ABSTRACT
Fruit characteristics of 10 mango varieties (Mangifera indica cv ‘Chok Anan,’ ‘Gold Nugget,’ ‘Hong Jin Long,’ ‘Jin Sui,’ ‘Keitt,’ ‘Mallika,’ ‘Nam Doc Mai,’ ‘Neelum,’ ‘Tainong No. 1ʹ, and ‘Zihua’), grown in a greenhouse, were evaluated during postharvest ripening. Mango fruits were harvested at 130 days after full bloom and then ripened for 3, 5, and 7 days after harvest (DAHs) at ambient temperature and relative humidity. All fruit skin colors became redder except for ‘Keitt’ and ‘Tainong No. 1ʹ, as reflected in increasing a* values (a color space coordinate describing green-red coloration). Of the 10 varieties, fruit weights of eight varieties did not significantly change among DAHs. Fruit weights of ‘Nam Doc Mai’ and ‘Zihua’ decreased at 7 DAH. Only the flesh weights of ‘Zihua’ changed among all varieties. Of all the varieties, fruit lengths and fruit diameters of eight varieties did not change among DAHs. Soluble solid contents did not change in four varieties and increased in six varieties. Titratable acidity decreased in six varieties but did not change in the other varieties. The fruit skin firmness decreased in all varieties among DAHs. Changes in fruit characteristics depended on mango varieties or the length of postharvest ripening period or both. Principal component analysis showed that fruit diameter, SSC, L*, b*, and firmness were essential variables. These findings could be used to provide consumers with information about fruit characteristics associated with the ripening of various mango cultivars.
Introduction
Mango (Mangifera indica L.) is the popular tropical fruit in the world. Mango is known as the “king of fruits” due to its pleasant flavor, nutritional value, attractive color, distinct taste, and aroma (Farina et al., Citation2020; Nunes et al., Citation2007; Tharanathan et al., Citation2006). The fruit also has a high nutritional value and health benefit due to phytochemical components (Maldonado-Celis et al., Citation2019; Torres-León et al., Citation2016). The fruit is cultivated in about 100 countries with an annual production of over 55 Mt in 2019 (FAO, Citation2021) and third in the global trade of tropical fruit (FAO, Citation2020). The production occurs mainly in tropical and subtropical regions, including India, China, Thailand, Indonesia, Philippines, Brazil, Pakistan, and Mexico (FAO, Citation2020; Farina et al., Citation2020). Today, the production is spread outside the traditional geographical regions of Central and South America, Australia, Southeast Asia, Hawaii, Egypt, Israel, and South Africa (Farina et al., Citation2020; Han et al., Citation2016).
The characteristics of mango fruit were affected by climatic conditions (Laxman et al., Citation2016; Normand et al., Citation2015; Tharanathan et al., Citation2006; Tiyagi et al., Citation2017), including solar radiation, temperature, and rainfall. During fruit development in the parental tree, solar radiation affects the nutritional composition and skin color of mango fruit (Jutamanee and Onnom, Citation2016). In the case of temperature, day, night, and their difference affect the skin color, acidity, and sweetness (Lobo and Sidhu, Citation2017). High rainfall decreases the fruit firmness involved in shelf-life and increases skin cracking disorders (Tiyagi et al., Citation2017). With the interaction of these climatic conditions, the fruit qualities of mango are also affected by geographical regions from tropic to subtropic and temperate regions (Hofman et al., Citation1997; Tiyayon and Paul, Citation2017).
Recently, mango cultivation has continuously expanded to temperate regions, including Korea and Japan, due to increasing their domestic consumption (Han et al., Citation2016). Due to climate change, the climatic conditions of temperate regions become a preferred condition to grow mango and economic benefit. However, since mango tree is vulnerable to cold, the tree should be grown in a greenhouse to prevent chilling injury during the winter (Han et al., Citation2016; Lim et al., Citation2020). While the ripening of mango fruit is mainly studied openly grown in subtropical or tropical regions, including India, Thailand, and the Philippines (Gentile et al., Citation2019; Liguori et al., Citation2020; Ramírez et al., Citation2014), it was not well studied in a greenhouse grown in any geographical regions (Farina et al., Citation2020; Han et al., Citation2016). For these reasons, in a greenhouse cultivation of mango, cultivar selection and farm expansion are limited. In this study, we evaluated fruit characteristics involved in commodity during postharvest ripening in 10 mango varieties grown in a greenhouse. We also investigated the relationship between varieties and postharvest ripening periods and fruit characteristics. The fruit characteristics and these relationships are expected to contribute to a commercially valuable database related to fruit ripening in various mango varieties grown in a greenhouse of temperate regions.
Materials and Methods
Plant Materials and Environmental Conditions
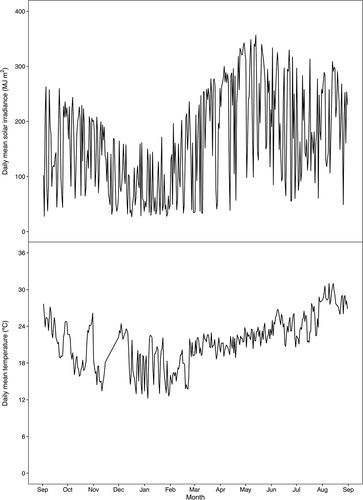
Ten varieties of eleven-year-old mango trees were used in this study: ‘Chok Anan,’ ‘Gold Nugget,’ ‘Hong Jin Long,’ ‘Jin Sui,’ ‘Keitt,’ ‘Mallika,’ ‘Nam Doc Mai,’ ‘Neelum,’ ‘Tainong No. 1ʹ, and ‘Zihua.’ The fruit trees were grown in a greenhouse at the experimental orchard of the National Institute of Horticultural and Herbal Science, Rural Development Administration, Jeju (33° 28ʹ N, 126° 31ʹ E), Republic of Korea. These trees were cultivated under a standard guideline for mango cultivation (Jeon et al., Citation2013). To prevent mango trees from chilling injury, the minimum temperature of winter season was maintained in the greenhouse at 10°C by using hot air blowers, according to the guideline (Jeon et al., Citation2013). Daily mean solar irradiance and temperature in the greenhouse were measured and recorded from September 2019 to August 2020 () using Data loggers (WatchDog 2450, Spectrum Technologies, Inc., Aurora, IL, USA).
Figure 1. Daily mean solar irradiance and temperature at the greenhouse of the experimental site (33° 28ʹ N, 126° 31ʹ E) from the 1st September 2019 to the 31th August 2020
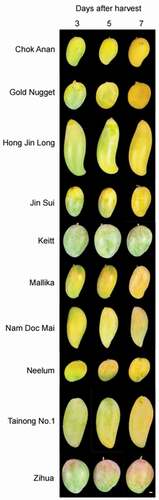
Fruits, still green and not physiologically ripe, were harvested at 130 days after full bloom and ripen, considering a guideline (Jeon et al., Citation2013) and farmer practices in the experimental location. To evaluate fruit morphological and physiological traits involved in commodity, fruits are stored during 3, 5, or 7 days after harvest (DAH) () at ambient temperature and RH conditions () in a storage room, and then their traits were measured on three biological replicates of three fruits each per DAH.
Table 1. Minimum and maximum ambient temperatures, and ambient relative humidity at the storage room during 7 days after harvest
Measurement of Fruit Morphological Traits
Fruit skin color was measured at using a colorimeter (CM-700d, Konica Minolta, Tokyo, Japan) and described using CIELAB L*, a*, and b* color space coordinates (McGuire, Citation1992). The L* value represented the lightness of the color, with a range of 0 to 100 (0, black; 100, white). The a* value is negative for green and positive for red. The b* value is negative for blue and positive for yellow. For each fruit, these values were measured at three different points along the fruit equator. The fruit and flesh fresh weights (FW) were measured using a digital scale (Jw-1-2000, ACOM, Republic of Korea). Fruit diameter and length were measured using digital calipers (CD-15APX, Mitutoyo, Japan).
Measurement of Fruit Physiological Traits
Fruit soluble solid content (SSC) was determined using a handheld digital refractometer (HI96801, Hanna, Romania). Titratable acidity of the aqueous extract of the fruit pulp was determined via titration against 0.16 M NaOH and was expressed as mg citric acid (ca) equivalent (eq.)·100 g−1 FW, according to the method of Han et al. (Citation2016). Fruit skin firmness was measured on one side of fruit using a texture analyzer (TA-XT express, Stable Micro System, UK). A puncture test was carried out using a 2.0 mm diameter cylindrical probe at three different points along the fruit equator with a cross-head speed of 2.0 mm s−1 and a penetration depth of 5.0 mm. The equatorial region of mango for the measurement of textural parameters was selected as it gave more consistent results than any other region on the fruit surface (Gunness et al., Citation2009). The maximum force was applied to break up the fruit skin was taken as fruit skin firmness (N) (Jha et al., Citation2011).
Statistical Analyses
Statistical analyses were performed with packages on R 4.0.2 (R Core Team, Citation2021) and RStudio 1.3 (Rstudio, Citation2020) software. Statistically significant differences were determined using analysis of variance with the agricolae v1.3.3 package (De Mendiburu, Citation2020). Means were compared using Scheffe’s tests at p < .05.
The principal component was determined to obtain correlation among fruit characteristics and relationship between fruit characteristics and postharvest ripening using factoextra v1.0.7 package (Kassambara and Mundt, Citation2020). The variables were standardized by subtracting the mean and then dividing by the standard deviation of each original variable to give each weight in the analysis.
Results and Discussion
Fruit Skin Color
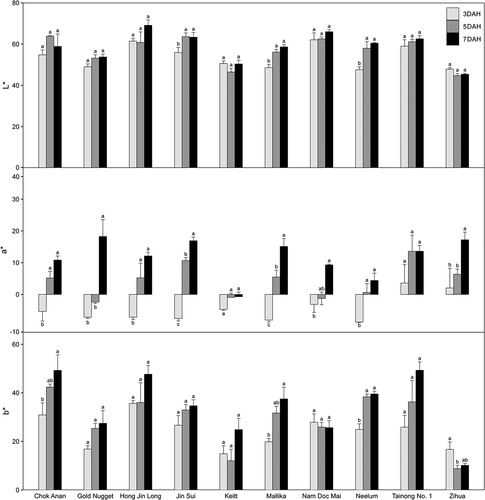
During postharvest ripening, changes in fruit skin color according to variety were different based on changes in L*, a*, and b* ( and 3). Of the 10 varieties, the L* values of ‘Chok Anan,’ ‘Gold Nugget,’ ‘Hong Jin Long,’ ‘Keitt,’ ‘Nam Doc Mai,’ ‘Tainong No. 1ʹ, and ‘Zihua’ did not change during postharvest ripening (). The L* values of the other three varieties, ‘Jin Sui,’ ‘Mallika,’ and ‘Neelum,’ increased from 3 to 5 DAHs and maintained up to 7 DAH. The a* values of all varieties, except for ‘Keitt’ and ‘Tainong No. 1ʹ, increased (i.e., were redder). b* Values of the fruits of ‘Chok Anan’ and ‘Mallika’ increased (i.e., were more yellow) from 3 to 7 DAHs; those of ‘Neelum’ increased from 3 to 5 DAHs. The b* values of ‘Zihua’ decreased from 3 to 5 DAHs and those of the other varieties did not change among DAHs. The pattern of ‘Zihua’ was similar in the fruits of ‘Alphonso,’ ‘Arka Anmol,’ ‘Banganapalli,’ ‘Janardhan Pasand,’ and ‘Irwin’ during ripening (Karanjalker et al., Citation2018; Nambi et al., Citation2015; Ueda et al., Citation2000). Depending on variety, fruit skin color can change from dark green to olive and is sometimes reddish, orange-yellow or yellowish during postharvest ripening (Singh et al., Citation2013). Changes in mango fruit skin color are associated with changes in chlorophylls, carotenoids, and anthocyanins (Ranganath et al., Citation2018; Singh et al., Citation2013). Ranganath et al. (Citation2018) reported that β-carotene, violaxanthin, and cis-β-carotene were the major three carotenoids in fruit skin 12 mango varieties, which were categorized into green, yellow, and red skin-colored types. These varieties also had anthocyanin compounds belonging to cyanidin, peonidin, petunidin, delphinidin, and pelargonidin groups. During ripening, chlorophylls in fruit skin was degraded, carotenoids increased significantly, anthocyanins maintained. With these changes, yellow colored varieties including ‘Chock Anan,’ ‘Gold Nugget,’ and ‘Peach’ accumulated high carotenoid levels (Kienzle et al., Citation2011; Kudachikar et al., Citation2001; Ranganath et al., Citation2018) and red colored varieties including ‘Tommy Atkins,’ ‘Lalmuni,’ and ‘Gulabi’ accumulated high anthocyanin levels (Ranganath et al., Citation2018). The accumulation of pigment patterns needs to be studied in further study since color changes were different from cultivars during ripening ( and 3),
Fruit Weight, Flesh Weight, Fruit Length, and Fruit Diameter
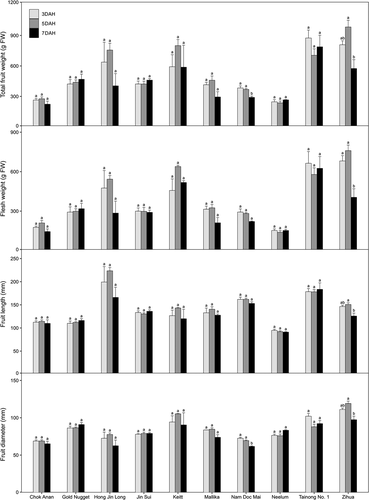
The fruit weights of the 10 varieties were from 236.2 (‘Neelum’ at 5 DAH) to 983.4 g FW (‘Zihua’ at 5 DAH) during postharvest ripening (). Of the 10 varieties, the fruit weights of ‘Nam Doc Mai’ and ‘Zihua’ decreased from 5 to 7 DAHs by 21.1 and 41.6%, respectively, and those of others did not change significantly. The flesh weights of the 10 varieties were from 138.7 (‘Neelum’ at 5 DAH) to 760.0 g FW (‘Zihua’ at 5 DAH) during postharvest ripening (). Flesh weight decreased in ‘Zihua’ only from 5 to 7 DAHs by 46.7%; flesh weight did not change in the other varieties among DAHs. Fruit length and diameter changed only in ‘Nam Doc Mai’ and ‘Zihua.’ From 5 to 7 DAHs, the diameter of ‘Nam Doc Mai’ decreased and the diameter and length of ‘Zihua’ decreased. The volume shrinkage of ‘Nam Doc Mai’ and ‘Zihua’ would mean a decrease in shelf life compared to other cultivars, since volume shrinkage and weight loss are sign of water loss during postharvest ripening of fresh fruits, including apples (Tu et al., Citation2000), blueberries (Paniagua et al., Citation2013) and mangoes (Rathore et al., Citation2007). Water loss depends on genetic characteristics (e.g., associated with variety; Rathore et al., Citation2007) and storage conditions (e.g. temperatures and RH; Paniagua et al., Citation2013). The fruit of ‘Dosehari’ had 9.64% water loss by 9 DAH, when stored at 32–35°C with 53.6–78.8% RH (Rathore et al., Citation2007). The effects of RH during postharvest ripening are not well studied in mango fruits. However, high humidity conditions during postharvest ripening can reduce water loss in various fruits including blueberries (Paniagua et al., Citation2013), grapes (Pereira et al., Citation2018), and litchis (Reichel et al., Citation2017). In our study, fruit weights and flesh weights did not change in any variety until 5 DAH (). Further studies are required to determine the changes in water loss associated with postharvest ripening conditions including temperatures and RH.
SSC, Titratable Acidity, and Fruit Skin Firmness
During postharvest ripening, the SSCs of the 10 varieties were from 10.1 (‘Keitt’ at 3 DAH) to 26.5 °Bx (‘Chok Anan’ at 7 DAH) (). SSCs did not change in ‘Hong Jin Long,’ ‘Keitt,’ ’Mallika’, or ‘Nam Doc Mai,’ but did increase in the other six varieties (). The SSCs of the six varieties increased to a mean of 30.2% and ranged from 13.3% (‘Jin Sui’ from 3 to 5 DAHs) to 62.5% (‘Chok Anan’ between 3 and 7 DAHs). Increases in SSC during postharvest ripening occurred in the fruits of many mango varieties including ‘Alphonso,’ ‘Haden,’ and ‘Tommy Atkins’; the patterns in the increases were different due to storage conditions and variation associated with varieties (Gil et al., Citation2000). During the ripening of mango fruits, increases in SSC might be due to alterations in cell wall structure and breakdown of carbohydrates into simple sugars, including sucrose, fructose, and glucose (Rathore et al., Citation2007). Storage temperatures can also affect increasing rate of SSCs during postharvest ripening of mango fruit (Gil et al., Citation2017). One study of the ‘Haden’ fruits found that SSC was 14.2 °Bx at 25°C and 16.6 °Bx at 12°C at 20 DAH (Manzano et al., Citation1997).
Figure 5. Changes in soluble solid contents, titratable acidities, and fruit skin firmness of ten Mango varieties at 3, 5, and 7 days after harvest (DAHs). Error bars indicated standard errors of the mean values. Different letters indicate significant differences using Scheffe’s test
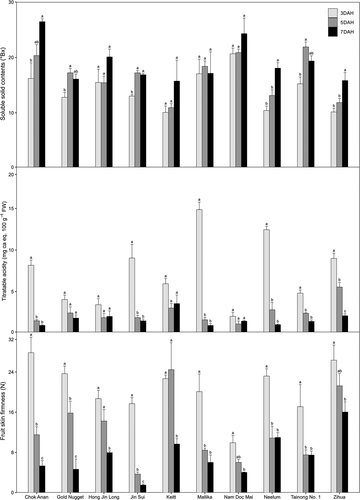
Titratable acidity of the 10 varieties were from 0.9 (‘Mallika’ at 7 DAH) to 12.4 mg ca eq. 100 g−1 FW (‘Neelum’ at 3 DAH) during postharvest ripening (). The titratable acidity did not change in ‘Gold Nugget,’ ‘Hong Jin Long,’ ‘Keitt,’ ‘Nam Doc Mai’ and decreased from 3 to 5 DAHs in the other six varieties. Of these six varieties, the titratable acidity of ‘Zihua’ also decreased from 5 to 7 DAHs. These results were consistent with the results of Doreyappa-Gowda and Huddar (Citation2001). They found that fruits of eight mango varieties (‘Alphonso,’ ‘Amrapali,’ ‘Arka Aruna,’ ‘Arka Amnmol,’ ‘Arka Neelkiran,’ ‘Arka Puneet,’ ‘Mallika,’ and ‘Ratna’) stored at 18–34°C decrease in acidity from 2.71% to 0.04% during postharvest ripening. These changes coincided with a series of physico-chemical changes. Our results were also consistent with the findings of Srinivasa et al. (Citation2002) During postharvest ripening, titratable acidity values of ‘Alphonso’ fruits decreased from 2.17% to 0.08% at 12 DAHs when stored at 27°C with 65% RH. Citric, succinic, malic, and tartaric acids are the organic acids present in mature mango fruit; citric acid is present at the highest concentration and tartaric acid is present at the lowest concentration (Medlicott and Thompson, Citation1985). During ripening, fruit experiences a substantial loss of organic acids. Citric acid is the major constituent in several mango varieties and a loss in concentration leads to decreased acidity during ripening (Singh et al., Citation2013).
During postharvest ripening, the skin firmness of ‘Chok Anan,’ ‘Gold Nugget,’ and ‘Jin Sui’ decreased among DAHs; however, those of the other varieties decreased between specific DAHs. The firmness of ‘Mallika,’ ‘Neelum,’ and ‘Tainon No. 1ʹ decreased only from 3 to 5 DAHs, and those of ‘Nam Doc Mai’ and ‘Zihua’ decreased between 3 and 7 DAHs. The firmness of ‘Hong Jin Long’ and ‘Keitt’ maintained from 3 to 5 DAHs and decreased up to 7 DAH. Many studies reported a drastic decrease in fruit skin firmness in mango during the first week of postharvest ripening (Jha et al., Citation2010; Nambi et al., Citation2015; Wen et al., Citation2006). Jha et al. (Citation2010) found that fruit skin firmness decreased in eight mango varieties during postharvest ripening at 27°C with 55% RH. The varieties exhibited a gradual decrease in skin firmness until 6 DAH and a rapid decrease from 6 to 8 DAHs. However, from 8 DAH onwards, the skin firmness decreased at a very slow rate. In ‘Hong Jin Long’ and ‘Wacheng’ fruit, skin firmness decreased significantly by 6 DAH (Wen et al., Citation2006). In ‘Alphonso’ and ‘Banganapalli,’ drastic decrease was also observed in the fruit skin firmness till 7 DAH (Nambi et al., Citation2015). The changes in the skin firmness of mango fruit during ripening were associated with gradual textural softening and reflected the degree of the ripening with other biochemical and physicochemical changes (Tharanathan et al., Citation2006; Yashoda et al., Citation2006). The polysaccharides of the cell wall are degraded during ripening, endogenously catalyzed by various carbohydrate hydrolases (Tharanathan et al., Citation2006). Turgor pressures decrease due to osmotic dehydration and degradation of storage polysaccharides (Sulistyawati et al., Citation2018). Accordingly, the fruit cell wall is disassembled during ripening, contributing to decreased fruit skin firmness.
During postharvest ripening of mango fruits, the characteristics were significantly different between varieties and/or DAHs (). Fruit skin colors, as reflected in changing L*, a*, and b* values, were significantly different between varieties, DAHs, and interactions between varieties and DAHs. Taken together, these results indicated that the skin colors of mango fruits can be used as an index of ripening stages. As with the relationships between fruit weight, flesh weight, length, and diameter with variety and/or DAH, the rate of weight loss and volume shrinkage during postharvest ripening would also be variety-dependent ( and ). During postharvest ripening, SSC, titratable acidity, and fruit skin firmness were significantly different between variety and DAH. However, we found that SSC was not significantly different when we examined the interaction between variety and DAH.
Table 2. Two-way factorial analysis of variance, using type III sums of squares, of fruit characteristics with variety, days after harvest (DAHs), and their interaction
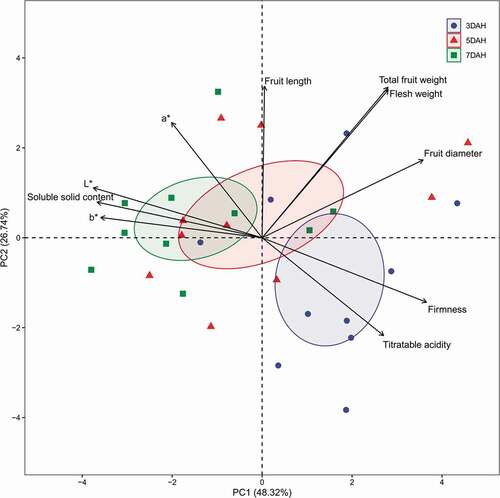
Principal Component Analysis of Fruit Characteristics during Postharvest Ripening
Fruit characteristics of mango were located in each principal component, which were arranged by the size of variance (). The five principal components explained 94.46% of all the variance, and PC1 and 2 explained 75.06% (). PC1 explained 48.32% and represented five characteristics (fruit diameter, SSC, L*, b*, and firmness) based on the eigenvalue of 4.83. These characteristics were 0.83 of the correlation value on absolute average and contributed to 71.12% in PC1. Of these characteristics, fruit diameter and firmness were positively correlated with PC1, while SSC, L*, and b* were negative. Although changes in SSC, L*, and b* were different among DAHs and fruit diameter was not significant ( and ), they are important variables for describing ripening patterns. PC2 explained 26.74% and represented total fruit weight, fruit diameter, and flesh weight based on the eigenvalue of 2.67. These three characteristics were positively correlated with PC2 and had 0.76 of the correlation value on average with contribution to 64.44% in PC2. During ripening of mango fruit, 95% confidence of ellipse for the mean of each DAH was shifted from positive to negative along PC1 axis () while from negative to positive along PC2. The principal component analysis showed a distinct tendency of during ripening of mango fruit, regardless of cultivars.
Table 3. Correlation (Corr) and contribution (Contrib) values of fruit characteristics at each principal components (PC) from PC analysis of ten Mango cultivars at 3, 5, and 7 days after harvest (DAH). Eigenvalues and their contribution to total variation are listed at the bottom of columns
Figure 6. Plot of fruit qualities and position of principal component (PC) scores of means on the first two PC axes for ten Mango cultivars at 3, 5, and 7 days after harvest (DAHs) with 95% confidence of ellipses for the mean of each DAH, respectively. Percentages in parenthesis represent the variance of each PC
Conclusion
Ripening of mango fruit is a process with stages well differentiated in their physicochemical properties. We confirmed that mangoes have distinct color changes during ripening, which may help the consumer determine the optimal time to consume a mango fruit. Ripening of mango fruit grown in a greenhouse showed a similar pattern and period compared to previous studies for postharvest ripening in tropical and subtropical regions. A further study on biochemical and transcriptional changes, including phytohormone interaction and metbolite biosynthesis, may be essential to understand the effects on fruit ripening of environmental conditions.
Acknowledgments
The authors would like to acknowledge public service workers in our laboratory for its collaboration in the research activity.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Core Team, R. 2021. R: A language and environment for statistical computing. 18 May. R Foundation for Statistical Computing. https://www.R-project.org.
- De Mendiburu, F. 2020. agricolae: Statistical procedures for agricultural research. R package version 1.3.3. https://CRAN.R-project.org/package=agricolae.
- Doreyappa-Gowda, I.N., and A.G. Huddar. 2001. Studies on ripening changes in mango (Mangifera indica L.) fruits. J. Food Sci. Tec. Mysore 38(2):135–137.
- FAO. 2020. Major tropical fruits: Preliminary market results 2019. http://www.fao.org/publications/card/en/c/CA7566EN
- FAO. 2021. FAOSTAT. Food and Agriculture Organization of the United Nations. 14 May. http://faostat.fao.org/faostat/en/#data/QC.
- Farina, V., C. Gentil, G. Sortino, G. Gianguzzi, E. Palazzolo, and A. Mazzaglia. 2020. Tree-ripe mango fruit: Physicochemical characterization, antioxidant properties and sensory profile of six mediterranean-grown varieties. Agronomy 10(6):884. doi: https://doi.org/10.3390/agronomy10060884.
- Gentile, C., E.D. Gregorio, V.D. Stefano, G. Mannino, A. Perrone, G. Avellone, G. Sortino, P. Inglese, and V. Farina. 2019. Food quality and nutraceutical value of nine cultivars of mango (Mangifera indica L.) fruits grown in mediterranean subtropical environment. Food Chem. 277:471–479. doi: https://doi.org/10.1016/j.foodchem.2018.10.109.
- Gil, M.A., I.F. Duarte, I. Delgadillo, I.J. Colquhoun, F. Casuscelli, E. Humpfer, and M. Spraul. 2000. Study of the compositional changes of mango during ripening by use of nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 48(5):1524–1536. doi: https://doi.org/10.1021/jf9911287.
- Gil, P.P.S., S.K. Jawandha, N. Kaur, and N. Singh. 2017. Physico-chemical changes during progressive ripening of mango (Mangifera indica L.) cv. Dasehari under different temperature regimes. J. Food Sci. Technol. 54:1964–1970. doi: https://doi.org/10.1007/s13197-017-2632-6.
- Gunness, P., O. Kravchuk, S.T. Nottingham, B.R. D’arcy, and M.J. Gidley. 2009. Sensory analysis of individual strawberry fruit and comparison with instrumental analysis. Postharvest Biol. Technol. 52:164–172. doi: https://doi.org/10.1016/j.postharvbio.2008.11.006.
- Han, S.-H., J.-S. Kim, T. Teruya, Y. Teruya, I. Moromizato, and C.K. Lim. 2016. Comparison of the fruit qualities, the free radical scavenging activities and mangiferin content of the mango, cv. Irwin cultivated in Jeju and Okinawa. Korean J. Hortic. Sci. Technol 34(4):634–643.
- Hofman, P.J., L.G. Smith, G.F. Meiburg, and J.E. Giles. 1997. Production locality affects mango fruit quality. Aust. J. Exp. Agr. 37:801–808. doi: https://doi.org/10.1071/EA97058.
- Jeon, S.G., J.H. Park, S.C. Kim, E.Y. Song, J.H. Joa, K.S. Choi, C.K. Lim, and M.S. Kim. 2013. Greehouse cultivation for mango trees, p. 105–114. In: G.D. Ko, D.M. Oh, H.D. Lee, T.W. Jeong, I.M. Choi, and S.C. Kim (eds.). Agriculture technical guideline: Mango. ISBN: 978-89-480-2380-0-95520. Rural Development Administration, Jeonju, Republic of Korea.
- Jha, S.K., S. Sethi, M. Srivastav, A.K. Dubey, R.R. Sharma, D.V.K. Samuel, and A.K. Singh. 2010. Firmness characteristics of mango hybrids under ambient storage. J. Food Eng. 97(2):208–212. doi: https://doi.org/10.1016/j.jfoodeng.2009.10.011.
- Jha, S.N., P. Jaiswal, N. Kairam, P.K. Poonam, A.K. Singh, and R. Kumar. 2011. Textural properties of mango cultivars during ripening. J. Food Sci. Technol. 50:1047–1057. doi: https://doi.org/10.1007/s13197-011-0431-z.
- Jutamanee, K., and S. Onnom. 2016. Improving photosynthetic performance and some fruit quality traits in mango trees by shading. Photosynthetica. 54:542–550. doi: https://doi.org/10.1007/s11099-016-0210-1.
- Karanjalker, G.R., K.V. Ravishankar, K.S. Shivashankara, M.R. Dinesh, T.K. Roy, and D.V. Sudhakar Rao. 2018. A study on the expression of genes involved in carotenoids and anthocyanins during ripening in fruit peel of green, yellow, and red colored Mango cultivars. Appl. Biochem. Biotechnol 184(1):140–154. doi: https://doi.org/10.1007/s12010-017-2529-x.
- Kassambara, A., and F. Mundt. 2020. factoextra: Extract and visualize the results of multivariate data analyses. R package version 1.0.7. https://CRAN.R-project.org/package=factoextra.
- Kienzle, S., P. Sruamsiri, R. Carle, S. Sirisakulwat, W. Spreer, and S. Neidhart. 2011. Harvest maturity specification for mango fruit (Mangifera indica L. ‘Chok Anan’) in regard to long supply chain. Postharvest Biol. Technol 61(1):41–55. doi: https://doi.org/10.1016/j.postharvbio.2011.01.015.
- Kudachikar, V., S.G. Kulkarni, M.N. Keshava Prakash, M.S. Vasantha, B. Aravinda Prasad, and K.V.R. Ramana. 2001. Physico-chemical changes during maturity of mango Mangifera indica L. variety ‘Neelum.’ J. Food Sci. Technol 38(5):540–542.
- Laxman, R.H., C.J. Annapoornamma, and G. Biradar. 2016. Mango, p. 169–181. In: N. Rao, K. Shivashankara, and R. Laxman (eds.). Abiotic stress physiology of horticultural crops. ISBN: 978-81-322-2723-6. Springer, New Delhi.
- Liguori, G., C. Gentile, G. Sortino, P. Inglese, and V. Farina. 2020. Food quality, sensory attributes and nutraceutical value of fresh ‘Osteen’ mango fruit grown under mediterranean subtropical climate compared to imported fruit. Agriculture 10(4):103. doi: https://doi.org/10.3390/agriculture10040103.
- Lim, C.K., H.S. Oh, H.J. Ahn, and M.K. Jeon. 2020. Growth stage and flowering characteristics of mango in response to different night air temperature. Annual Autumn Conference of the Korean Society for Horticulture Science, 5–11 November, e-Conference, Republic of Korea. p. 140.
- Lobo, M.G., and J.S. Sidhu. 2017. Biology, postharvest physiology, and biochemistry of mango, p. 37–59. In: M. Siddiq, J.K. Brecht, and J.S. Sidhu (eds.). Handbook of Mango fruit: Production, postharvest science, procession technology, and nutrition. ISBN: 978-11-190-1435-5. Wiley, Oxford, UK.
- Maldonado-Celis, M.E., E.M. Yahia, R. Bedoya, P. Landázuri, N. Loango, J. Aguillón, B. Restrepo, and J.C.G. Ospina. 2019. Chemical composition of mango (Mangifera indica L.) Fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 10:1073. doi: https://doi.org/10.3389/fpls.2019.01073.
- Manzano, J.E., Y. Perez, and E. Rojas. 1997. Coating waxes on haden mango fruits (Mangifera indica L.) varieties for export. Acta Hortic. 445:738–746. doi: https://doi.org/10.17660/ActaHortic.1997.455.94.
- McGuire, R.G. 1992. Reporting of objective color measurements. HortScience 27(12):1254–1255. doi: https://doi.org/10.21273/HORTSCI.27.12.1254.
- Medlicott, A.P., and A.K. Thompson. 1985. Analysis of sugars and organic acids in ripening mango fruits (Mangifera indica L. var Keitt) by high performance liquid chromatography. J. Sci. Food. Agric 36(7):561–566. doi: https://doi.org/10.1002/jsfa.2740360707.
- Nambi, V.E., K. Thangavel, and D.M. Jesudas. 2015. Scientific classification of ripening period and development of color grade chart for Indian mangoes (Mangifera indica L.) using multivariate cluster analysis. Sci. Hortic 193(22):90–98. doi: https://doi.org/10.1016/j.scienta.2015.05.031.
- Normand, F., P.E. Lauri, and J.M. Legave. 2015. Climate change and its probable effects on mango production. Acta Hortic. 1075:21–31. doi: https://doi.org/10.17660/ActaHortic.2015.1075.1.
- Nunes, C.N., J.P. Emond, S. Bredcht, S. Dea, and E. Prooulx. 2007. Quality curves for mango fruit (cv. Tommy Atkins and Palmer) stored at chilling and non-chilling temperature. J. Food Qual 30(1):104–120. doi: https://doi.org/10.1111/j.1745-4557.2007.00109.x.
- Paniagua, A.C., A.R. East, J.P. Hindmarsh, and J.A. Heyes. 2013. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 79:13–19. doi: https://doi.org/10.1016/j.postharvbio.2012.12.016.
- Pereira, E., R.G.B. Silva, W.A. Spagnol, and V. Silva Junior. 2018. Water loss in table grapes: Model development and validation under dynamic storage conditions. Food Sci. Technol. 38(3):473–479. doi: https://doi.org/10.1590/1678-457x.08817.
- Ramírez, F., T.L. Davenport, G. Fischer, J.C.A. Pinzón, and C. Ulrichs. 2014. Mango trees have no distinct phenology: The case of mangoes in the tropics. Scientia Hortic. 168:258–266. doi: https://doi.org/10.1016/j.scienta.2014.01.040.
- Ranganath, K.G., K.S. Shivashankara, T.K. Roy, M.R. Dinesh, G.A. Geetha, K.C. Pavithra, and K.V. Ravishankar. 2018. Profiling of anthocyanins and carotenoids in fruit peel of different colored Mango varieties. J. Food Sci. Technol. 55:4566–4577. doi: https://doi.org/10.1007/s13197-018-3392-7.
- Rathore, H.A., T. Masud, S. Sammi, and A.H. Soomro. 2007. Effect of storage on physico-chemical composition and sensory properties of mango (Mangifera indica L.) variety Dosehari. Pak. J. Nutr 6(2):143–148. doi: https://doi.org/10.3923/pjn.2007.143.148.
- Reichel, M., J. Welhofer, R. Triani, P. Sruamsiri, R. Carle, and S. Neidhart. 2017. Postharvest control of litchi (Litchi chinensis Sonn.) pericarp browning by cold storage at high relative humidity after enzyme-inhibiting treatments. Postharvest Biol. Technol. 125:77–90. doi: https://doi.org/10.1016/j.postharvbio.2016.10.002.
- Rstudio. 2020. Open source and professional software for data science teams. 24 Nov. https://rstudio.com.
- Singh, Z., K.S. Rajesh, A.S. Vidhu, and N. Pravendra. 2013. Mango: Postharvest biology and biotechnology. Crit. Rev. Plant Sci. 32:217–236. doi: https://doi.org/10.1080/07352689.2012.743399.
- Srinivasa, P.C., R. Baskaran, M.N. Ramesh, K.V.H. Prashanth, and R.N. Tharanathan. 2002. Storage studies of mango packed using biodegradable chitosan film. Eur. Food Res. Technol. 215:504–508. doi: https://doi.org/10.1007/s00217-002-0591-1.
- Sulistyawati, I., M. Dekker, V. Fogliano, and R. Verkerk. 2018. Osmotic dehydration of mango: Effect of vacuum impregnation, high pressure, pectin methylesterase and ripeness on quality. LWT. 98:179–186. doi: https://doi.org/10.1016/j.lwt.2018.08.032.
- Tharanathan, R.N., H.M. Yashoda, and T.N. Prabha. 2006. Mango (Mangifera indica L.), “The king of fruits”: An overview. Food Rev. Int. 22:95–123. doi: https://doi.org/10.1080/87559120600574493.
- Tiyagi, S., S. Sahay, M. Imran, K. Rashmi, and S.S. Mahesh. 2017. Pre-harvest factors influencing the postharvest quality of fruits: A review. Curr. J. Appl. Sci. Technol. 23(4):1–12. doi: https://doi.org/10.9734/CJAST/2017/32909.
- Tiyayon, C., and R.E. Paul. 2017. Biology, postharvest physiology, and biochemistry of mango, p. 17–35. In: M. Siddiq, J.K. Brecht, and J.S. Sidhu (eds.). Handbook of mango fruit: Production, postharvest science, procession technology, and nutrition. ISBN: 978-11-190-1435-5. Wiley, Oxford, UK.
- Torres-León, C., R. Rojas, J.C. Contreras-Esquivel, L. Serna-Cock, R.E. Belmares-Cerda, and C.N. Aguilar. 2016. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 55:109–117. doi: https://doi.org/10.1016/j.tifs.2016.06.009.
- Tu, K., B. Nicolai, and J. De Baerdemaeker. 2000. Effects of relative humidity on apple quality under simulated shelf temperature storage. Sci. Hortic. 85(3):217–229. doi: https://doi.org/10.1016/S0304-4238(99)00148-X.
- Ueda, M., K. Sasaki, N. Utsunomiya, K. Inaba, and Y. Shimabayashi. 2000. Changes in physical and chemical properties during maturation of mango fruit (Mangifera indica L. ‘Irwin’) cultured in a plastic greenhouse. Food Sci. Technol. Res 6(4):299–305. doi: https://doi.org/10.3136/fstr.6.299.
- Wen, Q., R. Mao, Q. Dong, and Y. Xin. 2006. Studies on postharvest physiology and the storage technology of mango (Mangifera indica L.). J. Food Process. Preserv. 30:670–683. doi: https://doi.org/10.1111/j.1745-4549.2006.00097.x.
- Yashoda, H.M., T.N. Prabha, and R.N. Tharanathan. 2006. Mango ripening: Changes in cell wall constituents in relation to textural softening. J. Sci. Food. Agric. 86(5):713–721. doi: https://doi.org/10.1002/jsfa.2404.