biochemical methods are both time consuming and are influenced by the environment (Bernard et al., Citation2018b). During the past 20 years, many genetic studies of walnut have been conducted using molecular biology techniques, such as DNA-based markers, which provides accurate results independently of environmental influences. These studies aimed to examine the diversity (Doğan et al., Citation2014; Shah et al., Citation2018a; Vyas et al., Citation2003), determine relationships within or among germplasm collections and populations (Ali et al., Citation2016; Christopoulos et al., Citation2010; Ji et al., Citation2014; Mozaffarian et al., Citation2008), elucidate phylogenetic and origin (Pollegioni et al., Citation2017; Roor et al., 2017), build genetic map (Bernard et al., Citation2020; Woeste et al., Citation1996; You et al., Citation2012; Zhu et al., Citation2015) and to investigate biotic or abiotic stress (Luo and Liu, Citation2019; Zerillo et al., Citation2014; Zhu et al., Citation2015). In these studies, various molecular markers were used such as Restriction Fragment Length Polymorphisms (RFLPs) Randomly Amplified Polymorphic DNAs (RAPDs), Inter Simple Sequence Repeats (ISSRs), Simple Sequence Repeats (SSRs), Amplified Fragment Length Polymorphisms, and SNPs. Compared to other marker types, these techniques can provide relevant results regarding the genetic diversity in all tissues at all stages. ISSR is one of the simplest and widely used markers for molecular fingerprinting and population structure analysis, because of their stability, reproducibility, and easy detection by PCR (Vijayan, Citation2005).
ISSR markers have been applied in several genetic studies in walnut, including genetic diversity studies (Christopoulos et al., Citation2010; Ji et al., Citation2014; Potter et al., Citation2002), to determine the origin of a genotype (Malvolti et al., Citation2010) and genetic mapping studies (Malvolti et al., Citation2001). In general, ISSR markers have been usually used for local Juglans regia germplasm characterization by Iranian (Ghanbari et al., Citation2019; Rashnodi et al., Citation2019), Kashmir (Shah et al., Citation2019), Greek (Ji et al., Citation2014; Miltiadis et al., Citation2010), China (Ji et al., Citation2014) and Italian walnut (Pollegioni et al., Citation2003b). The walnut (Juglans regia L.) is a rustic and traditional tree widespread throughout the temperate regions of the world due to its adaptation capabilities. The walnut production worldwide leaders are China, the USA, Turkey, Iran, Mexico, and Ukraine; All together ensure 85% of the worldwide production (FAO, 2018). In Morocco, the walnut occupies 7702 ha with a mean production of 12467 tons (FAO, 2018), and its cultivation is spread over many local agroecosystems. Owing to the fact that walnut trees have been seed-propagated in Moroccan traditional orchards and mountains for decades, which involved considerable genetic variation, there is a remarkable lack of studies focusing on walnut genetic variability through molecular markers. The current study aims to assess the molecular variability within the local walnut trees germplasm. For this purpose, the genetic diversity of a set of preselected genotypes from two large walnut regions (high and middle Atlas) was analyzed using ISSR markers. Thus, the specificity of this genetic material is studied via a comparison with some Bulgarian varieties, conserved, in the INRA experimental station. This work aims to provide new data on the species local diversity, of great interest for its conservation and orientation of the genetic improvement program.
Materials and Methods
Plant Material
The investigated accessions consisted of 33 seedling clones prospected in two contrasted agroecosystems in the high and middle Atlas Mountains in Morocco (El-Haouz and Midelt regions, respectively), compared to 8 Bulgarian varieties, grafted on Juglans regia rootstock. The prospected clones were preselected on the basis of their agronomic traits, while Bulgarian genotypes were ex-situ conserved, at the experimental station of National Institute for Agricultural Research (INRA) of Meknes-Morocco.
Denominations, attributed codes, and geographical origins of the studied accessions are listed in . Samples consisted of young leaves randomly collected from each tree, immediately lyophilized, and stored at −80°C before ISSR analysis.
Table 1. List of walnut local clones and Bulgarian varieties included in this study
DNA Extraction
Total DNA was extracted from 40 mg of lyophilized leaves using the CTAB technique described by Saghai-Maroof et al. (Citation1984). The purified total DNA was assessed by measuring the optical density (OD) at 260 nm using spectrophotometer (WPA, Biowave, S2100, Diode Array spectrophotometer). The amount of proteins contained in the DNA suspension was measured at 280 nm. Finally, the OD (260–280) ratio served as to assess the quality or purity of extracted DNA.
ISSR Amplification
DNA of each sample was screened with 13 ISSR primers listed in . Amplifications were performed in a volume of 25 μL containing 20 ng genomic DNA, 5 μM of ISSR primers, 2 units of DNA polymerase (PROMEGA), 1x Taq buffer; 1.5 mM MgCl2 and 0.2 mM for each dNTP.
Table 2. List of ISSR primers used and their annealing temperature
Initially, 48 ISSR primers were selected based on the literature. The optimum annealing temperatures of ISSR primers were determined through gradient PCR using a gradient thermal cycler. After a preliminary screening of these primers, 13 primers were selected for subsequent analysis (). In order to obtain high intensity, resolution, and sharpness DNA fragments with a low background in the ISSR profiles, polymerase chain reaction (PCR) was carried out using the Eppendorf Master Cycler gradient thermocycler. After 5 min at 94°, 35 cycles were performed with 1 min at 94°C, 1 min at either 42°C, 52°C, or 56°C (Annealing temperature for each primer in ) and 1 min at 72°C, followed by a final extension step of 5 min at 72°C. Amplified products were detected using electrophoresis on a 2% agarose gel with 1X TBE buffer and under a voltage of 4 V/cm. bands revelation was made by staining with ethidium bromide and visualized using an UV transilluminator related to an imaging system.
Data Scoring and Statistical Analysis
Qualitative analysis was performed phenotypically using a binary matrix, where the DNA bands, amplified by a given primer, were scored as present (1) or absent (0) for all of the samples. The size of each band produced by ISSR was calculated using pro-Mesurim software, where the algorithm is compiled based on marker bands sizes. Since the markers are dominant, each band represents the phenotype of a biallelic locus. To evaluate the genetic polymorphism and informativeness of the ISSR markers used, the following genetic indexes were calculated: similarity matrix, using the number of polymorphic bands (Nei and Li, Citation1979) and simple matching coefficient for genetic distances using the clustering calculator program (Brzustowski, Citation2002). On this basis, a histogram for comparing individuals in pairs according to the number of ISSR markers that distinguish them was established. A hierarchical ascending classification dendrogram for ISSR markers was constructed on the basis of simple matching coefficient, using the NTSYS-pc ver software. 2.11 (Rohlf, Citation2000), and this similarity matrix was used to construct a cluster based on Unweighted Pair-Group Method with Arithmetic average (UPGMA) method. In order to determine the effectiveness of used markers, the characterization of primers for their ability to differentiate the genotypes was assessed by calculating polymorphic information content (PIC). The PIC, linked to the genetic diversity for each primer, was evaluated according to the formula of De Riek et al. (Citation2001).
where f is the band frequency in the data set.
PIC value of each primer was calculated as the averaged PIC values of its bands.
The Effective multiple ratio (EMR) was calculated according to formula of Powell et al. (Citation1996)
where np is the number of polymorphic loci and n is the total number of loci.
Moreover, the Marker index (MI) was determined as the product of PIC value and the (EMR) MI = PIC x EMR (Chesnokov and Artemyeva, Citation2015b). Marker index (MI) is a statistical parameter used to estimate the total utility of the maker system.
Results and Discussion
Selection of Primers
The 48 ISSRs used in this study were chosen for their high polymorphism in previous studies. Among them 13 primers (27% of initial primers used) were retained for their high polymorphic amplification. By comparison with past results, this number is generally higher than those used in previous walnut genetic studies. For instance, 7 ISSR primers were used to characterize local cultivars of Greek walnut (Christopoulos et al., Citation2010), 9 primers for Italian varieties (Pollegioni et al., Citation2003b) and 8 primers were used by Potter et al. (Citation2008) in 48 walnut cultivars genetic variability assessment.
Genotypes Characterization
The 13 selected primers exhibited 120 clear and reproducible ISSR markers, where their number per primer varied between 7 for the primers F2, F13, and UBC 818X and 13 bands generated by IMA12, with an average of 9 bands per primer (). This number reflects a high level of polymorphism within the studied genotypes. Previous work has revealed 54 bands by eight primers in 48 cultivars (Potter et al., Citation2008), others have found 93 by 7 primers in the study of 56 walnut accessions (Christopoulos et al., Citation2010). In addition, 115 bands were revealed by nine primers in the characterization of 48 varieties of walnut (Pollegioni et al., Citation2003b).
Table 3. ISSR primers, optimal annealing temperatures, and amplification results of ISSR primers in 41 walnut genotypes
These results are comparable to those reported by Potter et al. (Citation2008) who found a number of markers ranging from 5 to 9 markers with an average of 6.8. Doǧan et al., 2014 used 25 ISSR primers with an average of 8 bands, whereas in the characterization of 56 walnut genotypes, Christopoulos et al. (Citation2010) reported between 7 and 20 markers. Similarly, Pollegioni et al. (Citation2003b) showed an average of 12 bands with a range of 9 and 15. Compared to other tree species, carob for example, the number of bands ranged from 1 to 19 was revealed using 15 primers (Konate, Citation2007). This number can be significantly influenced by the analyzed plant species and the nature of the migration gel used (Nagaraju et al., Citation2002; Wiesner and Wiesnerová, Citation2003).
The size-range of the fragments generated by the 13 primers ranged between 167pb (UBC808) and 2834 (F5) (). Over 101 fragments, 75 were revealed polymorphic, with an average polymorphism rate of 75.2%. Previous studies have shown that the size of the bands has varied from 150 to 1100 bp (Sica et al., Citation2005), from 140 to 1500 bp (Nagaraju et al., Citation2002) and from 250 to 2800 bp (Pradeep et al., Citation2005). They also noted that more polymorphism percentage is close to 100%, more the primer is polymorphic and useful in the study of genetic diversity. Primers 1MA5-3 and F8 had the highest diversity index (100%), which amplified 10 and 8 polymorphic bands, respectively. However, no monomorphic band was revealed.
The average polymorphism percentage was consistent compared to previous work of Pollegioni et al. (Citation2003b), who found an average value of about 65%. However, it was greater than that of Potter et al. (Citation2008), who reported a value of 57%, and lower than that of Christopoulos et al. (Citation2010) who found polymorphism percentage of 83%. The difference between the results above might be linked to the number and type of primers used or the nature of the plant material characterized and the specific PCR test used. Besides the above-mentioned reasons, the differences can be attributed to some probable errors in collection labeling or in sampling method (Christopoulos et al., Citation2010).
The polymorphic information content, effective multiple ratio, and marker index values are presented in .
Regarding the diversity index (PIC), the results showed that all primers generated highly polymorphic profiles, with a PIC ranging from 0.212 for the primer F14 to 0.370 for the ‘primer F5, with an average of 0.32 (). Generally, for dominant markers, it has been reported that PIC values range from 0 to 0.5 (Chesnokov and Artemyeva, Citation2015a). The PIC value of each primer was calculated as the average value of its bands. De Riek et al. (Citation2001) reported that the PIC confirms the good ability to discriminate primers as a maximum value of 0.5 for the dominant markers. Christopoulos et al. (Citation2010), reported similar results in walnut genotypes using ISSR markers, where PIC varied between 0.209 and 0.350. These results are in concordance with those of Topçu et al., (Citation2015) and Ahmed et al. (Citation2012), who found an average PIC of 0.40 and 0.39, respectively.
EMR is another parameter used in our study. The highest EMR (10) was obtained from 1MA5-3 and the lowest (2.29) from F2, with an average of (5.33) per primer. MI was calculated for each ISSR primer to determine the overall usefulness of the marker system used. The MI of the primers ranged from 1.27 (F14) to 3.11 (IMA12), with an average of 2.18. The MI values with an average of 2.18 was higher than that obtained in Indian walnut by Noor Shah et al. (Citation2018) (0.24), but lower than that showed by Christopoulos et al. (Citation2010) (3.19) and also reported in Greek walnut by Shamasbi et al. (Citation2018) using ISSR (28.9) for Indian walnut. The high value of the MI for ISSRs is the result of highlights the distinctive nature of these markers. The higher the MI, the better method is used (Rahimi et al., Citation2018).
Previous work for other plant species has reported similar results for EMR and IM settings with small differences (Giachino (Citation2019); Mukherjee et al. (Citation2013), Kumar et al. (Citation2014) and Tagizad et al. (Citation2010)).
Genetic Similarity and Clustering Analysis
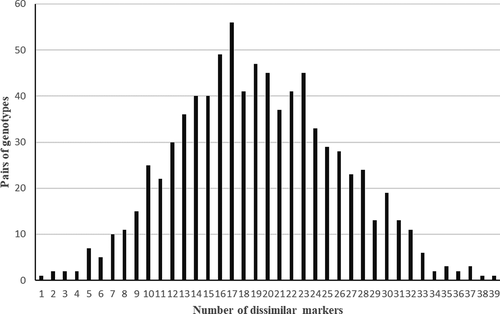
To estimate the putative relatedness, the results show the pairwise comparison between the 41 genotypes gave a total pairs number of 1171. The unimodal distribution indicating an only 10 pairs of genotypes were differentiated by less than 7 markers. The other pairs were distinguished by 8 to 39 markers, where most of the differences comprised between 8 and 32 markers (). These large differences are mainly due to the fact that the genotypes were seed-propagated, which involved considerable genetic variation. The pairwise comparison has been widely used in previous genetics studies (Lynch and Ritland, Citation1999; Pew et al., Citation2015; Ritland, Citation1996; Wang, Citation2002).
It is noted that genetic distances have ranged from 0.083 for (NMDT3/NMDT1), which indicates a probable similarity to 0.417 (NIM1/NMA1), showing a distinguished dissimilarity between the accessions (). This finding is not surprising, since genotypes (NMDT3/NMDT1) are from the same agroecosystem in the Middle Atlas (Barram, Midelt). For the NIM1 and NMA1 genotypes, although they are from the same region of the High Atlas but not from the same village, are genetically spaced. This can be explained either by the fact that farmers share walnut seeds regardless of their origins, that are often related to specific social practices (Hssaini et al., Citation2019) and also because of seedling propagation which explodes genetic diversity. Christopoulos et al. (Citation2010) reported a genetic distance between 0.13 and 0.93, while Mahmoodi et al. (Citation2013) indicated a distance of 0.13 to 0.76 in the study of 21 walnut cultivars. Similarly, Ipek et al. (Citation2018), reported an average genetic similarity of 0.688 in Kenyan walnut trees. These differences can be attributed to the geographical origin factor.
Table 4. Heatmap of distances matrix between the 41 genotypes of walnut
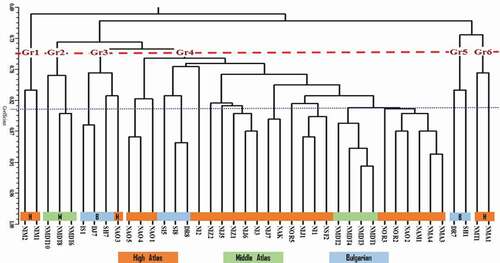
Furthermore, 90 useful polymorphic markers resulted in the construction of a dendrogram using the unweighted pair group method with arithmetic averages (UPGMA) based on genetic similarity coefficient (), which illustrated the genetic relationships between the 41 genotypes studied. The first observation is that most of clones growing in the same agro-ecosystem were clustered in the same subset, which probably indicates that this genetic material was from the same genetic pool of nut seeds. On the other hand, some other clones were clustered together despite their geographical origin difference. This plant material may be exchanged between farmers from different localities. The same findings were reported by Ipek et al. (Citation2018) who reported that this kind of genetic similarities are due to transfer of seeds between different regions. Maghsoodi et al. (Citation2018) studied the genetic diversity in seven Persian walnut populations using ISSR and SRAP Markers, and found that the populations studied are genetically differentiated depending on the geographical distances between genotypes. Generally, the difference in distance between the genotypes shown by previous studies may be explained by the variation in the number of genotypes object of study and the number of primers used.
This classification (CAH) made it possible to highlight six distinct groups according to a similarity index (SI), which is of 0.775. Group 1 include only two genotypes from the High Atlas Mountains, which showed that they belong to a very small gene pool. The second group brings together three genotypes from the Middle Atlas Mountains, while the third group comprised four genotypes, three of which are of Bulgarian origin and one originated in Moroccan (High Atlas), suggesting that these genotypes may have belonged to the same genetic pool. The fourth group include a large number of studied genotypes with the dominance of clones growing in the high Atlas Mountains. Three genotypes from Bulgaria and four genotypes from the middle atlas are inserted between the genotypes from the high atlas. Indeed, the exchange of plant material and more particularly the fruit can explain between Moroccan regions in particular. Two genotypes of Bulgarian origin are gathered in the 5th group, whereas the 6th group contained two genotypes from the high Atlas Mountains. This classification suggests that there is a remarkable geographic structure. Similar results are reported by Ipek et al. (Citation2018), who showed that 6 main groups were revealed by the ISSR markers in the characterization of 8 genotypes and 9 nut cultivars (Juglans regia L) in Konya, showing great diversity genetic. An in-depth analysis of the dendrogram showed that Gr 1 (NIM1 & NIM2) and Gr 6 (NMT1 & NMA1) are genetically very distant, which indicates that these two groups probably belong to a very distinct gene pool. These two groups are usually from the same region (High Atlas) but in different places. This is explained by the fact that these genotypes were seed-grown, which differs from gene pool views. Until recently, walnut was nearly always propagated by seedling (Ebrahimi et al., Citation2011), which allowed each geographic region to maintain diverse populations (Ebrahimi et al., Citation2015). Generally, genotypes gathered in the provinces of Midelt and Marrakech were divided into separate groups. Tabasi et al. (Citation2020a) showed similar results, where they showed that the populations collected from Semnan, Markazi, and Alborz provinces were almost divided into separate subsets. This is in agreement with Pollegioni et al. (Citation2014) who concluded that space and landscape feature act simultaneously on gene flow, influencing the genetic structure of the walnut populations. Previous studies on genetic diversity in walnut have shown similar similarity coefficients (Ahmed et al., Citation2012; Mahmoodi et al., Citation2013; Salieh et al., Citation2013). The Bulgarian varieties fell into three groups Gr5, Gr3, and Gr4. This distinction of the foreign genetic pool confirms the specificity and the authenticity of the local genetic inheritance of the walnut tree. This genetic structure of the Moroccan walnut and its genetic specificity is comparable to that of the Moroccan almond tree, which was able to acquire a distinct genetic pool through the propagation by seedling of the almonds (El Hamzaoui et al., Citation2013). The clustering of few Moroccan and Bulgarian genotypes in the same subgroups is probably due to two factors: i) some Moroccan genotypes are in fact Bulgarian varieties which were distributed by the Ministry of Agriculture during the 2000s to promote walnut production in highlands; and ii) the used nut seeds were collected from Bulgarian varieties or originating from pollination between foreign varieties and local clones.
On the other hand, the four Bulgar genotypes SH1, SH7, DR7, and DR8 were clustered distinctively regardless of their similar denominations (Sheinovo for SH types and Drianoski for DR types). This suggests that they are genetically distant and did not belong to the same genetic pool. This can be due to labeling problems during their introduction to Morocco or could be attributed to gene flow. The research by Christopoulos et al. (Citation2010) showed similar results. However, the genotypes (SI5 and SI8) had a common name (Silistrenski). This is confirmed by the molecular study. These two genotypes can be derived from a common ancestor or they are the clones of the same genotype.
In many places, walnut genotypes are known under different names so they can be the same. This is confirmed by Tabasi et al. (Citation2020a). Precise identification of walnut genotypes is an absolute requirement for good management and the use of genetic material in breeding and selection programs (Maghsoodi et al., Citation2018). The obtained results showed that the ISSR markers are effective in identifying genotypes, assessing genetic diversity, and can be used for DNA fingerprinting of walnut populations (Tabasi et al., Citation2020a). This study will help map the associations in the walnut once the economic characteristics are associated with molecular discoveries. LI et al. (Citation2011) reported the interest of ISSR markers, to evaluate the genetic variation and genetic structure to provide a theoretical basis and technical support for appropriate conservation and application of existing genetic resources of walnut.
The interest of the ISSR markers in these study could be a help to explore the diversity, population genetics for improving walnut future breeding practices, besides providing a piece of useful genetic resource information for studying and understanding the genomic aspect of walnut.
CONCLUSION
The molecular study has shown that the genetic resources in two walnut populations in Morocco (High and Middle Atlas) are genetically diverse and that they include distinct genetic pools. This higher genetic variation is due to seedling propagation or the nature of the walnut cross-pollinated mode of reproduction which is generally anemophilous pollination. A phylo-geographic structure is to be elucidated between the two areas and also between the villages as well, as in relation to the Bulgarian varieties. These last were found genetically close with some Moroccan genotypes, which has been largely linked to the genetic flows between these two genetic pools and the great adoption of foreign varieties by farmers. The study outputs showed that ISSR markers can be successfully used to determine the diversity of walnut genotypes. This study confirmed the effectiveness of molecular markers in the walnut genetic assessment. The genetic pools identified in this study should be used as crossing parents to facilitate walnut breeding in the Morocco mountain area.
Authors contribution
Karim Houmanat wrote the manuscript and interpreted the data. Jamal Charafi designed the methodology of the research and performed the statistical analysis. Lahcen Hssaini helped in the interpreted the data and reading the article. Abdellah Kajji and Rachid Razouk helped in the methodology and provided the technical support for designing and conducting research. Hafida Hanine performed the review.
Declaration of interest
The authors declare no conflict of interest.
References
- Ali, A.M., S.J. Zubair, A.M. Abbas, and J.M. Jubrael. 2012. SSR and RAPD analysis of genetic diversity in walnut (Juglans regia L.) genotypes from Jammu and Kashmir, India. Physiol. Mol. Biol. Plants. 18:149–160. doi: https://doi.org/10.1007/s12298-012-0104-z.
- Ali, A.M., S.J. Zubair, A.M. Abbas, and J.M. Jubrael. 2016. Genetic diversity among Walnuts (Juglans regia) population in Kurdistan Region–Iraq using AFLP-PCR. ZANCO. J. Pure Appl. Sci. 28:50–55.
- Bernard, A., A. Marrano, A. Donkpegan, P.J. Brown, C.A. Leslie, D.B. Neale, et al. 2020. Association and linkage mapping to unravel genetic architecture of phenological traits and lateral bearing in Persian walnut (Juglans regia L.). BMC Genomics. 21:1–25. doi: https://doi.org/10.21203/rs.2.18573/v2.
- Bernard, A., F. Lheureux, and E. Dirlewanger. 2018b. Walnut: Past and future of genetic improvement. Tree. Genet. Genomes. 14:1. doi: https://doi.org/10.1007/s11295-017-1214-0.
- Bernard, A., T. Barreneche, F. Lheureux, and E. Drilewanger. 2018a. Analysis of genetic diversity and structure in a worldwide walnut (Juglans regia L.) germplasm using SSR markers. PLoS ONE 13:1–19.
- Brzustowski, J. 2002. Clustering Calculator “fast clustering algorithms, including UPGMA and Saitou-Nei neighbor joining” Department of Biological Sciences, University of Alberta; Canada. Accessed 30 March, 2020. http://www.biology.ualberta.ca/jbrzusto/cluster.php
- Charafi, J., A. El Meziane, A. Moukhli, B. Boulouha, C. El Modafar, and B. Khadari. 2008. An ancient Moroccan olive germplasm collection identified by a SSR locus-based genetic study. Genet. Resour. Crop Evol. 55:893–900. doi: https://doi.org/10.1007/s10722-007-9294-6.
- Chesnokov, Y.V., and A.M. Artemyeva. 2015a. Evaluation of the measure of polymorphism information of genetic diversity. Agric. Biol. 50:571–578.
- Chesnokov, Y.V., and A.M. Artemyeva. 2015b. Evaluation of the measure of polymorphism information of genetic diversity. Agric. Biol. 50:571–578.
- Christopoulos, M.V., D. Rouskas, E. Tsantili, and P.J. Bebeli. 2010. Germplasm diversity and genetic relationships among walnut (Juglansregia L.) cultivars and Greek local selections revealed by Inter-Simple Sequence Repeat (ISSR) markers. Sci. Hort. 125:584–592. doi: https://doi.org/10.1016/j.scienta.2010.05.006.
- De Riek, J., E. Calsyn, I. Everaert, E. Van Bockstaele, and M. De Loose. 2001. AFLP based alternatives for the assessment of distinctness, uniformity and stability of sugar beet varieties. Theor. Appl. Genet. 103:1254–1265. doi: https://doi.org/10.1007/s001220100710.
- Doğan, Y., S. Kafkas, M. Sütyemez, Y. Akça, and N. Türemiş. 2014. Assessment and characterization of genetic relationships of walnut (Juglans regia L.) genotypes by three types of molecular markers. Sci. Hort. 168:81–87. doi: https://doi.org/10.1016/j.scienta.2014.01.024.
- Ebrahimi, A., A. Khadivi-Khub, Z. Nosrati, and R. Karimi. 2015. Identification of superior walnut (Juglans regia) genotypes with lateleafing and high kernel quality in Iran. Sci. Hortic. 193:195–201. doi: https://doi.org/10.1016/j.scienta.2015.06.049.
- Ebrahimi, A., R. Fatahi, and Z. Zamani. 2011. Analysis of genetic diversity among some Persian walnut genotypes (Juglans regia L.) using morphological traits and SSRs markers. Sci. Hortic. 130:146–151. doi: https://doi.org/10.1016/j.scienta.2011.06.028.
- El Hamzaoui, A., A. Oukabli, J. Charafi, and M. Moumni. 2013. Moroccan almond is a distinct gene pool as revealed by SSR. Sci. Hortic. 154:37–44. doi: https://doi.org/10.1016/j.scienta.2013.02.022.
- Ghanbari, A., M. Faraji, M. Behnamian, A. Estaji, A. Pyrayesh, and S. Fahim. 2019. Genetic diversity evaluation of some Walnut (Juglans regia L.) Genotypes in Meshkin-Shahr by ISSR Marker. J. Nuts. 10:1–8. doi: https://doi.org/10.22034/jon.2019.664205.
- Giachino, R.R.A. 2019. Investigation of the genetic variation of anise (Pimpinella anisum L.) using RAPD and ISSR markers. Genet. Resour. Crop Evol. doi: https://doi.org/10.1007/s10722-019-00861-y.
- Hssaini, L., H. Hanine, R. Razouk, S. Ennahli, A. Mekaoui, A. Ejjilani, and J. Charafi. 2020. Assessment of genetic diversity in Moroccan fig (Ficus carica L.) collection by combining morphological and physicochemical descriptors. Genet. Resour. Crop Evol. 67(2):457–474. doi: https://doi.org/10.1007/s10722-019-00838-x.
- Hssaini, L., H. Hanine, R. Razouk, S. Ennahli, A. Mekaoui, and J. Charafi. 2019. Characterization of local fig clones (Ficus carica L.) collected in Northern Morocco. Fruits. 74:55–64. doi: https://doi.org/10.17660/th2019/74.2.1.
- Ipek, M., Ş. Arıkan, L. Pırlak, and A. Eşitken. 2018. Phenological, morphological and molecular characterization of some promising Walnut (Juglans regia L) Genotypes in Konya. Erwerbs-Obstbau. 61:149–156. doi: https://doi.org/10.1007/s10341-018-0411-9.
- Ji, A., Y. Wang, G. Wu, W. Wu, H. Yang, and Q. Wang. 2014. Genetic diversity and population structure of North China mountain walnut revealed by ISSR. Am. J. Plant Sci. 5:21–3194. doi: https://doi.org/10.4236/ajps.2014.521335.
- Kabiri, G., S. Bouda, M. Elhansali, and A. Haddioui. 2018. Morphological and pomological variability analysis of Walnut (Juglans regia L.) genetic resources from the middle and high atlas of Morocco. Atlas J. Biol. 575–582. doi: https://doi.org/10.5147/ajb.v0i0.179.
- Khadari, B., J. Charafi, A. Moukhli, and M.A. Ater. 2008. Substantial genetic diversity in cultivated Moroccan olive despite a single major cultivar: A paradoxical situation evidenced by the use of SSR loci. Tree Genet. Genomes. 4:213–221. doi: https://doi.org/10.1007/s11295-007-0102-4.
- Kodad, O., G. Estopañán, T. Juan, I. Socias, R. Company, and M. Sindic. 2016. Genotype and year variability of the chemical composition of walnut oil of Moroccan seedlings from the high Atlas Mountains. Grasas Aceites. 67:e116. doi: https://doi.org/10.3989/gya.0256151.
- Konate, I. 2007. Diversité Phénotypique et Moléculaire du Caroubier (Ceratonia siliqua L.) et des Bactéries Endophytes qui lui sont Associées. Thèse, Biotechnologie et Biologie Moléculaire, Universite Mohammed V-Agdal, Faculte des Sciences, Rabat, 196p.
- Kumar, A., P. Mishra, S.C. Singh, and V. Sundaresan. 2014. Efficiency of ISSR and RAPD markers in genetic divergence analysis and conservation management of Justicia adhatoda L., a medicinal plant Subhash. Plant Syst Evol. 300:1409–1420.
- LI, C., S.P. LUO, B. ZENG, J. LI, and G. LI. 2011. Analysis of genetic diversity of germplasm resources of walnut (Juglans regia L.) revealed by ISSR in Xinjiang of China. Sci. Agr. Sinica 44:1871–1879.
- Luo, X., and J. Liu. 2019. Transcriptome Analysis of Acid-Responsive Genes and Pathways Involved in Polyamine Regulation in Iron Walnut. Genes. 10:8–605. doi: https://doi.org/10.3390/genes10080605.
- Lynch, M., and K. Ritland. 1999. Estimation of pairwise relatedness with molecular markers. Genetics 152:1753–1766.
- Maghsoodi, M., M. Sheidai, and F. Koohdar. 2018. Population genetic study in Juglans regia L. (Persian walnut) and its taxonomic status within the genus Juglans L. Phytotaxa. 376:154–166. doi: https://doi.org/10.11646/phytotaxa.376.4.1.
- Mahmoodi, R., F. Rahmani, and R. Rezaee. 2013. Genetic diversity among Juglans regia L. genotypes assessed by morphological traits and microsatellite markers. Spanish J. Agric. Res. 11:431–437.
- Malvolti, M.E., B. Fornari, E. Maccaglia, and F. Cannata. 2001. Genetic linkage mapping in an intraspecific cross of walnut (Juglans regia L.) using molecular markers. Acta Hortic. 544:179–185.
- Malvolti, M.E., P. Pollegioni, A. Bertani, S. Mapelli, and F. Cannata. 2010. Juglans regia provenance research by molecular, morphological and biochemical markers: A case study in Italy. Biorem. Biodiv. Bioavail. 4:84–92.
- Miltiadis, V.C., D. Rouskasb, A. Eleni Tsantili, and P.J. Bebelic. 2010. Germplasm diversity and genetic relationships among walnut (Juglans regia L.) cultivars and Greek local selections revealed by Inter-Simple Sequence Repeat (ISSR) markers. Sci. Hortic. 125(2010):584–592.
- Mozaffarian, F., M. Mardi, A. Sarafrazi, and G.N. Ganbalani. 2008. Assessment of geographic and host-associated population variations of the carob moth, Ectomyelois ceratoniae, on pomegranate, fig, pistachio and walnut, using AFLP markers. J. Insect Sci. 8:1–6. doi: https://doi.org/10.1673/031.008.0601.
- Mukherjee, A., B. Sikdar, B. Ghosh, A. Banerjee, E. Ghosh, M. Bhattacharya, and S.C. Roy. 2013. RAPD and ISSR analysis of some economically important species, varieties, and cultivars of the genus Allium (Alliaceae). Turk. J. Bot. 37:605–618.
- Nagaraju, J., M. Kathirvel, R.R. Kumar, E.A. Siddiq, and S.E. Hasnain. 2002. Genetic analysis of traditional and evolved Basmati and non-Basmati rice varieties by using fluorescence-based ISSR-PCR and SSR markers. PNAS. 99:5836–5841. doi: https://doi.org/10.1073/pnas.042099099.
- Nei, M., and W.H. Li. 1979. Modèle mathématique de la variation de l’étude génétique en ce qui concerne les endonucléases de restriction. Proc. Natl. Acad. Sci. USA 76:5269–5273.
- Noor Shah, U., J.I. Mir, N. Ahmed, and K.M. Fazili. 2018. Assessment of germplasm diversity and genetic relationships among walnut (Juglans regia L.) genotypes through microsatellite markers. J. Saudi Soc. Agri. Sci. 17:339–350.
- Pew, J., P.H. Muir, J.L. Wang, and T.R. Frasier. 2015. Related: An R package for analysing pairwise relatedness from codominant molecular markers. Mol. Ecol. Resour. 15:557–561.
- Pollegioni, P., K. Woeste, F. Chiocchini, S. Del Lungo, M. Ciolfi, I. Olimpieri, et al. 2017. Rethinking the history of common walnut (Juglans regia L.) in Europe: Its origins and human interactions. PloS One. 12. doi:https://doi.org/10.1371/journal.pone.0172541.
- Pollegioni, P., Woeste, K.E., Chiocchini, F., Olimpieri, I., Tortolano, V., Clark, J., Hemery, G.E., Mapelli, S., Malvolti, M.E. 2014. Landscape genetics of Persian walnut (Juglans regia L.) across its Asian range. Tree Genet. Genomes. 10:1027–1043. doi: https://doi.org/10.1007/s11295-014-0740-2.
- Pollegioni, P., S. Bartoli, F. Cannata, and M.E. Malvolti. 2003b. Genetic differentiation of four Italian walnut (Juglans regia L.) varieties by inter simple sequence repeat (ISSR). J. Genet Plant. Breed. 57:231–240.
- Potter, D., F. Gao, G. Aiello, C. Leslie, & G. McGranahan. 2002. Intersimple sequence repeat markers for fingerprinting and determining genetic relationships of walnut (Juglans regia) cultivars. J. Am. Soc. Hortic. Sci. 127:75–81.
- Potter, D., F. Gao, G. Aiello, C. Leslie, and G. Mc Granahan. 2008. Intersimple Sequence Repeat Markers for Fingerprinting and Determining Genetic Relationships of Walnut (Juglansregia) Cultivars. J. Amer. Soc. Hort. Sci. 127:75–81.
- Powell, W., M. Morgante, C. Andre, M. Hanafey, J. Vogel, S. Tingey, and A. Rafalski. 1996. The utility of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol. Breeding 2:225–238.
- Pradeep, A.R., S.H. Chatterjee, and C.V. Nair. 2005. Genetic differentiation induced by selection in an inbred population of the silkworm Bombyx mori, revealed by RAPD and ISSR markers systems. J. Appl. Genet. 46:219–298.
- Rahimi, M., H. Hatami, and M.M. Maleki. 2018. Identification of informative markers of agronomic traits in different ecotypes of sand plantain (Plantago psyllium). BIOLOGIJA 63(4):325–333.
- Rashnodi, N., J.E. Moghadam, and A. Arash Fazeli. 2019. The Screening of Persian Walnut Genotypes Based on the Quantitative and Qualitative Characters and the Investigation of Genetic Diversity among Promising Samples Using ISSR Marker. Plant Prod. 42(2):279–294.
- Ritland, K. 1996. Estimators for pairwise relatedness and individual inbreeding coefficients. Genet. Res. 67:175–185.
- Rohlf, F.J. 2000. Taxonomie Numérique NTSYS-PC. Système d’analyse multivariée. Version 2.1. EXETER software, Setauket, New York.
- Saghai-Maroof, M.A., K.M. Soliman, R.A. Jorgensen, and R.W. Allard. 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. PNAS 81:8014–8018.
- Salieh, F.M.H., N.A.R. Tahir, and J.M. Faraj. 2013. Assessment of genetic relationship among some Iraqi walnut genotypes (Juglans regia L.) in Sulaimani region using RAPD and SSR molecular markers. Jordan J. Agric. Sci. 9(3).
- Shah, U.N., J.I. Mir, N. Ahmed, and K.M. Fazili. 2018a. Assessment of germplasm diversity and genetic relationships among walnut (Juglans regia L.) genotypes through microsatellite markers. J. Saudi Soc. Agric. Sci. 17:339–350. doi: https://doi.org/10.1016/j.jssas.2016.07.005.
- Shah, U.N., J.I. Mir, N. Ahmed, and K.M. Fazili. 2019. Genetic Diversity Analysis of Walnut (Juglans regia L.) from Kashmir Valley Using RAPD and ISSR Markers. Agrotechnology. 8:185. doi: https://doi.org/10.35248/2168-9881.19.8.185.
- Shamasbi, F.V., N. Nasiri, and E. Shokri. 2018. Genetic diversity of Persian ecotypes of Indian Walnut (Aeluropus littoralis (Gouan) Pari.) by AFLP and ISSR Markers. Cytol Genet. 52(3):222–230.
- Sica, M., G. Graziella, S. Montieri, L. Gaudio, and S. Aceto. 2005. ISSR markers show differentiation among Italian populations of Asparagus acutifolius L. Genetics. 6:1–7. doi: https://doi.org/10.1186/1471-2156-6-17.
- Tabasi, M., M. Sheidai, D. Hassani, and F. Koohdar. 2020a. DNA. fingerprinting and genetic diversity analysis with SCoT markers of Persian walnut populations (Juglans regia L.) in Iran. Genet. Res. Crop Evol. doi: https://doi.org/10.1007/s10722-020-00914-7.
- Tagizad, A., J. Ahmadi, R. Haddad, and M. Zarrabi. 2010. A comparative analysis of ISSR and RAPD markers for studying genetic diversity in Iranian pistachio cultivars. Iran J. Genet. Plant Breed 1(1):9–13.
- Topçu, H., Ikhsan, A. S., Sütyemez, M., Çoban, N., Güney, M., & Kafkas, S. 2015. Development of 185 polymorphic simple sequence repeat (SSR) markers from walnut (Juglans regia L.). Sci Horti, 194: 160–167.
- Torokeldiev, N., M. Ziehe, O. Gailing, and R. Finkeldey. 2019. Genetic diversity and structure of natural Juglans regia L. populations in the southern Kyrgyz Republic revealed by nuclear SSR and EST-SSR markers. Tree. Genet. Genomes. 15:1–5. doi: https://doi.org/10.1007/s11295-018-1311-8.
- Vijayan, K. 2005. Inter simple sequence repeat (ISSR) polymorphism and its application in mulberry genome analysis. Int. J. Ind. Entomol. 10:79–86.
- Vyas, D., S.K. Sharma, and D.R. Sharma. 2003. Genetic structure of walnut genotype using leaf isozymes as variability measure. Sci. Hortic. 97:141–152. doi: https://doi.org/10.1016/S0304-4238(02)00129-2.
- Wang, J. 2002. An estimator for pairwise relatedness using molecular markers. Genetics 160:1203–1215.
- Wiesner, I., and D. Wiesnerová. 2003. Effect of resolving medium and staining procedure on inter-simple sequence-repeat (ISSR) patterns in cultivated flax germplasm. Genet. Res. Crop Evol. 50:849–853. doi: https://doi.org/10.1023/A:1025942518475.
- Woeste, K., G. McGranahan, and R. Bernatzky. 1996. The identification and characterization of a genetic marker linked to hypersensitivity to the cherry leafroll virus in walnut. Mol. Breed. 2:261–266. doi: https://doi.org/10.1007/BF00564203.
- Wu, Y., D. Pei, S. Xi, and J. Li. 2000. A study on the genetic relationship among species in Juglans L. using RAPD markers. Acta Hortic. 27:17–22.
- Yang, L.X. 2005. Effect of water extracts of larch on growth of Manchurian walnut seedings. J. Forest. Res. 16:285–288. doi: https://doi.org/10.1007/BF02858190.
- You, F.M., K.R. Deal, J. Wang, M.T. Britton, J.N. Fass, D. Lin, A.M. Dandekar, C.A. Leslie, M. Aradhya, M.C. Luo, J. Dvorak. 2012. Genome-wide SNP discovery in walnut with an AGSNP pipeline updated for SNP discovery in allogamous organisms. BMC Genomics. 13:1–354. doi: https://doi.org/10.1186/1471-2164-13-354.
- Zerillo, M.M., J.I. Caballero, K. Woeste, A.D. Graves, C. Hartel, J.W. Pscheidt, J. Tonos, K. Broders, W. Cranshaw, S.J. Seybold and N. Tisserat. 2014. Population structure of Geosmithia morbida, the causal agent of thousand cankers disease of walnut trees in the United States. PloS One. 9–11. doi:https://doi.org/10.1371/journal.pone.0112847.
- Zhu, Y., Y. Yin, K. Yang, J. Li, Y. Sang, L. Huang & S. Fan. 2015. Construction of a high-density genetic map using specific length amplified fragment markers and identification of a quantitative trait locus for anthracnose resistance in walnut (Juglans regia L.). BMC Genomics. 16:1–61.
- Zietkiewicz, E., A. Raflski, and D. Labuda. 1994. Genome fingerprinting by simple sequence repeat (SSR) anchored Polymerase Chain Reaction Amplification. Genomics 20:176–183.