?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The objective of this study was to evaluate the phytochemical, antimicrobial, and insecticidal properties of tannins derived from Cydonia oblonga fruit.
Qualitative and quantitative methods were used for the determination of the phytochemicals of Cydonia oblonga fruits. The tannins of this fruit were extracted using solvents and salts, and their antimicrobial activity against S. aureus ATCC 25923 and E.coli ATCC 25322 and C.albicans was investigated.
The lethal effect of tannins extract was evaluated on Tribolium confusum under controlled laboratory conditions (at temperature 28 ± 2°C, relative humidity 75 ± 5%, and photoperiod of 16:8).
The obtained results showed very interesting levels of tannins extraction (9.66%, 7.33%) using solvents and salts, respectively. The obtained tannins exerted no antimicrobial activity for the tested strains.
On the other hand, all doses (50 μl, 100 μl, and 200 μl) of tannins tested on Tribolium confusum adults by the inhalation method showed a bio-insecticidal effect with an LD50 of 109.64 μl of tannin extract. Cydonia oblonga fruit can be an alternative for the development of natural drugs and insecticides with an important role in the fight against parasites.
Introduction
Quince fruits have become in recent years a matter of considerable economic importance and are used in many countries for pharmaceutical, cosmetic (perfumes), food and agricultural applications. Chemically, these fruits are known for their richness in vitamin C, minerals (Rop et al., Citation2011), and secondary metabolites such as essential oils and phenolic compounds (Erdogan et al., Citation2012; Oliveira et al., Citation2012; Osman et al., Citation2010; Siqueira et al., Citation2012).
Various epidemiological studies have demonstrated a beneficial effect of quince fruit pulp in reducing the risk of some major diseases such as certain types of cancer (Gosse et al., Citation2005; Nomoto et al., Citation2004), cardiovascular diseases (Yamanaka et al., Citation1997), colon and kidney problems (Carvalho et al., Citation2010; Fattouch et al., Citation2007; Hamauzu et al., Citation2008; Magalhães et al., Citation2009). Quince tannins act as antiseptic agents in pulmonary infections thanks to their inhibitory action on the growth of bacteria, fungi, and viruses (Adel et al., Citation2010; Ayoub, Citation1982; Latte and Kolodziej, Citation2000). Besides, quince seeds are traditionally used for the treatment of diarrhea, cough, dysentery, constipation, and bronchitis (Duke et al., Citation2002; Prajapati et al., Citation2006).
As far as we know, the richness of quince fruit in tannins is responsible for the astringent property, which makes it less palatable to humans and animals.
Numerous research works have been conducted on the phenolic compounds derived from this plant since it might have beneficial effects as antimicrobial and insecticidal (Mollashahi et al., Citation2017; Rampadarath et al., Citation2014).
Moreover, phytochemical studies carried out on quince fruit revealed the presence of secondary metabolites. However, none of the studies have tested the ability of tannins quince fruit as insecticidal against food pest insect Tribolium confusum.
T.confusum is one of the major pest insects of cereals and their by-products, infesting in particular mills, silos, and other storage facilities. This beetle causes considerable damage both at the larval and adult stages by feeding on and contaminating food with their feces, exuviae, dead insects, and the strong noxious odor they produce (Timothy, Citation2009), leading to a serious loss of market value (Trematerra et al., Citation2011).
Chemical pesticides have several effects on the environment and human health, therefore, the use of natural insecticides derived from plants contributes to the biological fight against pests while protecting human health.
The main objective of the current study was to evaluate the phytochemical, biochemical, antimicrobial and insecticidal properties of Cydonia oblonga fruit.
Materials and Methods
Plant and Biological Materials
The Maliformis variety (Cydonia oblonga var.) studied was purchased at a supermarket located in Tizi-Ouzou region during the period of March-April 2017. summarizes some physicochemical parameters of this fruit.
Table 1. Some physico-chemical parameters of Cydonia oblonga fruit (n = 3)
Tribolium confusum adult insects used in this research were provided by the laboratory of Entomology, Plant Biology Department from Mouloud Mammeri University of Tizi-Ouzou Algeria.
Two bacteria Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25322 and a yeast Candida albicans were used to determine the antimicrobial activities of Cydonia oblonga fruit tannins extracts. These strains were delivered by the Microbiology Laboratory at Mouloud Mammeri University of Tizi-Ouzou.
Methods
Chemical Characterization of the Fruit
Chemical analyses were conducted to determine various nutritional parameters of Cydonia oblonga pulp, namely: pH, acidity, water content, ashes, Total Polyphenol Content (TPC), total flavonoid content, and tannin content as described below.
Quince fruit was cleaned and cut into 5 mm thick pieces, which were suspended in distilled water 1:5 (W/V), and then filtered to remove pulp and seeds and finally centrifuged for 10 min. The supernatant was then used for measuring pH through a pH meter (Owen and Johns, Citation1999).
The water content was determined by drying Cydonia oblonga pulp in an oven at 103 ± 2°C for 15 min. This allows determining the dry weight until it has a constant weight (Audigie et al., Citation1978).
The ashes were obtained after calcining quince pulp at 550°C for 5 h in a muffle furnace (Nabertherm B170) according to the method described by Laurent (Citation1975).
The degree of acidity was determined by titrimetric method, where a sample of 5% was used in titration by a solution of 0.1 N sodium hydroxide (NaOH). Acidity is expressed in grams of citric acid per 100 g of sample.
The TPC extraction method adopted was suggested by Owen and Johns (Citation1999). 2 g of sample were added to 20 ml of ethanol: water solvent (ranged between 0% and 100% ethanol and pure distilled water) for 72 h on a shaker at 150 rpm and then kept in a dark refrigerator. Further, the supernatant was collected and evaporated by keeping it on water, both at 45°C, using a rotary evaporator (LABOROTA 4000 HEIDOLPH, Germany). The dry extracts were then kept at 4°C until use (Mau et al., Citation2001). The obtained extracts were then used for the determination of TPC and total flavonoid content.
TPC was determined using Folin-Ciocalteu reagent according to Juntachote et al. (Citation2005). 0.5 ml of extract was added to the flask containing 5 ml distilled water, followed by addition of 0.5 ml of Folin Ciocalteu. The solution was mixed, and then 0.5 ml 20% sodium carbonate (Na2CO3) was added. The obtained mixture was stored at room temperature for two hours. The absorbance was measured at 760 nm. From the graph, the amount of TPC present in the sample tube was calculated and expressed as mg Gallic Acid Equivalents/g fresh sample. The analysis was conducted in triplicate.
Total flavonoid contents were determined based on Aluminum Chloride (AlCl3) colorimetric assay described by John et al. (Citation2013). An aliquot of the dried extract was added to a flask containing 4 ml of distilled water. Next, 0.3 ml of 5% Sodium nitrite (NaNO2) was added to the flask, followed by the addition of 0.3 ml 10% (AlCl3) after 5 min, and later 2 ml of 1 M Sodium hydroxide (NaOH). The whole mixture was made up of 10 ml distilled water and absorbance was measured at 510 nm. The Total flavonoid content in the sample was calculated from the calibration curve expressed in mg Quercetin Equivalents/g fresh sample. The analysis was conducted in triplicate.
Tannin contents were optimized by two precipitation methods as described by Biaye (Citation2002) and Bruneton (Citation1999). Precipitation in the first method was provoked by salts with 2% Sodium bisulfite (NaHSO3) and 0.5% Sodium bicarbonate (NaHCO3) and in the second method by solvents (methanol and diethyl ether). The tannins extraction yield was then calculated using the formula described by Fellah et al. (Citation2008).
Where W1: Weight of dried extracted tannins, W0: Weight of sample used.
The tannin contents in the extract were determined by the casein colorimetric method of Biaye (Citation2002). 1 g of casein was added to 6 ml of sample extract in the flask after 12 ml of distilled water was added. The solution was mixed for 3 h on a shaker at 150 rpm. The mixture was then filtered, and the filtrate was made up to 25 ml with distilled water. The absorbance was read at 760 nm. Tannin contents were given by the difference between TPC content and TPC obtained after tannin complexation by casein.
Antimicrobial Activity of Tannins Extracts
The antimicrobial activity of three tannins ethanolic extracts (10%, 20%, and 30%) against three strains (Escherichia coli ATCC 25322, Staphylococcus aureus ATCC 25923, and Candida albicans) was determined. 20 μl of each extract were tested by the agar disc diffusion method (Rahal, Citation2005). A suspension containing 107CFU/ml of bacteria was spread on Mueller-Hinton Agar, (MHA, Oxoid) and 104CFU/ml of fungi was spread on OGA Agar. All the tested strains were provided by the Microbiology laboratory of Mouloud Mammeri University of Tizi-Ouzou.
The sensitivity of the three strains tested concerning the tannin extract (20 μl) was evaluated according to the method described by Ponce et al. (Citation2003).
Breeding Insects
T.confusum breeding was carried out in glass jars containing grains, flour, and other cereal-based products, which were previously put in a freezer for 24 or 48 hours to get rid of any eggs that could infest the substrate. After the jars were removed and left in ambient air, adult insects (females and males) were added (30 to 40 individuals). The jars were then closed with a fine-mesh fabric lid held in place with a ribbon to allow the insects to breathe.
Insects breeding was performed under controlled laboratory conditions at a temperature of 28 ± 2°C, relative humidity of 75 ± 10%, photoperiod of 8 hours of light, and 16 hours of darkness to allow optimal growth of the larvae.
The jars were visited every day, and seven-day adult insects were collected in new jars and used for each treatment.
Lethal Concentration of 50% (LC50) of Tannins Extract
Cydonia oblonga derived tannins obtained from salt extraction method were tested on 7-day T. confusum adults. The insects were infected by fumigation method as described by Papachristos and Stamopoulos (Citation2002), so that filter paper discs (Ѳ = 2.5 cm) were suspended through a wire to the center of the inside surface of the lids of the glass vials (6.5 cm long and 3.5 cm wide).
The lethal concentration of 50% (LC50) of the tannins fruit extracts was determined. Indeed, three doses (50 µl, 100 µl, 200 µl) corresponding to concentrations of 0.01 g, 0.02 g, 0.04 g of tannins extracts leading to mortality were investigated in this study with three replicates for each concentration.
These concentrations were prepared from an initial solution of 2 g of sample added to 10 ml of dimethyl sulfoxide (DMSO) and then injected into the discs. Conversely, for control treatment only distilled water was used.
The number of dead T. confusum adults was evaluated with increasing tannins concentrations in all treatments () until the death of all the individuals (), and the average values of mortality were calculated.
Table 2. Number of killed T. confusum adult according to the tannins concentration (n = 3)
Table 3. Exposure time, average mortality rate, correlation coefficient and variance dependent dose
Before calculating the LC50, the percentage of the observed mortality was corrected compared to the control using the formula described by Abbot (Citation1925).
Where CM (%): percentage of the corrected mortality, M1: percentage of the observed mortality of control insects, M2: mortality percentage of the treated insects.
The average values of mortality, the corrected mortality, and the probits relative to the decimal logarithms of tannins extract were estimated through the regression curve of the corrected mortality of T.confusum depending on the logarithm of tannins concentration.
Statistical Analysis
Statistical analyses of data were conducted using Microsoft Excel statistical function expressed as the mean value ± SD. ANOVA method was used to determine correlation (R2) between the observed mortality and the corresponding time and tannin concentration.
The variance was also determined to estimate the dispersion degree of the observed mortality overtime versus the average mortality rate recorded for each dose tested.
Results
Phytochemical analysis of Cydonia oblonga fruit shows the presence of secondary metabolites (tannins, gallic tannins, leuco-anthocyanins, and glucosides) with important amounts. Flavonoids and alkaloids were also found but in lower quantities. On the other hand, no anthocyanins, free quinones, saponifies, and coumarins were detected.
The chemical composition (carbohydrates, phenolics compounds) and extraction yields of tannins are shown in . Cydonia oblonga fruit is a rich source of polyphenols (96.82 ± 1.7 mg AGE/g F.M) based on the fresh fruit. Compared to other fruits, this value is better than that discovered in apple fruit, which vary between 0.4–1.8 mg AGE/g FM as reported by Brat et al. (Citation2006) and Colin-Henrion (Citation2008).
Tannins extraction method using solvent was shown to be more effective than those using salts. The tannin yields were estimated to be 9.66 ± 0.007% and 7.33 ± 0.008% based on fresh fruit using methanol and salts, respectively.
These results were supported by the similar chemical composition revealed by Infra-Red spectra (). The latter showed that tannins derived from solvent extraction were characterized by the highest intensity of peaks. The extracted tannins reveal the presence of an intense broad band at 3431 cm−1 attributed to the hydroxyl group (O-H). In addition, the signals observed at 2926 cm−1 and 2934 cm−1 can be attributed to C-H. Both HCO3− and HSO3− ions can be absorbed in this zone. Furthermore, low intensity of amine functions was observed due to the low solubility of amine in the solvent.
Figure 1. IR spectrum of tannins extracted from Cydonia oblonga fruit, tannins derived from salts extraction (in blue color) and methanol extraction (in red color)
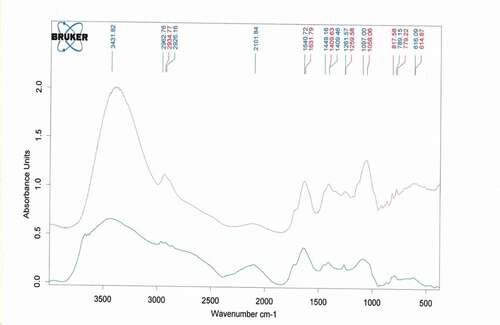
The absorptions observed around (1640 cm−1and 1409 cm−1) are assigned to the amide groups, and more precisely those recorded at 1259 cm−1 are specific to the ether group. On the other hand, asymmetric acetylenic hydrocarbons bond vibrations were found between 789 cm−1 and 779 cm−1. Generally, the bands observed around 616 cm−1 and 617 cm−1 are attributed to the iron oxides magnetite (Fe3O4) and maghemite (ƴ-Fe2O3), respectiveIy (Namduri and Nasrazadani, Citation2008).
The ethanolic extracts of tannins do not affect as antimicrobial, and no inhibition was observed against the tested E.coli ATCC 25322, S. aureus ATCC 25923, and C. albicans strains.
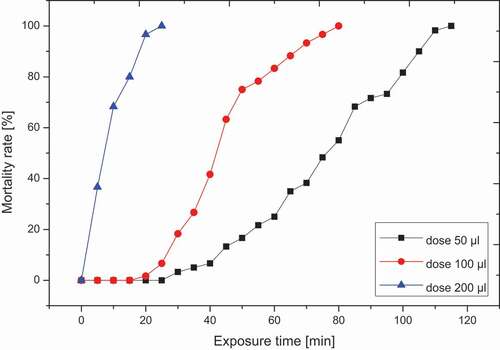
The results obtained from the experiments show a significant increase in the mortality of T.confusum with increasing concentration of fruit tannins ().
The mortality rate increased with increasing tannin concentrations in all treatments (), with doses ranging from 50 µl (115 min) to 200 µl (25 min).
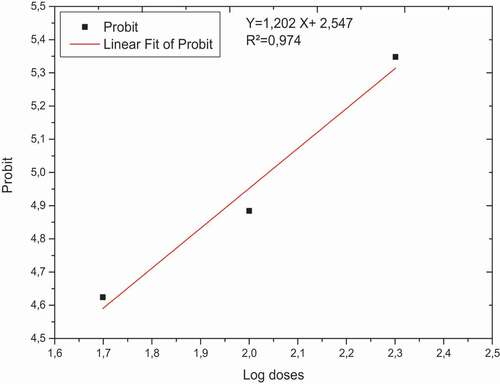
shows the mortality average values, the corrected mortality, and the probits relative to the decimal logarithms of the tested doses.
According to the regression equation Y = 1.2024X + 2.5474 (). Replacing “Y” by 5 (The probit which corresponds to 50% of mortality), we find the value of X that corresponds to the decimal logarithm of the LD50 of 109.64 µl of tannin extract.
Figure 3. Regression line for corrected mortality of T.confusum as a function of the doses decimal logarithms
Positive linearity is observed between the mortality rates recorded and the exposure time of the T.confusum adults according to the doses tested. Which explains a significant reduction in exposure time depending on the doses from 50 µl (115 min) to 200 µl (25 min).
The variances () recorded for the three doses differ slightly. This result highlights the distribution similarity of the mortality rates compared to the average mortality rates recorded overtime for each dose.
Table 4. Average mortality values, corrected mortality and probits as a function of the decimal logarithms of the doses
It should be noted that the 200 µl dose exerts a better insecticidal effect against T.confusum with total mortality after 25 min compared to individuals of the negative control (Zero mortality). These results in a strong slope inclination characterizing the 200 µl dose compared to those of the other tested doses.
Discussion
The availability of phenolic compounds depends on a number of factors such as extrinsic factors (geographic and climatic), eco-physiological state (harvest period), shelf-life, and genetic factors (Aganga and Mosase, Citation2001), together with the experimental conditions (temperature, amount of solvent) (Baustita et al., Citation2007).
The low tannin yields obtained can be explained by the solubility of tannins, which is dependent on the nature of the extracted tannins, their molecular weight, and their polymerization degree (Jean-Blain, Citation1998; Makkar et al., Citation1991). Compared to the results using other extraction methods, Makkar et al. (Citation1991) proved that acetone – water or (methanol – water) solutions were more effective for the tannin extraction process.
The resistance of the tested strains against the ethanolic extracts can be explained firstly by the concentration and the nature of tannins. Studies of Mcleod (Citation1974) have demonstrated that the condensed tannins showed a very effective antimicrobial effect as hydrolysable tannins. On the other hand, by defense mechanisms, bacteria can inactivate tannins by synthesizing glycoproteins to degrade and use them according to Reed (Citation1995).
Secondly, plant compounds are often considered as a means of plant defense against pathogens and pests. These include alkaloids, terpenes, and tannins (Auger et al., Citation2008).
Plant based volatile substances are known to attract insects and alter their behavior. One example is the male T. confusum pheromones, which contain quinones that cause a strong noxious odor and thus makes the infested food unfit for human consumption (Timothy, Citation2009; Verheggen et al., Citation2007). Indeed, the aggregation of pheromones can provoke behavioral responses in males and can potentially be useful when combined with other compounds such as tannins to fight insect pests (Athanassiou et al., Citation2006; El-Sayed et al., Citation2009).
Therefore, it can be said that the tannin extract exerts considerable insecticidal activity through different mechanisms. Tannin compounds are known for their hardening properties, which limit transfer into the cells.
Furthermore, tannins possess larvicidal and repellent properties; they influence the growth, development, and fecundity of several phytophagous insects (Acheuk et al., Citation2014). Indeed, ingestion or fumigation of tannins affect the integrity of the digestive tract of phytophagous insects (Ayres et al., Citation1997), and cause death and malformations in offspring (Carpinella et al., Citation2003).
The toxicity of tannins in T. confusum adults varies according to the concentration and duration of treatment. Similar results were obtained by Aouina and Khelifi (Citation2018), who reported that adults were more sensitive than larvae to insecticides. In the present study, the mortality caused by fumigation of tannins in adults revealed that the toxic effects of tannins depend on the dose applied and the exposure time. Indeed, a relatively high dose of 200 μl and an exposure time of 25 h cause the mortality of all the individuals. Similarly, the toxicity of the oil of L. spica and linalool varies according to the stage of development and duration of treatment. Indeed, young larvae are the most sensitive to the toxic effects of linalool after 24 hours of exposure, while older larvae were little affected by fumigation. Linalool resulted in high mortality in eggs but lower mortality in pupae and adults (Kheloul et al., Citation2020). Direct toxicity was observed from monoterpenoids and tannins; the exposure of females to the vapors was found to cause a decrease in fecundity and hatching rate (Stamopoulos et al., Citation2007). Moreover, the insecticidal activity of Triterpenoids extracted from Melia azedarach (Meliaceae) against adults of T. castaneum manifests itself by an anti-nutritional effect and inhibition of food intake in phytophagous insects.
The presented results correlate those found in the literature (Rampadarath et al., Citation2014), where phenolic compounds (flavonoids and tannins) were reported to exert antimicrobial and insecticidal effects.
The same insecticidal effect was found using soyasapogenol, and hederagenin glycosides derived from Alfalfa roots, which are rich in medicagenic acid. They were markedly toxic to the flour beetle (T.castaneum) (Fenwick et al., Citation1991). Therefore, Alfalfa saponins acted as natural feeding barriers for phytophagous insects and were found to be toxic compounds to many insects (Golawska, Citation2007).
The LC 50 value found is significantly lower in comparison to that of the C. colocynthis plant tested on adult grasshopper, which was calculated to be 18.58 milligrams per ml (Mollashahi et al., Citation2017).
Conclusion
Cydonia oblonga fruit has several active compounds (polyphenols, flavonoids, tannins). This research highlights the characteristics of tannin extract of this fruit as an insecticide against T.confusum adults.
This fruit can constitute an alternative for improving the properties of synthetic drugs and natural insecticide, which is a major subject for future research.
List of abbreviations
A.A.S:Atomic Absorption Spectroscopy
Abs:Absence
FM:Fresh Mass
HRC:High Reconstitution Capacity
IR:Infra-Red
R:Resistant
TPC:Total Phenolic Content
Disclosure of potential conflicts of interest
All the authors declare no conflict of interest, financial or otherwise
Acknowledgments
This work was supported by grants from:
Laboratory of Chemistry and Microbiology of Mouloud Mammeri University of Tizi-Ouzou (Algeria), Laboratory of Entomology, Plant Biology Department from Mouloud Mammeri University of Tizi-Ouzou Algeria.
Laboratory of the Research Unit, Materials, Processes & Environment (UR-MPE) of the University of Boumerdes.
References
- Abbot, W.S. 1925. A method of computing the effectiveness of an insecticide. J.Economic Entomol. 18(2):265–267.
- Acheuk, F., K. Abdellaoui, L. Bendifallah, A. Hammichi, and E. Semmar. 2014. Effects of crude ethanolic extract of Solenostemma argel (Asclepiadaceae) on 5th instar larvae of Locusta migratoria. AFPP Tenth International Conference on Pests in Agriculture Montpellier-October 22 and 23, 2014.
- Adel, A.M., Z.H. Abdel – Wahab, A.A. Ibrahim, and M.T. Al – Shemy. 2010. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresource. Tech. 101(12):4446–4455. doi: https://doi.org/10.1016/j.biortech.2010.01.047.
- Aganga, A.A., and K.W. Mosase. 2001. Tannin content, nutritive value and dry matter digestibility of Lonchocarpus capassa, Zizyphus mucronata, Sclerocarya birrea, Kirkia acuminata and Rhus lancea seeds. Animal. Feed. Sci. Tech. 91(1–2):107–113. doi: https://doi.org/10.1016/S0377-8401(01)00235-8.
- Aouina, A., and N. Khelifi. 2018. Evaluation of the repellent effect of Cuminum cyminum L. and Foeniculum vulgare mill on stored cereals insect Tribolium castaneum (Herbst). Academic Master’s Thesis Biodivesity and plat Physiology, University of M’sila, 50.
- Athanassiou, C.G., N.G. Kavallieratos, S.N. Xyrafidis, and P. Trematerra. 2006. Behavioural responses of Tribolium confusum Jacquelindu Val (Coleoptera: Tenebrionidae) to flour previously infested or contaminated by Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Semiochemicals, p. 441–445. In: Biology. Behavior. And pest detection on stored grain. Brazil: Brazilian Post-Harvest Association (ABRAPOS) Passo Fund BBrazil.
- Audigie, C., J. Figarella, and F. Zonszain. 1978. Biochemical analysis manipulation. p. 247. Doin (Ed.). Paris.
- Auger, J., I. Arnault, and E. Thibout. 2008. Sulfur-containing substances from Alliums and crucifers: Phytosanitary potential and applications to biofumigation, p. 101–123. In: Biopesticides of plant origin. Paris: Tec and Doc Lavoisier.
- Ayoub, H.S.M. 1982. Molluscicidal properties of Acacia nilotica. Planta. Medica. 46(11):23–29. doi: https://doi.org/10.1055/s-2007-971210.
- Ayres, M.P., P.H. Clausen, S.F. MacLean, A.M. Redman, and P.B. Reichart. 1997. Diversity of structure and antiherbivore activity in condensed tannins. Ecol. 78(6):1696–1712. doi: https://doi.org/10.1890/0012-9658(1997)078[1696:DOSAAA]2.0.CO;2.
- Baustita, O., F. Fernandez, R. Lopez, and P. Gomes. 2007. The effects of enological practices in anthocyanins, phenolic compounds and wine color and their dependence on grape characteristic. J. Food. Comp. Analysis. 20(7):546–552. doi: https://doi.org/10.1016/j.jfca.2007.04.008.
- Biaye, M. 2002. Pharmacological Action of Tannins pharmacologiques des tanins. Doctoral Thesis. Cheikh Anta Diop University of Dakar.
- Brat, P., S. George, A. Bellamy, L. Du Chaffaut, A. Scalbert, L. Mennen, N. Arnault, and M.J. Amiot. 2006. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. 136(9):2368–2373. doi: https://doi.org/10.1093/jn/136.9.2368.
- Bruneton, J. 1999. Pharmacognosie, phytochemistry medicinal plants. 3rd. Tech and Doc. Lavoisier, Paris. p. 227–494.
- Carpinella, M.C., M.T. Defago, G. Valladares, and S.M. Palacios. 2003. Antifeedant and insecticide properties of a limonoid from melia azedarach (Meliaceae) with potential use for pest management. J. Agric. Food Chem. 51(2):369–374. doi: https://doi.org/10.1021/jf025811w.
- Carvalho, M., B.M. Silva, R. Silva, P. Valentão, P.B. Andrade, and M.L. Bastos. 2010. First report on cydonia oblonga miller anticancer potential: Differential antiproliferative effect against human kidney and colon cancer cells. J. Agric. Food. Chem. 58(6):3366–3370. doi: https://doi.org/10.1021/jf903836k.
- Colin-Henrion, M. 2008. From apple to processed apple: Impact of the process on two nutritional compounds. Physical and sensory characterization of processed products. Ph.D thesis; Angers University (France). 254.
- Duke, J.A., M.J. Bogenschutz-Godwin, J. Ducelliar, and P.A.K. Duke. 2002. Handbook of medicinal herbs. 2nd ed. CRC Press, Boca Raton, FL.
- El-Sayed, A.M., D.M. Suckling, J.A. Byers, E.B. Jang, and C.H. Wearing. 2009. Potential of lure and Kill in long-term pest management and eradication of invasive species. J. Economic. Entomol. 102(3):815–835. doi: https://doi.org/10.1603/029.102.0301.
- Erdogan, T., T. Gonenç, Z.S. Hortoglu, B. Demirci, K.H.C. Başer, and B. Kivçak. 2012. Chemical composition of the essential oil of quince (Cydonia Oblonga Miller) leaves. Med. Aromat. Plants. 1:134. doi: https://doi.org/10.4172/2167-0412.1000e134..
- Fattouch, S., P. Caboni, V. Coroneo, C.I.G. Tuberoso, A. Angioni, S. Dessi, N. Marzouki, and P. Cabras. 2007. Antimicrobial activity of Tunisian quince (Cydonia Oblonga Miller) pulp and peel phenolic extracts. J. Agr. Food. Chem. 55(3):963–969. doi: https://doi.org/10.1021/jf062614e.
- Fellah, H., R. Ksouri, K. Chaieb, N. Karray-Bouraoui, N. Trabelsi, M. Boulaaba, and C. Abdelly. 2008. Phenolic composition of Cynara cardunculus L. Organs, and their biological activities. CR. Bio. 331(5):372–379. doi: https://doi.org/10.1016/j.crvi.2008.02.008.
- Fenwick, G.R., K.R. Price, and C. Tsukamoto. 1991. Saponins, p. 285–327. In: J.P. Felix D’Mello, C.M. Duffus, and J.H. Duffus (eds.). Toxic substances in crop plants. Royal Society of Chemistry, Cambridge.
- Golawska, S. 2007. Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris. J.Chem. Ecol. 33(8):1598–1606. doi: https://doi.org/10.1007/s10886-007-9333-y.
- Gosse, F., S. Guyot, S. Roussi, A. Lobstein, B. Fisher, N. Seiler, and F. Raul. 2005. Chemoproventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of edon carcinogenesis. Carcinogenesis 26(7):1291–1295. doi: https://doi.org/10.1093/carcin/bgi074.
- Hamauzu, Y., M. Irie, M. Kondo, and T. Fujita. 2008. Anti-ulcerative properties of crude polyphenols and juice of apple and Chinese quince extracts. Food. Chem. 108(2):488–495. doi: https://doi.org/10.1016/j.foodchem.2007.10.084.
- Jean-Blain, C. 1998. Nutritional and toxicological aspects of tannins. Rev. Med. Vet. 149:911–920.
- John, B.V., R.V. Reddy, and C.T. Sulaiman. 2013. Total phenolics and flavonoids in selected Justicia species. J.Pharmacogn. Phytochemistry. 2(4):72–73.
- Juntachote, T., E. Berghofer, S. Siebenhdl, and F. Bauer. 2005. The antioxidative properties of holy basil and galangal in cooked ground pork. Meat. Sci. 72(3):446–456. doi: https://doi.org/10.1016/j.meatsci.2005.08.009.
- Kheloul, L., S. Anton, C. Gadenne, and A. Kellouche. 2020. Fumigant toxicity of Lavandula spica essential oil and linalool on different life stages of Tribolium confusum (Coleoptera: Tenebrionidae). J. Asia-Pacific Entomol. 23(2):320–326. doi: https://doi.org/10.1016/j.aspen.2020.02.008.
- Latte, L.P., and H. Kolodziej. 2000. Antifungal effect of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Natur. Forch. 5(5–6):467–472.
- Laurent, S. 1975. Comparative study of different methods of extracting and measuring tannins in some Pteridophytes. Arch. Int. Physiol. Biochim. 83(4):735–752. doi: https://doi.org/10.3109/13813457509081892.
- Magalhães, A.S., B.M. Silva, J.A. Pereira, P.B. Andrade, P. Valentão, and M. Carvalho. 2009. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food. Chem. Toxicol. 47(6):1372–1377. doi: https://doi.org/10.1016/j.fct.2009.03.017.
- Makkar, H.P., R.K. Dawra, and B. Singh. 1991. Tannin levels in leaves of some oak species at different stages of maturity. J. Sci. Food. Agric. 54(4):513–519. doi: https://doi.org/10.1002/jsfa.2740540403.
- Mau, J.L., G.R. Chao, and K.T. Wu. 2001. Antioxidant properties of methanolic extracts from several ear mushrooms. J. Agric. Food. Chem. 49(11):5461–5467. doi: https://doi.org/10.1021/jf010637h.
- Mcleod, M.N. 1974. Plant tannins. Their role in forage quality. Nutr. Abstr. Rev. 44:803–815.
- Mollashahi, H., A. Mirshekari, M. Ghorbani, and A. Tarrah. 2017. Insecticidal effect of the fruit extract bitter melon (citrullus colocynthis) on locust chrotogonus trachypterus (Orth: Pyrgomorphidae). Biosci. Biotech. Res. Asia. 14(4):1285–1289. doi: https://doi.org/10.13005/bbra/2571.
- Namduri, H., and S. Nasrazadani. 2008. Quantitative analysis of iron oxide using Fourier transform infrared spectophotometry. Corrosion. Sci. 50(9):2493–2497. doi: https://doi.org/10.1016/j.corsci.2008.06.034.
- Nomoto, H., M. Ligo, H. Hamada, S. Kojima, and H. Tsuda. 2004. Chemoprevention of colorectal cancer by grape seed proanthocyanidin is accompanied by a decrease in proliferation and increase in apoptosis. Nutr. Cancer. 49(1):81–88. doi: https://doi.org/10.1207/s15327914nc4901_11.
- Oliveira, A.P., R.M. Costa, A.S. Magalhães, J.A. Pereira, M. Carvalho, P. Valentão, P.B. Andrade, and B.M. Silva. 2012. Targeted metabolites and biological activities of Cydonia oblonga Miller leaves. Food. Res Int. 46(2):496–504. doi: https://doi.org/10.1016/j.foodres.2010.10.021.
- Osman, A.G., M. Koutb, and A.E.D.H. Sayed. 2010. Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet-A radiation on African catfish Clarias gariepinus (Burchell, 1822). J. Photochem. Photobiol. 99(1):1–8. doi: https://doi.org/10.1016/j.jphotobiol.2010.01.002.
- Owen, P.L., and T. Johns. 1999. Xanthipe oxidase inhibitory activity of northeastern North America plant remedies used for gout. J. Ethnopharmacol. 64(2):149–160. doi: https://doi.org/10.1016/S0378-8741(98)00119-6.
- Papachristos, D.P., and D.C. Stamopoulos. 2002. Rappelent toxic and reproduction inhibitory effects of essential oils vapours on Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae. J. Stored. Products. Res. 38(2):117–128. doi: https://doi.org/10.1016/S0022-474X(01)00007-8.
- Ponce, A.G., R. Fritz, C. Del Valle, and S.I. Roura. 2003. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. Food. Sci. Tech. 36:679–684.
- Prajapati, N.D.S.S., A.K. Purohit, and T.K. Sharma. 2006. A handbook of medicinal plants. Jodhpur: Agrobios. Section II. Vol. 86. India: Agro Bios
- Rahal, K. 2005. Standardization of the antibiogramme in human medicine on a national scale according to recommendations of WHO. Ministry for health, the population and the hospital reform: 257. Geneva: Word Health Organization edited by Hilary Cdman Lindsay Martinez.
- Rampadarath, S., D. Puchooa, and V.M. Ranghoo-Sanmukhiya. 2014. A comparison of polyphenolic content, antioxidant activity and insecticidal properties of Jatropha species and wild Ricinus communis L. found in mauritius. Asian. Pac. J. Trop. Med. 7(Suppl 1):S384–S390. doi: https://doi.org/10.1016/S1995-7645(14)60263-7.
- Reed, J.D. 1995. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 73(5):1516–1528. doi: https://doi.org/10.2527/1995.7351516x.
- Rop, O., J. Balik, V. Reznicek, T. Jurikova, P. Skardova, P. Salas, J. Sochor, J. Mlcek, and D. Kramarova. 2011. Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech. J. Food. Sci. 29(No. 1):65–73. doi: https://doi.org/10.17221/212/2009-CJFS.
- Siqueira, C.F., D.L. Vasconcelos Cabral, T.P. Sobrinho, E.L. Cavalcanti De Amori, J. Melo, T. Araujo, and U.P. Albuquerque. 2012. Levels of tannins and flavonoids in medicinal plants: Evaluating bioprospecting strategies. J. Evid. Based. Complement. Alternat. Med. 2012: 1–7.
- Stamopoulos, D.C., P. Damos, and G. Karagianidou. 2007. Bioactivity of five monoterpenoid vapours to Tribolium confusum (du Val)(Coleoptera: Tenebrionidae). J. Stored Products Res. 43(4):571–577. doi: https://doi.org/10.1016/j.jspr.2007.03.007.
- Timothy, J.D. 2009. Effects of crowding on the loss weight of sorghum flour and on the survival and development of adult confused flour beetle, Tribolium confusum in sorghum. New York Sci J. 2:56–61.
- Trematerra, P., V. Stejskal, and J. Hubert. 2011. The monitoring of semolina conatamination by insect fragments using the light filth method in an Italian mill. Food. Control. 22(7):1021–1026. doi: https://doi.org/10.1016/j.foodcont.2010.11.026.
- Verheggen, F., C. Ryne, P.-O.C. Olsson, L. Arnaud, G. Lognay, H.-E. Hogberg, D. Persson, E. Haubruge, and C. Lofstedt. 2007. Electrophysiological and behavioral activity of secondary metabolites in the confused flour beetle, Tribolium confusum. J. Chem. Ecol. 33(3):525–539. doi: https://doi.org/10.1007/s10886-006-9236-3.
- Yamanaka, N., O. Oda, and S. Nagao. 1997. Green tea catechins such as (–) - epicatechin and (–)- epigallocatechin accelerate cu+2- induced low density lipoprotein oxidation in propagation phase. FEBS. Lett. 401(2–3):230–234. doi: https://doi.org/10.1016/S0014-5793(96)01455-X.