ABSTRACT
In this study, effects of foliar Zn sprays at different periods on yield, fruit quality attributes, bioactive compounds and micro-macro elements of ‘Granny Smith’ (Malus x domestica Borkh.) apples grafted on MM111 rootstocks were investigated. Experimental treatments were arranged as control (without Zn sprays), Zn-1 [Zn sprays 1 month after full bloom (when the fruit had a size of a walnut)], Zn-2 (Zn sprays 1 and 2 months after full bloom) and Zn-3 (Zn sprays 1, 2 and 3 months after full bloom). Experimental trees were sprayed with 0.2% Zn (ZnSO4.7H2O). Compared to control, Zn sprays increased yields. SSC values of Zn-2 and Zn-3 treatments were lower than the control and Zn-1 treatments. The total phenolics and total flavonoids of Zn-treated fruit were lower than the control fruit. The greatest vitamin C and antioxidant activity were obtained from Zn-2 and Zn-3 treatments, respectively. Zn-2 and Zn-3 treatments had greater zinc and phosphorus concentrations than the control treatments and Zn-sprayed fruit had greater nitrogen and potassium concentrations than the control fruit.
It was concluded based on present findings that Zn treatments could be used as an effective tool to increase yield, antioxidant activity and mineral elements of ‘Granny Smith’ apples.
Introduction
Micro element deficiencies, especially zinc (Zn) deficiency, are common in world and Turkish soils. Zn deficiency is generally common in agricultural fields of arid and semi-arid regions with low soil pH and lime levels. Previous studies revealed that Zn-deficiency was encountered in about 30% of agricultural fields worldwide (Alloway, Citation2009). Soil pH is the most significant factor designating the availability of soil Zn. Soil Zn can easily be adsorbed onto cation exchange surfaces at high pH levels. The other factors influencing soil Zn levels include soil texture, high lime contents, type of clay, soil moisture content, organic matter content and plant uptake (Marschner, Citation1995). Since zinc is an important micro nutrient for plants, Zn-deficiency has negative impacts on various metabolic processes in plants (Ozturk and Tarakcıoglu, Citation2016). Previous studies indicated the most significant function of Zn in plants as direct participation into protein synthesis. It was also indicated that Zn played direct or indirect roles in synthesis of more than 300 enzymes (Alloway, Citation2013; Cakmak, Citation2000). Plant meristematic growth points require high Zn levels. Therefore, in plants with Zn-deficiency, biochemical processes such as cell elongation, division and differentiation are negatively influenced (Cakmak et al., Citation1989). In Zn-deficient plants, phytohormones, especially auxin (IAA) exist at low concentrations. Under zinc deficiency, peroxidase activity and free oxygen radicals increase, and thus oxidative degradation occurs in plant cells (Cakmak et al., Citation1996). Zn deficiency can be eliminated through soil and/or foliar Zn fertilizations. Zinc fertilizer sources can be gathered under four main groups as of inorganic, synthetic chelates, natural organic compounds and inorganic compounds. Inorganic zinc fertilizer sources include: ZnO, ZnCO3, ZnSO4, Zn(NO3)2 and ZnCl2 (Mortvedt and Gilkes, Citation1993; Wedekind et al., Citation1994). Among these inorganic Zn sources, ZnSO4 is more commonly used to eliminate Zn deficiency as compared to the other sources (Mortvedt, Citation1991) since ZnSO4 is more soluble in water, cheap and easy to supply (Mortvedt, Citation1991; Schulte and Walsh, Citation1982). Previous studies revealed that foliar Zn treatments not only improve growth and development of fruit trees but also significantly influence fruit yields (Aglar et al., Citation2016; Ozturk et al., Citation2019a; Sahota and Arora, Citation1981; Soliemanzadeh and Mozafari, Citation2014). Foliar Zn treatments increase fruit carbohydrate levels and activate carbohydrate enzymes (Yogaratnam and Johnson, Citation1982). Also many cultural practices such as irrigation, pruning, including application of nutrients affect yield and fruit quality in the following year (Milosevic and Milosevic, Citation2015).
This study was conducted to determine the effects of foliar Zn sprays at different periods on yield, quality attributes, bioactive compounds and mineral elements of ‘Granny Smith’ (Malus x domestica Borkh.) apples grafted on MM111 rootstocks.
Materials and Methods
Plant Materials
Nine-years old uniform ‘Granny Smith’ apple trees (Malus x domestica Borkh.) grafted on MM111 rootstock in Karaman Province (37° 44ʹ 55.22”N latitude, 33° 51ʹ 57.31”E longitude and 1010 m altitude) in Central Anatolia Region of Turkey were selected as the plant material of the experiments. The trees were planted at 2.5 m × 5 m spacing and trained by a central leader system. Standard cultural practices such as irrigation, fertilization, and disease control were performed regularly throughout the experimental period. Each tree was supplied with 300 g N, 150 g P2O5, 50 g K2O and 25 g FeSO4.7H2O. Half of N and all of P, K and Fe were applied in February and the remaining half of N was applied when the fruit reached to a size of a walnut.
Experimental soils had 0.64 mg kg−1 Zn (insufficient), 4.11 mg kg−1 Fe (insufficient), 8.20 kg da−1 P2O5 (insufficient), 86.0 kg da−1 K2O (sufficient), 2.08% organic matter (medium), 0.42 dS/m salt (unsaline), 8.20 pH (alkaline), 54.7% lime (high) and soil texture was clay-loam (CL). Soil analysis was performed in accordance with Lindsay and Norvell (Citation1978), texture with Bouyoucous (Citation1951), lime with Çaglar (Citation1949), pH, organic matter and salt with Jackson (Citation1959).
Experimental Design and Treatments
Experiments were conducted in randomized plots design with five replications and with four trees in each replicate. In each replicate, 1 tree was used as control, 1 tree was used for Zn-1 treatments [Zn sprays 1 month after full bloom (when the fruit had a size of a walnut)], 1 tree was used for Zn-2 treatments (Zn sprays 1 and 2 months after full bloom) and 1 tree was used for Zn-3 treatments (Zn sprays 1, 2 and 3 months after full bloom). Zn sprays were applied early in the morning of a non-windy day without a forecast of rain within 24 h with a hand-sprayer as to wet the entire tree. ZnSO4 was applied to trees at 0.2% dose and in the form of ZnSO4.7H2O. To improve the efficiency of treatments, 0.01% Tween-20 was used as surfactant. Only water+0.01% Tween 20 solution was sprayed to control trees.
All fruit were manually harvested at the estimated harvest time (180 days after full bloom). Fruit was placed into plastic boxes and transported to laboratory for analyses with a frigorific vehicle (at 10 °C and 85% relative humidity).
Yield, Fruit Mass, Geometric Mean Diameter, Firmness
All fruit harvested from each tree were weighed with a digital scale (Radwag PS 4500/C/1, Poland) and the fruit yield was expressed in kg tree−1. Fruit mass was calculated as the ratio of fruit yield to number of fruit harvested from each tree and expressed in g. Fruit length (L), width (W) and thickness (T) were measured with a digital caliper (± 0.01 mm) (SPI Tronic 6”, USA). Geometric mean diameter (Dg) was determined by using the formula [Dg = (LWT)1/3] defined by Mohsenin (Citation1970). For fruit firmness, peel was removed from three equilateral sections with a peeler and a hand penetrometer (FT–327, McCormick Fruit Tech., WA, USA) with an 11.1 probe was used. Results were expressed in Newton (N).
Color Characteristics
L*, chroma and hue angles were measured with a colorimeter (Konica-Minolta, CR-400, Japan). CIE (Commission Internationale de l’Eclairage system) was used in color measurements. L*, chroma and hue angle measurements were made on 15 fruit randomly taken from each tree of each replicate. Then, the XYZ values were converted to L*, a* and b* coordinates using the equations corresponding to illuminant D65 and standard observer 10°. Chroma was calculated with C* = (a*2 + b*2)1/2 and hue angle with h° = tan−1 b*/a*.
Soluble Solids Content (SSC), Titratable Acidity, Starch Index
Fifteen fruit harvested from each tree were initially washed through distilled water and unpeeled with a sharp stainless knife. Then, a slice was taken from each fruit, chopped in an electrical blender and homogenized. Resultant homogenate was filtered through a cheese cloth. SSC of the filtrate was measured in a digital refractometer (PAL-1, Atago, USA) and expressed in %. About 10 ml sample was taken from each extract and supplemented with 10 ml distilled water. Then the mixture was titrated with 0.1 N sodium hydroxide (NaOH) until a pH of 8.1. Titratable acidity was expressed in malic acid equivalent 100 mL−1. For starch index, fruit were divided into halves with a stainless knife and slice of 1 cm thick was cut from stalk side of the fruit (Ozturk et al., Citation2019b). Then, 0.5% iodized potassium iodine (IKI) was sprayed over the slice as to wet the whole slice. About 15 min later, level of dark blue color generated over the slice surface was assessed based on Starch-Iodine Index Chart (where 1 = 100% starch and 8 = 0% starch) (Blanpied and Silsby, Citation1992).
Vitamin C, Total Phenolics, Total Flavonoids and Antioxidant Activity
For vitamin C, sufficient quantity of extract and a refractometer (Merck RQflex plus 10, Turkey) was used in accordance with the method specified by Ozturk et al. (Citation2018). Results were expressed in mg 100 g−1. For total phenolics, total flavonoids and antioxidant activity, 15 g fruit sample was taken from the homogenate prepared to determine SSC, placed into a tube and preserved at −20 °C until the analyses. Then, frozen samples were thawed at 21 °C, and fruit juice of samples was separated from the pulp via centrifuging the slurry at 12 000 × g at 4 °C for 30 min. Prepared juice was diluted with distilled water and used for analyses of bioactive compounds.
Folin–Ciocalteu (FC) method was used to determine the total phenolics of the samples. Analytical-grade gallic acid was used as the standard. The total phenolic content was measured according to Singleton and Rossi (Citation1965). It was measured with an automated UV-VIS spectrophotometer (Shimadzu, Kyoto, Japan). Results were presented in mg GAE 100 g−1. Total flavonoids were determined in accordance with Zhishen et al. (Citation1999). Results were expressed in mg QE (quercetin equivalent) 100 g−1. The 1.1-diphenyl-2-picryl-hydrazil (DPPH) is a stable free radical. Thus, the free radical scavenging activity of methanol extract of fresh fruit was determined by DPPH as described in Blois (Citation1958). Results were expressed in mmol Trolox equivalent (TE) 100 g−1.
Mineral Element Analysis
Sufficient quantity of fruit sample was taken from the samples prepared for SSC, and they were sliced into small pieces and dried in an oven at 70 °C. Samples were then ground in a hand-mixer and subjected to microwave digestion (Kacar and Inal, Citation2008). P, K, Fe and Zn readings were performed on resultant extracts in an ICP-OES (Varia Vista) device. Nitrogen was determined in accordance with Kjeldahl distillation method (Bremner, Citation1965).
Statistical Analysis
Experimental data were subjected to analysis of variance (ANOVA) in accordance with randomized plot design. Significant means were compared with the aid of Duncan’s Multiple Range test (DMRT). Statistical analyses were conducted with the aid of SPSS 21.0 software. All analyses were performed with a 95% confidence level (p < .05).
Results and Discussion
Yield, Fruit Mass, Geometric Mean Diameter, Firmness
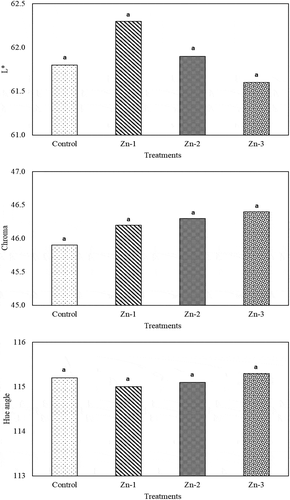
Several studies have been conducted with plant nutrients to get higher yield per unit area. It was observed in this study that foliar Zn sprays significantly increased yield per tree. Fruit mass increased with Zn-1 and Zn-2 treatments, but the differences in fruit mass of the treatments were not found to be significant. For geometric mean diameters, Zn-1 treatments had greater values than the control and the other Zn treatments. Zn treatments did not have significant effects on fruit firmness ().
Figure 1. Effects of foliar zinc sprays on yield, fruit mass, geometric mean diameter and fruit firmness of ‘Granny Smith’ apples. The means shown with the same lower case letter on the bar are no different according to DMRT at P < .05
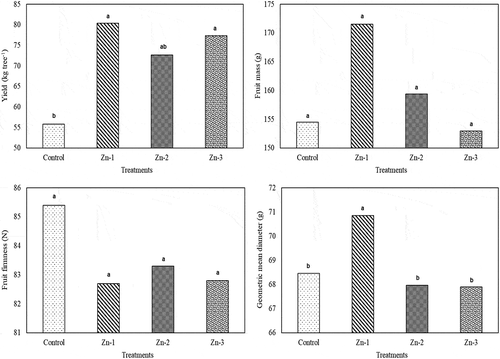
Gianguzzi et al. (Citation2017) indicated that 0.2% Zn treatments 30 days after full bloom significantly increased yield and fruit size of ‘Glaxy Gala’ apples, but did not have significant effects on fruit firmness. Zn has significant contributions to carbohydrate quantities of the fruit and plays a significant role in activation of carbohydrate enzymes (Yogaratnam and Johnson, Citation1982). Thus, parallel to increasing cellular activities, Zn treatments play a role in accumulation of storage substances, provide plant nutrients and thus increase fruit sizes (Eman et al., Citation2007). Such a case also increased yield and fruit size of the present study. Contrarily, insignificant effects of Zn treatments on fruit sizes were also reported for apples (Zhang et al., Citation2013)
Color Characteristics
Effects of Zn treatments on color characteristics were not different from the control treatments (). Similarly, Gianguzzi et al. (Citation2017) indicated that Zn (0.2% ZnSO4) treatments did not yield significant changes in fruit color of ‘Glaxy Gala’ apples. Contrarily, Aglar et al. (Citation2016) indicated that Zn (0.3% ZnSO4) treatments promoted red color development of ‘Jersey Mac’ apples. Differences in results of different studies were mostly attributed to cultivars and application doses.
Soluble Solids Content (SSC), Titratable Acidity, Starch Index
Significant changes were observed in SSC of ‘Granny Smith’ apples with foliar Zn sprays (P < .05). SSC values of Zn-2 (14.8%) and Zn-3 (14.2%) treatments were significantly lower than the SSC value of the control treatment (15.3%). Again, SSC value of Zn-3 treatment was also significantly lower than the SSC of the other Zn treatments. Although titratable acidity of the control (0.65), Zn-1 (0.68) and Zn-2 (0.63 g malic acid 100−1 ml) treatments were statistically similar, titratable acidity significantly decreased in Zn-3 treatments and reduced to 0.55 g malic acid 100−1 ml level. The starch index of Zn-treated fruit were not significantly different from the control fruit ().
Figure 3. Effects of foliar zinc sprays on SSC, titratable acidity and starch index of ‘Granny Smith’ apples. * 1 = 100% starch, 8 = 0% starch in scale. The means shown with the same lower case letter on the bar are no different according to DMRT at P < .05
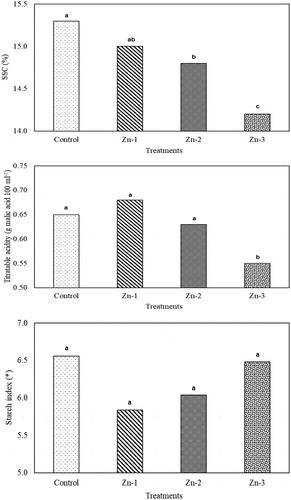
Fruit SSC values increase, but titratable acidity decrease with the progress of ripening (Ozturk et al., Citation2019a). In present study, SSC values significantly decreased with Zn-2 and Zn-3 treatments and titratable acidity significantly decreased only with Zn-3 treatments. This effect of zinc application can be explained by the possibility that the carbohydrate competition between the fruit and the shoot or leaf has changed against the fruit. It has been reported that zinc promotes auxin production at the shoot tips (Marschner, Citation1995). Auxins are thought to be involved in regulating the sink strength by controlling the import of assimilates to the sink (Hansen and Grossmann, Citation2000). Furthermore, the fruit sugar and acid content depend on the activity of their metabolic enzyme and their interaction (Tucker, Citation1993). Zn application may have caused this result by affecting the relevant enzyme activities. Although there are research results indicating that zinc application has an effect on the activity of enzymes involved in carbohydrate metabolism (Zhang et al., Citation2016) more detailed studies are needed on this subject. Zhang et al. (Citation2013) indicated that the effects of Zn treatments 21 days after full bloom on SSC of Gala apples were not significantly different from the control treatments, but titratable acidity decreased with Zn treatments as it was in the present study. In another study, SSC and acidity of apples treated with chelated-Zn (0.13 Zn%) were not significantly different from the values of the control apples (Rasouli and Saba, Citation2018). Similar findings were reported by Wojcik and Popinska (Citation2009) for pears. Differences in results of different studies were mainly resulted from Zn application dates, doses, fruit cultivars and species.
Vitamin C, Total Phenolics, Total Flavonoids and Antioxidant Activity
Total phenolics and total flavonoids of Zn-treated fruit were significantly lower than the control fruit. Decreasing effects of Zn-1 treatment on total phenolics were more remarkable than the effects of the other Zn treatments. Total flavonoids of Zn-1 treatments were significantly greater than the Zn-2 treatments. Antioxidant activity of Zn-3-treated apples was significantly greater than the control and the other Zn treatments. Effects of Zn treatments on vitamin C were similar with the control treatments. But, vitamin C of Zn-2 treatment was significantly greater than the Zn-1 treatment (). Contrary to present findings, Zn treatments significantly decreased vitamin C content of apples, but the effects of Zn on vitamin C have not been fully elucidated, yet (Rasouli and Saba, Citation2018; Zhang et al., Citation2013).
Figure 4. Effects of foliar zinc sprays on vitamin C, total phenolics, total flavonoids and antioxidant activity of ‘Granny Smith’ apples. The means shown with the same lower case letter on the bar are no different according to DMRT at P < .05
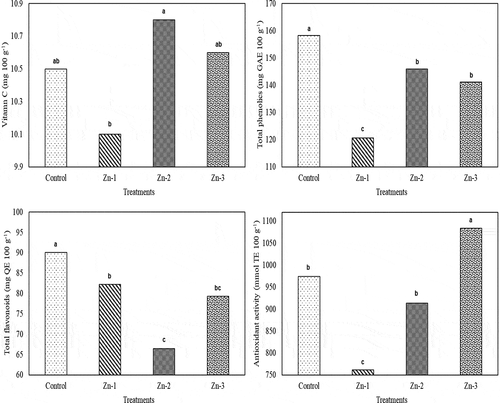
Phenolic compounds are secondary metabolites formed as a result of defense mechanism of the plants against stress conditions (Tomas‐Barberan and Espin, Citation2001). Alloway (Citation2008) indicated that Zn played a significant role in defensive reactions against photo-oxidative damages. Thus, in the present study, foliar Zn sprays increased plant nutrient (N, P, K) uptake of apples as compared to the control fruit (). Therefore, it was thought that stress conditions were not formed fully. Then, lower phenolics and flavonoids might have been measured from Zn-treated fruit. Contrarily, greater phenolics and flavonoids were measured from the control (without Zn treatments) fruit. Moreover, Peng et al. (Citation2005) reported greater phenolic compounds under lower nutrient conditions (as it was in control fruit of the present study). Contrary to present findings, there are some other studies indicating increasing total phenolics with Zn treatments (Quan et al., Citation2008) and insignificant differences between Zn-treated and the control fruit (Rasouli and Saba, Citation2018).
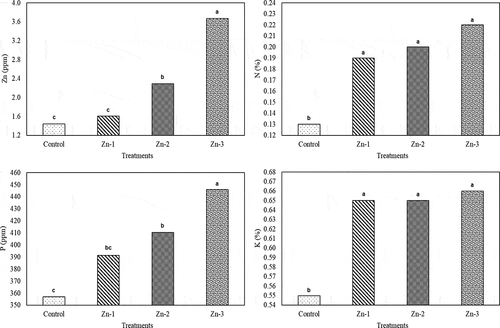
Figure 5. Effects of foliar zinc sprays on micro and macro elements such as zinc (Zn), nitrogen (N), phosphorus (P) and potassium (K) of ‘Granny Smith’ apples. The means shown with the same lower case letter on the bar are no different according to DMRT at P < .05
Zinc plays a significant role in control of production and detoxification of ROS able to damage membrane lipids and sulfides (Alloway, Citation2008). In present study, antioxidant activity only of Zn-3 treatment was greater than the control treatment. Chaoui et al. (Citation1997) and Rasouli and Saba (Citation2018) indicated that Zn activated some antioxidant enzymes and positively influenced antioxidant capacity. However, the antioxidant activity of Zn-1 treatment was lower than the control treatment. Similar findings were also reported by Aglar et al. (Citation2016) for Zn (0.3% ZnSO4)-treated Jersey Mac apples.
Mineral Elements
Foliar Zn treatments significantly increased fruit Zn (except for Zn-1), N, P (except for Zn-1) and K contents (). It was indicated in previous studies that foliar Zn treatments after full bloom may increase fruit Zn concentration (Hipps and Davies, Citation2001; Neilsen and Neilsen, Citation2002). It was reported that foliar Zn treatments increased fruit Zn content of three pear cultivars (Akça, Santa Maria and Deveci) about 3 folds (Erdem and Öztürk, Citation2012). Similar findings were also reported for Zn-treated apples (Aglar et al., Citation2016; Rasouli Sadaghiani et al., Citation2002; Zhang et al., Citation2013). There is a synergic relationship between N and Zn, thus increasing N-use efficiency was reported with increasing Zn nutrition levels (Marschner, Citation1995). Thus, in the present study, fruit N contents increased with Zn treatments. As it was in N concentration, fruit P and K concentrations also increased with increasing Zn concentrations. Fruit K concentration of 0.55% at control conditions increased to 0.66% with Zn-3 treatment, P concentration of 357 ppm in control treatment increased to 445.9 ppm with Zn-3 treatment. Similarly, Amiri et al. (Citation2008) reported that with Zn (8 g L−1 ZnSO4) treatments, apple N concentrations increased from 420 ppm to 441 ppm, K concentrations increased from 1320 ppm to 1386 ppm and P concentrations increased from 105 ppm to 110 ppm.
Conclusions
It was concluded based on present findings that foliar spray of zinc sources might be used as an efficient method to eliminate Zn deficiency in both field crops and horticultural crops. Such treatments also eliminate soil factors limiting Zn uptake of plant roots from the soils. Present Zn sprays promoted uptake of Zn and other nutrients. Foliar Zn sprays also improved unit area yields which is a significant factor in fruit culture. Again, especially with Zn-1 treatments, fruit sizes significantly increased. It was finally concluded that foliar Zn sprays could be used as an effective tool in apple production. However, there is a need for further researches to investigate the efficiency of Zn treatments in different fruit species and cultivars.
Authors’ Contributions
Halil Erdem and Ozgur Sahin, Burhan Ozturk, Yusuf Tutus and Sefa Gun. Planning, design, analysis of fruit and data analysis of experiment, and writing of the manuscript.
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Acknowledgments
The authors are grateful to Prof. Dr. Zeki Gokalp (a certified English translator and an expert in Biosystems Engineering) for his critical reading and through syntactic corrections of the manuscript.
References
- Aglar, E., K. Yildiz, Y. Ozkan, B. Ozturk, and H. Erdem. 2016. The effects of aminoethoxyvinylglycine and foliar zinc treatments on pre-harvest drops and fruit quality attributes of Jersey Mac apples. Sci. Hortic. 213:173–178. doi: https://doi.org/10.1016/j.scienta.2016.10.026.
- Alloway, B.J. 2008. Zinc in soils and crop nutrition. International Zinc Association, Brussels, p. 135.
- Alloway, B.J. 2009. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Hlth. 31:537–548. doi: https://doi.org/10.1007/s10653-009-9255-4.
- Alloway, B.J. 2013. Sources of heavy metals and metalloids in soils, p. 11–50. In: B.J. Alloway (ed.). Heavy metals in soils. Springer, Netherlands.
- Amiri, M.E., E. Fallahi, and A. Golchin. 2008. Influence of foliar and ground fertilization on yield, fruit quality, and soil, leaf, and fruit mineral nutrients in apple. J. Plant Nutr. 31:515–525. doi: https://doi.org/10.1080/01904160801895035.
- Blanpied, W.J., and K.J. Silsby. 1992. Predicting harvest date windows for apples. Cornell Cooperative Extension Inf. Bull. N. Y. 221:1–18.
- Blois, M.S. 1958. Antioxidant determinations by the use of a stable free radical. Nature 181(4617):1199. doi: https://doi.org/10.1038/1811199a0.
- Bouyoucous, G.A. 1951. Determination of particle size in soils. Agron. J. 42(438):44.
- Bremner, J.M. 1965. Inorganic forms of nitrogen 1. Methods Soil Anal. Part 2. Chem. Microbiol. Prop. 9:1179–1237.
- Çaglar, K.Ö. 1949. Soil science. No. 10. Ankara University’s Publish, Ankara.
- Cakmak, I. 2000. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 146:185–205. doi: https://doi.org/10.1046/j.1469-8137.2000.00630.x.
- Cakmak, I., H. Marschner, and F. Bangerth. 1989. Effect of zinc nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other phytohormones in bean (Phaseolus vulgaris L.). J. Exp. Bot 40(3):405–412. doi: https://doi.org/10.1093/jxb/40.3.405.
- Cakmak, I., L. Öztürk, S. Karanlik, H. Marschner, and H. Ekiz. 1996. Zinc‐efficient wild grasses enhance release of phytosiderophores under zinc deficiency. J. Plant Nutr. 19:551–563. doi: https://doi.org/10.1080/01904169609365142.
- Chaoui, A., S. Mazhoudi, M.H. Ghorbal, and E. El Ferjani. 1997. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 127:139–147. doi: https://doi.org/10.1016/S0168-9452(97)00115-5.
- Eman, A.A., A. El-Moneim, M.A. El-Migeed, A. Omayma, and M.M. Ismail. 2007. GA3 and zinc sprays for improving yield and fruit quality of Washington Navel orange trees grown under sandy soil conditions. Res. J. Agric. Biol. Sci. 3:498–503.
- Erdem, H., and B. Öztürk. 2012. Effect of foliar applied zinc on yield, mineral element contents and biochemical properties of pear varieties grafted to BA-29 rootstock. Süleyman Demirel University. J. Fac. Agric 5:93–106.
- Gianguzzi, G., G. Liguori, G. Sortino, G. Piva, and V. Farina. 2017. Effects of zinc foliar nutrition on ‘Gala’ Apple (Malus domestica Borkh.) fruit quality. Bulg. J. Agric. Sci 23:213–218.
- Hansen, H., and K. Grossmann. 2000. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 124:1437–1448. doi: https://doi.org/10.1104/pp.124.3.1437.
- Hipps, N.A., and M.J. Davies. 2001. Effects of foliar zinc applications at different times in the growing season on tissue zinc concentrations, fruit set, yield and grade out of culinary apple trees. Acta Hortic. 564:145–151. doi: https://doi.org/10.17660/ActaHortic.2001.564.16.
- Jackson, J.E. 1959. Quality control methods for several related variables. Technometrics. 1:359–377. doi: https://doi.org/10.1080/00401706.1959.10489868.
- Kacar, B., and A. Inal. 2008. Plant analysis. Nobel publication, Ankara.
- Lindsay, W.L., and W.A. Norvell. 1978. Development of a DTPA soil test for zinc, iron, manganese, and copper 1. Soil Sci. Soc. Am. J. 42:421–428. doi: https://doi.org/10.2136/sssaj1978.03615995004200030009x.
- Marschner, H. 1995. Mineral nutrition of higher plants. Second ed. Academic Press, London.
- Milosevic, T., and N. Milosevic. 2015. Apple fruit quality, yield and leaf macronutrients content as affected by fertilizer treatment. J. Soil Sci. Plant Nut. 15(1):76–83.
- Mohsenin, N.N. 1970. Physical properties of plant and animal materials. Gordon and Breach Sci Pub, New York, p. 51–87.
- Mortvedt, J.J. 1991. Use of industrial by-products containing heavy metal contaminants in agriculture, p. 861–870. In: R.G. Reddy, W.P. Imrie, and P.B. Queneau (eds.). Residues and effluents: Processing and environmental considerations: Proceedings of an international symposium. Minerals, Metals and Materials Society, Warrendale, PA.
- Mortvedt J.J., Gilkes R.J. (1993) Zinc Fertilizers. In: Robson A.D. (eds) Zinc in Soils and Plants. Developments in Plant and Soil Sciences, vol 55. Springer, Dordrecht. pp 33-44.
- Neilsen, G.H., and D. Neilsen. 2002. Effect of foliar Zn, form and timing of Ca sprays on fruit Ca concentration in new apple cultivars. Acta Hortic. 594:435–443. doi: https://doi.org/10.17660/ActaHortic.2002.594.56.
- Ozturk, A., K. Yildiz, B. Ozturk, O. Karakaya, S. Gun, S. Uzun, and M. Gundogdu. 2019b. Maintaining postharvest quality of medlar (Mespilus germanica) fruit using modified atmosphere packaging and methyl jasmonate. LWT-Food Sci. Technol. 111:117–124. doi: https://doi.org/10.1016/j.lwt.2019.05.033.
- Ozturk, B., E. Bektas, E. Aglar, O. Karakaya, and S. Gun. 2018. Cracking and quality attributes of jujube fruit as affected by covering and pre-harvest Parka and GA3 treatments. Sci. Hortic. 240:65–71. doi: https://doi.org/10.1016/j.scienta.2018.06.004.
- Ozturk, B., K. Yıldız, H. Erdem, O. Karakaya, A. Ozturk, and E. Aglar. 2019a. Aminoethoxyvinylglycine and foliar zinc treatments play a key role in pre-harvest drops and fruit quality attributes of William’s Pride apple. Acta Sci. Pol-Hortoru. 18(2):147–158.
- Ozturk, Y., and C. Tarakcıoglu. 2016. Seasonal changes of nutrient elements in the leaves of Palaz and Tombul hazelnut cultivars. Acad J. Agric. 5:87–96.
- Peng, Y., J. Yuan, F. Liu, and J. Ye. 2005. Determination of active components in rosemary by capillary electrophoresis with electrochemical detection. J. Pharmaceut. Biomed. 39:431–437. doi: https://doi.org/10.1016/j.jpba.2005.03.033.
- Quan, L.J., B. Zhang, W.W. Shi, and H.Y. Li. 2008. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integrat. Plant Biol. 50:2–18. doi: https://doi.org/10.1111/j.1744-7909.2007.00599.x.
- Rasouli, M., and M.K. Saba. 2018. Pre-harvest zinc spray impact on enzymatic browning and fruit flesh color changes in two apple cultivars. Sci. Hortic. 240:318–325. doi: https://doi.org/10.1016/j.scienta.2018.06.053.
- Rasouli Sadaghiani, M.H., M.J. Malakouti, and S.M. Samar. 2002. Effectiveness of different application methods of zinc sulfate on nutritional conditions of apple in calcareous soils of Iran. 17. World congress of soil science, Bangkok (Thailand), 14-21 Aug 2002.
- Sahota, G.S., and J.S. Arora. 1981. Effect of N and Zn on ‘Hamlin’ sweet orange (Citrus sinensis Osbeck). J. Jpn. Soc. Hortic. Sci. 50:281–286. doi: https://doi.org/10.2503/jjshs.50.281.
- Schulte, E.E., and L.M. Walsh. 1982. Soil and foliar applied zinc. Univ. Wisconsin Coop. Ext. Serv. A. 2528.
- Singleton, V.L., and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 16:144–158.
- Soliemanzadeh, A., and V. Mozafari. 2014. Response of pistachio trees to alternate bearing and foliar application of zinc and iron. Int. J. Fruit Sci. 14(2):174–187. doi: https://doi.org/10.1080/15538362.2013.818376.
- Tomas‐Barberan, F.A., and J.C. Espin. 2001. Phenolic compounds and related enzymes as determinants of quality in fruit and vegetables. J. Sci. Food Agric. 81:853–876.
- Tucker, G.A. 1993. Introduction, p. 1–51. In: G.B. Seymour, J.E. Taylor, and G.A. Tucker (eds.). Biochemistry of fruit ripening. Chapman and Hall, London.
- Wedekind, K.J., A.J. Lewis, M.A. Giesemann, and P.S. Miller. 1994. Bioavailability of zinc from inorganic and organic sources for pigs fed corn-soybean meal diets. J. Anim. Sci. 72:2681–2689. doi: https://doi.org/10.2527/1994.72102681x.
- Wojcik, P., and W. Popinska. 2009. Response of Lukasovka pear trees to foliar zinc sprays. J. Elementol. 14:181–187.
- Yogaratnam, N., and D.S. Johnson. 1982. The application of foliar sprays containing nitrogen, magnesium, zinc and boron to apple trees. II. Effects on the mineral composition and quality of the fruit. J. Hortic. Sci. 57:159–164.
- Zhang, Y., C. Fu, Y. Yan, Y.A. Wang, M. Li, M. Chen, S. Cheng, X. Yang, and S. Cheng. 2013. Zinc sulfate and sugar alcohol zinc sprays at critical stages to improve apple fruit quality. HortTechnology. 23:490–497. doi: https://doi.org/10.21273/HORTTECH.23.4.490.
- Zhang, Y., Y. Yan, C. Fu, M. Li, and Y.A. Wang. 2016. Zinc sulfate spray increases activity of carbohydrate metabolic enzymes and regulates endogenous hormone levels in apple fruit. Sci. Hortic. 211:363–368. doi: https://doi.org/10.1016/j.scienta.2016.09.024.
- Zhishen, J., T. Mengcheng, and W. Jianming. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559. doi: https://doi.org/10.1016/S0308-8146(98)00102-2.