?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Genus Garcinia shows high diversity of sexual systems; most of them are dioecious in nature. The other reported sexual systems of Garcinia include gynodioecious, androdioecious, monoecious, and andromonoecious. Garcinia indica (Thouars) Choisy was reported as facultative apomictic polygamodioecious species. There is a large diversity in floral types. Hence, a detailed study of various floral types found on different types of plants of G. indica and to correlate its functionality with sexual reproduction was undertaken. The study at Kokum Plot, BSSKVV, Dapoli, revealed that the male plants could be categorized into four types – androecious, functional males, andromonoecious, and hermaphrodites. The female plants displayed large variation in number and viability of stamens/staminodes. Each category showed significant difference in phenology, floral morphology and pollen types. In December, the percentage of staminate flowers (79.13 ± 2.93%) in androecious trees was significantly higher than functional males (58.61 ± 8.60%) or andromonoecious trees (56.36 ± 2.77%) (p ≤ .01). As female plants started flowering, all male morphotypes bore significantly higher numbers of staminate flowers and carpellate flowers were significantly reduced. Pollen viability was highest in androecious flowers at 90.11 ± 1.10% and lowest at 64.70 ± 2.09% in hermaphrodite trees. Earlier studies reported female flowers bore well-developed female gametophyte, but the embryo development was not observed. Thus, the fruit formation might be apomictic, but in present embryological investigation; more than 60% of female and andromonoecious flowers, a well-developed embryo was formed between 8 and 24 h after anthesis. Thus, the embryo formation might be sexual or apomictic. Floral types favoring sexual reproduction are essential for conservation of highly endemic species.
Introduction
Linnaeus named the genus Garcinia in honor of the French naturalist and Dutch Army surgeon Laurent Garcin (1683–1752) for his botanical contributions in the eighteenth century (Shameer et al., Citation2016). The genus Garcinia within the Family: Clusiaceae consists of over 800 species spread pantropically, chiefly in Asia, Africa, and Polynesia (Awachare and Upreti, Citation2020). Although the exact origin of this genus is not confirmed, it is believed to be originated in Southeast Asia (Richards, Citation2003). The center of diversity of Garcinia species is the Malaysian region, with some species reaching India and the Micronesian Islands and also extending to tropical Africa and the Neotropics (Shameer et al., Citation2016).
Garcinia is reported to show high diversity of sexual systems; most are dioecious in nature (Sweeney, Citation2008). Other reported sexual systems of Garcinia include gynodioecious, androdioecious, monoecious, and andromonoecious species (Joseph and Murthy, Citation2015). In Dioecism, plants bear either male or female flowers, whereas in monoecism, plants bear both male and female flowers. In Gynodioecism, plants bear either female or bisexual flowers whereas in Androdioecism, plants bear either male or bisexual flowers. In Andromonoecism, plants bear bisexual and male flowers both whereas in Gynomonoecism, plants bear bisexual and female flowers (Bawa and Beach, Citation1981). Garcinia atroviridis Griff. ex T. Anderson is reported to be gynodioecious in which female but hermaphrodite individuals co-occur (Pangsuban et al., Citation2007). The mangosteen (Garcinia mangostana L.) trees produce perfect flowers that are functionally female only, they produce stamens with aborted pollen grains and male trees are not found (Joseph and Murthy, Citation2015; Nuanjunkong and Meesawat, Citation2008). Leal et al. (Citation2013) reported the presence of an andromonoecious individual for the first time in a dioecious male-biased population of Garcinia brasiliensis Mart. Garcinia imberti Bourd. is functionally dioecious, self-incompatible and the male and female flowers are separate on the different trees which are dependent on pollinators for fruit set and insect vectors are essential for successful reproduction (Kandasamy et al., Citation2017). In case of Garcinia gummi-gutta (L.) Robs., it is dioecious, producing male and female flowers on separate plants. George et al. (Citation1992) reported the androdioecious character of this plant, that is coexistence of male and bisexual plants of Garcinia gummi gutta but recently no bisexual individuals have been recorded by different authors (Aswathi, Aswani, and Sabu, Citation2018). Garcinia travancorica Bedd. and Garcinia imberti are dioecious, the tree produces male and female flowers on two different individuals but the female tree bearing bisexual flowers at peak flowering time. Thus, it is gynomonoecious in nature (Manikandan, Citation2016). Garcinia celebica L. bears perfect flowers with numerous stamens surrounding the pistillode. Female flowers on separate trees have been observed, thus, showing androdioecism. (Sutthinon et al., Citation2018).
Dike and Deodhar (Citation2019) elaborately described different floral types of different trees found in G. indica. According to their observations, G. indica is polygamodioecious with three types of plants: no fruit yielding or male, high fruit yielding or functional females, and low fruit yielding bisexual.
Male or no fruit yielding plants bear flowers in the month of November–January. These flowers possess elongated receptacle: four petals and four sepals which are reddish cream in color. Stamens are numerous, fertile, and cohere at the base forming anthophore. Carpels are absent or small and rudimentary pistils are observed.
Female or high fruit yield plants produce flowers slightly late, in the period from December–February. The flowers on female plants are comparatively broader with short pedicel than male flower; four sepals and pale yellowish-green corolla. These flowers have fewer stamens or staminodes which are arranged in two, four, and eight tufts surrounding pistils. Ovary of the flower is larger with four or eight functional ovules on axile placenta.
Bisexual or low fruit yielding plants bear the typical male flowers as well as flowers with a ring of stamens or staminodes around carpel. The carpel of the flower is fertile, and its petals are reddish in color. These plants produce spindle-shaped fruits but rather in very low in number. But the male female or bisexual plants bear varied morphotypes of flowers which can be arranged in increasing complexity of stamens or decreasing complexity of carpels (Karnik, Citation1979). Hence, an elaborate study was undertaken for detailed study of morphotypes found on various G. indica plants and correlating its functionality with sexual reproduction.
Materials and Methods
Study Site
The detailed study of floral biology of Garcinia indica (Choisy) was carried out at Kokum Plot (17°45ʹ13.1”N 73°10ʹ33.6”E), Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Rantagiri, Maharashtra during the period 2019–2021(November to March). The Kokum plot has a total of 269 Garcinia indica (Choisy) trees planted in the year 1972 spread over an area of 1.29 ha. planted in 10 rows and 30 columns with spacing of 8 m x 6 m.
Floral Biology
Flowers of male and female trees were collected from lower sections of canopy during their flowering seasons. Male flowers were collected twice – in early (December) and late (January) flowering season. Around 70–100 flowers from each tree were collected.
The flowers were observed off-field for their characteristic morphology and percentage of occurrence for respective trees. Flowers were seen under light microscope to observe internal floral structure. Various floral types, such as male, female and hermaphrodite flowers were recorded, respectively. The trees were then identified as male, female, or hermaphrodite based on the percentage and type of flowers collected. Few selected female individuals were inspected throughout the season for their flowers and respective androecial arrangement. Floral colors recorded and compared with the Colors of the Royal Horticultural Society Color Charts Edition V in sRGB scale in the charts of Yellow-red, Turquoise-green and Brown-gray.
Pollen Morphology
Pollen grains from randomly collected 20 well developed flowers from each identified floral type were taken to study their morphology. The pollen grains found in mature flowers of various floral types were observed under compound microscope at 10x and 45x magnification.
Pollen Viability
Pollen viability was measured using Trypan blue exclusion Method. Pollen viability was then interpreted to classify the presence of stamen or staminode in each floral type
Sexual Reproduction
Female flowers were tagged on the day of anthesis and fixed in FAA (formalin: acetic acid: 90% ethanol in 1: 1: 9). To study various embryological stages, the flowers were fixed at pre-anthesis Day (−5, −3, −1) or, at anthesis (Day 0) and post-anthesis Day (+1 and +2) at an interval of eight hours for two days. For sectioning, the outer whorls of calyx, corolla were removed to expose the ovule for sectioning. Sections were mounted distilled water and observed under a bright field microscope and images were taken via an attached camera (Olympus CX31 Microscope with Olympus C-330 camera). Sections were taken manually and observed under light microscope.
Statistical Analysis
All values were expressed as Mean ± SD. To compare different male morphotypes, the data was statistically analyzed using One-way ANOVA and post hoc Tukey’s test. (α = 0.05 and 0.01) wherever applicable. The values were considered significant for p ≤ .05 and p ≤ .001.
Results and Discussions
Description of Study Site
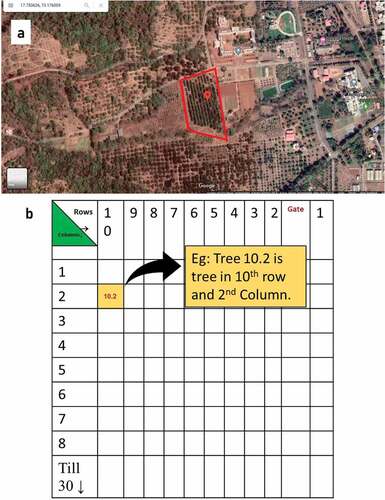
In present investigation, the study was concentrated at Kokum Plot (17°45ʹ13.1”N 73°10ʹ33.6”E), Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Rantagiri in which 269 Garcinia indica trees were planted in the year 1972. There were 145 Male and 124 were Female trees. Thus, the Male: Female ratio was initially 145:124. Over the course of time, 14 Males died due to various reasons so the current ratio in the plot being 131:124. As depicted in the (), Kokum trees in the plot were planted in 10 rows and 30 columns with spacing of 8 m x 6 m. All Kokum trees were tagged with plastic tags and labeled based on their position on site (Row. Column) – e.g.: Tree 10.2 is Tree in 10th row and 2nd Column ().
Figure 1. (a) Kokum plot (Marked) at Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli (Courtesy: Google Maps) and (b) Illustrated Map of Kokum Plot with tree coding system.
Climatic data for the year 2020 were obtained from Department of Agronomy, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Dapoli, Rantagiri. These data included temperature, precipitation, and relative humidity readings. The maximum mean annual temperature (Tmax) was 31.6°C whereas minimum mean annual temperature (Tmin) was 19.9°C. Mean maximum annual relative humidity (RH%) was 91.9% whereas minimum annual relative humidity (RH%) was 67.6%. The mean annual wind speed was 4.3 Kmph. Annual precipitation was 4145.4 mm and there were 110 rainy days. Mean annual Bright Sunshine (BSS) hours were 6.7 hours.
These trees on plot exhibited lot of variation in floral diversity. Hence, an elaborate study was undertaken for detailed study of morphotypes found on various plants.
Diversity in Floral Morphology in Male Plants of G. indica
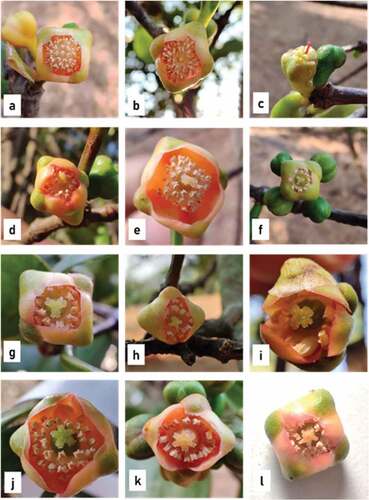
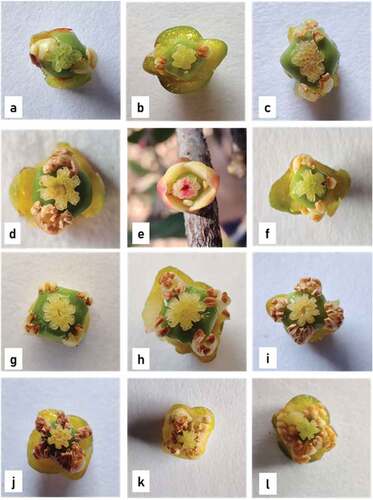
Various floral morphotypes – Staminate, rudimentary carpellate and carpellate male flowers were found on male trees. The staminate flowers on male trees could be segregated into two types: small and large staminate flowers based on their size. The mean diameter of the small staminate flowers was 0.74 ± 0.05 cm, and the mean diameter of the large staminate flowers was 0.95 ± 0.02 cm. They bear numerous stamens which cohere to form the anthophore (Internodal elongation of floral axis/thalamus between corolla and androecium is called Anthophore) (). Some flowers bore a rudimentary carpel on staminate flower. They are described as rudimentary male flowers. The apex of the anthophore bore a rudimentary carpel whose structure varied. It might be a pistillode which creates an empty circular area at the center of thalamus which is surrounded by numerous stamens (). The carpel is filiform (), not differentiated into ovary, style, and stigma. Sometimes, the carpel showed bilobed stigma and a distinct ovary, which internally did not show any locule or ovule development (). Staminate flowers with a well-developed carpel were described as carpellate male flowers. They have distinct bifid and trifid stigma and well-developed ovary which is internally differentiated in bilocular or trilocular carpel. The stylar canals seemed to be well developed in bifid and trifid carpellate flowers (). The number of stigmatic lobes corresponded to the number of locules present in the ovary. Carpellate flowers bore well developed carpel, globular in shape, and green in color. Internally, the carpel possessed distinct locules (). Hermaphrodite flowers were perfect carpellate flowers (). They possessed a well-developed ovary with a ring of stamens surrounding it which could further undergo fruit formation.
Various Types of Male Plants of G. indica
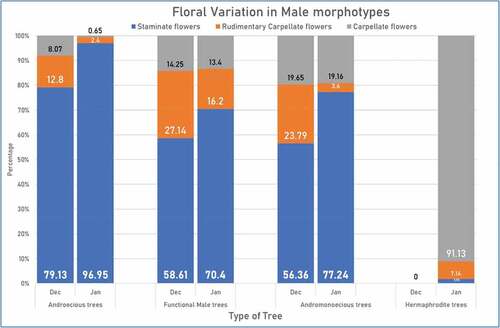
Detailed study of male trees was undertaken and the number of staminate, rudimentary carpellate and carpellate flowers were noted and tabulated in early and late flowering season (December and January respectively). Depending on percentage of different flowers the male trees were classified into different types viz. Androecious trees, functional male trees, Andromonoecious males, and Hermaphrodites. The percentage of different flowers born on trees was noted in Mean ± SD ().
Androecious Trees
Only three androecious trees (Trees coded: 10.3, 10.4, 4.19) were blooming during the initial period of flowering season i.e. December. The mean number of staminate flowers found was 79.13 ± 2.93%. About 12.8 ± 1.77% rudimentary carpellate flowers were present along with staminate flowers. The flowers with well-developed carpel were also observed in small numbers (8.07 ± 3.06%). In January, the number of androecious trees in bloom was considerably more (). Two Androecious individuals (Trees coded: 10.2,10.3) that were in full bloom earlier were on the verge of the end of their phenological period in January. Four flowering plants (Trees coded: 7.4, 7.5, 9.5, 10.5) were randomly selected for statistical analysis. It was noted that some trees showed small morphotype staminate flowers only in the earlier season started showing both small and large staminate flowers later. Also, the percent staminate flowers were 98.10 ± 2.27% along with 1.47 ± 2.55% rudimentary carpellate flowers. Carpellate flowers were only 0.43 ± 0.74%.
Functional Male Trees
Some male trees showed higher percentage of rudimentary flowers as well as carpellate flowers than androecious trees. Such trees were grouped under the category as ‘functional Males.’ When above floral type percentage variation was analyzed using One-way ANOVA with post hoc Tukey test (p = .05 and p = .01), it was noted that the mean percent of staminate flowers in functional male trees was significantly lower than androecious trees (). The mean percent of staminate flowers in December was only 58.61 ± 8.60% in functional males whereas it was significantly higher in androecious trees i.e. 79.13 ± 2.93% both at p ≤ .05 and p ≤ .01.
Figure 4. Floral type variation amongst different male morphotypes (n = 3, mean ± SD).* signifies statistically significant difference in percentage of floral types in male morphotypes (ANOVA and Tukey’s post hoc test: * p ≤ .05, ** p ≤ .01).
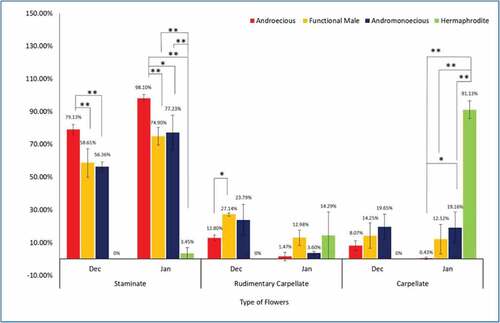
Similarly, the difference was also noted in percentage of rudimentary carpellate flowers. The mean percent of rudimentary carpellate flowers in functional males (December) was significantly higher (27.14 ± 0.90%) at p ≤ .05 than androecious plants where it was only 12.80 ± 1.77% ().
This difference was also prominently seen in the month of January. The percentage of staminate flowers significantly higher (98.10 ± 2.27%) in androecious trees than functional males which bore only 74.90 ± 5.37% staminate flowers at p≤ .05 and p≤ .01. Similarly, the androecious trees borne only 1.72 ± 3.45% of rudimentary carpellate flowers whereas in functional male plants the percentage of rudimentary carpellate flowers was much higher 12.98 ± 4.73% ().
The difference was also noted in proportion of carpellate flowers in different type of plants. In December, the percentage of carpellate flowers on functional male trees was 14.25 ± 7.69% and in January it was 12.12 ± 8.96%. Contrary to this in androecious trees, the percentage of carpellate flowers in December was 8.07 ± 3.06% which reduced to only 0.43 ± 0.74% in January ().
Andromonoecious Males
Andromonoecious males are low fruit yielding males that bore both staminate and carpellate flowers like functional males but the difference being – few carpellate flowers resulted in fruit formation. There are only three andromonoecious plants reported in the orchard of KKV, coded 3.1, 4.1 and 5.1. The mean percentage of staminate flowers in December was 56.36 ± 2.77% which was significantly lesser than androecious trees. There was no much difference in number of staminate flowers in andromonoecious males and functional males ().
The percentage of carpellate flowers was the highest in andromonoecious trees and some of the carpellate flowers had fertile ovary. In December it was 19.65 ± 7.66% and in January it was 19.16 ± 9.41%. Whereas in Androecious tree it was only 8.07 ± 3.06% in December which was reduced to 0.43 ± 0.74% in January. One fruit had already set in andromonoecious tree 4.1 in January. The ripened fruit bore well developed seeds ().
Hermaphrodite Plants
No hermaphrodites were flowering in December. In January, 2 hermaphrodite individuals were found to be flowering, they were, KKA 110/24 and 10.20. On an average, 91.13 ± 5.42% of the flowers were perfect carpellate flowers. They possessed a well-developed ovary with a ring of stamens surrounding it. Staminate flowers were rarely recorded (1.72 ± 3.45%). Carpellate flowers bore well developed carpel, globular in shape and green in color. Out of two trees, KKA 110/24 did not bear any fruits but 10.20 showed presence of one fruit only ().
Seasonal Variation in Floral Percentages Amongst Male Morphotypes
When the percentages of floral types were compared between early flowering season (December) and late flowering season (January) using One-way ANOVA with post hoc Tukey test (p = .05 and p = .01), significant differences were observed in staminate and rudimentary carpellate flowers of all male morphotypes ().
Figure 5. Season-wise mean floral variation between tree types (n = 3, mean ± SD).* signifies statistically significant difference amongst various floral types in male morphotypes (ANOVA and Tukey’s post hoc test: * p ≤ .05, ** p ≤ .01).
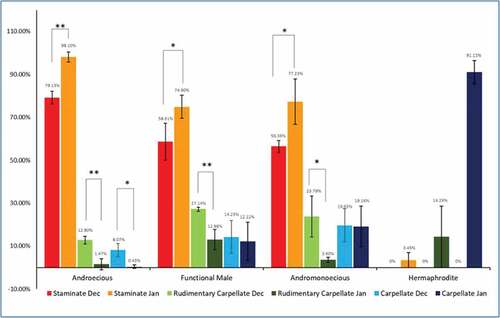
As flowering season advanced, the staminate flower percentage significantly increased in all morphotypes. In androecious trees it increased from 79.13 ± 2.93% in December to 98.10 ± 2.27% in January trees (p≤ .01). In functional males it was increased from 58.61 ± 8.60% to 74.90 ± 5.37% and 56.36 ± 2.77% to 77.23 ± 10.57% in Andromonoecious trees (p≤ .05) ().
As the season progressed, the percent rudimentary carpellate flowers significantly decreased in all morphotyes – from 12.80 ± 1.77% to 1.47 ± 2.55% in androecious trees, 27.14 ± 0.90% to 12.98 ± 4.73% in functional males (p≤ .01), 23.79 ± 9.57% to 3.60 ± 1.16% in andromonoecious trees (p≤ .05). There was a significant decrease in carpellate flowers of androecious trees. In December it was 8.07 ± 3.06% which was decreased to 0.43 ± 0.74% in January (p ≤ .05). whereas no significant difference was seen in functional male or andromonoecious trees ().
As said earlier, female flowering season starts later than male flowering. It was observed that as the flowering season advanced and the female trees started blooming, the number of pistillate and carpellate flowers in different type of male trees appeared to be significantly reduced whereas the number of staminate flowers was significantly higher. Thus, G. indica exhibited protandry to facilitate fertilization of flowers on female trees (Forrest, Citation2014). To generalize, male trees in G. indica can be categorized into four types – androecious males, functional males, Andromonoecious males (bore staminate flowers and carpellate flowers with functional ovary and sparingly produce fruit), and Hermaphrodites (bore only perfect flowers and sparingly produced fruits).
Diversity in Floral Morphology in Female Plants of G. indica
Flowering phenology of female plants begins in the month of December till month of late March- April. The mean monthly temperature in December varied from 14.5°C (Tmin) to 33.4°C (Tmax) with relative humidity between 67.75 and 92% whereas in the month of April, it varied from 20.7°C (Tmin) to 34.0°C (Tmax) with relative humidity between 51.5 and 87.5%. Female flowers like male flowers show tetramerous symmetry. However, color of calyx and corolla differ from male plants. Sepals of female flowers are Lime (144C) in color and the corolla is light yellow (2C) and devoid of red shades which are frequently observed in male flowers.
The difference in morphology of male and female flowers mainly lies in length of thalamus or receptacle. Generally, male flowers bear elongated thalamus which bear anthophore with numerous stamens. Female flowers bear a short receptacle, bearing a ring of stamens around round expanded ovary. Ovary has 5–7 locules with ovules on axile placenta. Stigma is sessile, flat ring like stellate structure. The number of stigmatic lobes vary in number from 6 to 10. Contrary to this the carpellate flowers of male plants bear trifid or tetrafid stigma. Each stigmatic lobe shows stigmatic canal which leads to stylar canal which are prominent.
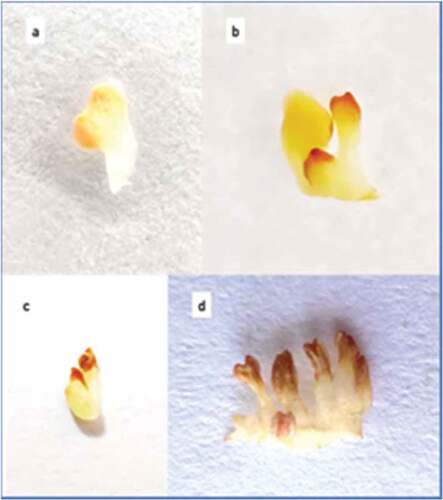
Stamens in female flowers produced rough pollen grains which were sterile. The filaments showed a lot of variation. Rarely they consisted of fleshy filaments bearing two anther lobes at the top ()). Many times, the filaments were fleshy branched and each branch formed the spathulate structure bearing anther lobes ()). This branching or degeneration of staminodes leaded to lot of variation in androecium (). The number of bundles varied.
Most frequently stamens form 2 or 4 bundles. In some flowers, staminodes were so much branched that they appeared forming a ring of stamens around ovary. shows androecium with 2 stamens in 2 bundles whereas shows how stamens in two bundles are branched and expanded to form spathulate structures. shows stamens forming 4 bundles whereas shows how the branching and expansion of filaments lead to formation of ring of stamens. are the examples how stamens forming 4 bundles degenerate forming 3 bundles.
Figure 7. Variation in number of bundles of stamens formed due to branching, expansion, and deletion of filaments of staminodes.
Few selected female individuals were inspected throughout the season for their flowers and respective androecial arrangement. From , Tree 5.7 possessed 66.67% of flowers with 4 bundles of stamens followed by 33.33% with 2 bundles of stamens. Tree 7.17 bore 70.00% flowers with 4 whorls of stamens and 30.00% with 3 whorls of stamens. Tree 10.18 bearing highest number of 2 whorles of stamens 64.71% the number of flowers with 4 whorls of stamens is only 17.65%.
Table 1. Percentage variation of female flowers amongst different female trees.
Here, the the special type of tree to be noted is – 8.13. In this plant, the number of flowers with 4 bundles is comparatively low (44.44%). It is bearing the flowers with 5 bundles which appear in form of ring. It is also showing all variations in androecium and the flowers with one two and 3 bundles are also noted. Similarly, tree 8.2 is also showing all androecial arrangements ranging from 2 bundles to 5 bundles.
Pollen Morphology in Various Floral Types of G. indica
In the present study, the pollen grains found in flowers of various floral types were observed under compound microscope at 10x and 45x magnification. The pollen grains obtained from staminate flowers in androecious males were tetrazonocolpate. The pollen grains were of two sizes. About 70% were of about 20 µm diameter and the others were little, smaller of about 15 µm in diameter (). The rudimentary carpellate flowers of androecious males also showed two types of pollen grains. But number of larger pollen grains was little lesser (48%) and small pollen grains was 52% (, ). In carpellate flowers of androecious males, all 100% pollen grains were of small size they were tetra aperturate and wall of pollen grain was comparatively thick.
Figure 8. Pollen grains of (a) staminate flowers, (b) rudimentary pistillate flowers, (c) carpellate flowers and (d) female flower.
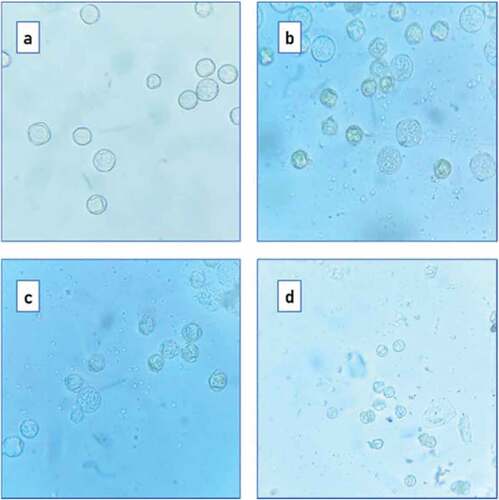
Table 2. Percentage pollen grain variation in male morphotypes.
A similar trend was observed in functional male plants. In staminate flowers of functional males had higher number (71.42%) of lager pollen grains. In rudimentary pistillate flowers, the larger pollen grains were only 34.37% and 65.62% pollen grains were of smaller size. In carpellate flowers all pollen grains were of small size. And the small pollen grains had comparatively thicker walls.
The unique observation was carpellate flowers on andromonoecious trees had 3 types of pollen grains. In addition to pollen grains of smaller and larger size, it also produced inaperturate thick-walled irregular pollen grains that were characteristic of female plants. To recall- the carpellate flowers of andromonocious plants were also bearing tri to tetralocular ovary bearing ovules on axile placenta and some of them resulted in fruit formation. Contrary to this, though the flowers on hermaphrodite plants also born bigger carpel, the pollen grains found on these flowers were mostly (76%) larger in size. Pollen grains observed on hermaphrodite plants were like pollen grains found on staminate flowers. All of them were of larger size and thin walled.
The pollen grains of all type of female flowers, two or four bundled possessing stamens or staminodes all were underdeveloped, smaller in size (approx. 10 µm) spiny thick walled and inaperturate ().
There are several reports of palynological studies on different species of Garcinia. The pollen grains of G. atroviridis were tricopolrate – small, 20–25 µm in diameter, with a scabrate pattern of exine (Pangsuban et al. Citation2007). The pollen grains of G. imberti were round with the average diameter of 20–36 µm (Kandhasamy et al., Citation2017). Garcinia celebica L. pollen grains showed triporate structure with clavate ornamentation (Sutthinon et al., Citation2018). Pollen grains of G. gummi-gutta were 24.6 ± 0.27 µm in diameter. An average of 4230 ± 603 pollen grains was produced per flower. Pollen grains are spherical in shape and porate, the exine surface is reticulate. Also, Bi, tetra and penta porate pollen grains were observed (Aswathi, Aswani, and Sabu Citation2018).
Pollen Viability in Floral Types of Male Plants of G. indica
Pollen Viability in different floral types was determined using Trypan Blue Exclusion Method. In androecious trees, the pollen viability in small staminate flowers was 90.11 ± 1.10% and in large staminate flowers was 86.66 ± 1.67% (). In flowers with rudimentary carpel, pollen viability was 86.36 ± 2.27%. Compared to pollen viability of staminate flowers, the pollen viability was significantly reduced in flowers with bifid and trifid stigma in androecious trees. In bifid carpellate flowers, it significantly decreased to 80.81 ± 2.08% (p < .01) and to 80 ± 4.00% in trifid carpellate flowers (p < .01) ().
Table 3. Pollen viability in male morphotypes.
Figure 9. Pollen viability in male morphotypes (n = 3, mean ± SD).* signifies statistically significant difference in pollen viability amongst various floral types in male morphotypes (ANOVA and Tukey’s post hoc test: * p < .05, ** p < .01).
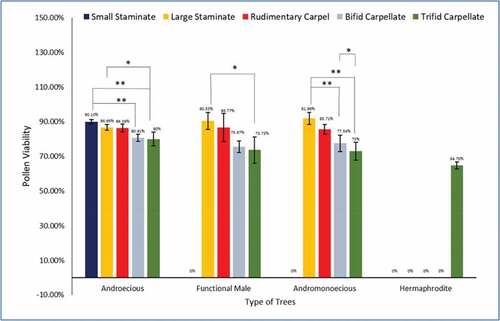
Similar observation was done by Dike and Deodhar (Citation2019), it was noted that pollen fertility was found to be dependent on size of carpel present in the male flowers. As the size of carpel increased, the number of viable pollen grains decreased. In the case of male flowers, with no carpel, 65% germination ability was observed, whereas in the case of flowers with carpel size about 1 mm and 2 mm, the pollen germination ability was decreased to 45% and 25%, respectively (Dike and Deodhar, Citation2019).
In the present study, the functional male trees bear only large staminate flowers and in these flowers the percentage viability was 90.53 ± 4.82%. In flowers with rudimentary carpel, the viability was 86.77 ± 8.05%, whereas in flowers with bifid stigma it reduced to 75.67 ± 3.34%. When compared to staminate flowers in functional male trees, the pollen viability in flowers having trifid stigma was significantly reduced to 73.73 ± 7.64% (p < .05).
Small staminate flowers in andromonoecious showed pollen viability of 91.96 ± 3.50% and showed 85.71 ± 2.86% in rudimentary carpellate flowers. Compared to pollen viability of staminate flowers in andromonoecious plants, it significantly reduced to 77.54 ± 4.81% and 73 ± 5.05% in bifid and trifid carpellate flowers respectively (p < .01). The viability of rudimentary carpel did not vary much amongst morphotypes- 86.36 ± 2.27%, 86.77 ± 8.05%, and 85.71 ± 2.86%, respectively.
The lowest viability was seen in hermaphrodite trees at 64.70 ± 2.09% which majorly possessed carpellate type of flowers. But it should be noted that the hermaphrodite plants produced flowers with fertile stamens and carpels. Contrary to this in all the type of female flowers only 1 to 2% pollens were viable.
Male pollen viability as high as 95.7% was recorded in Garcinia brasiliensis (Leal et al., Citation2013). Maximum pollen viability (89.16 ± 2.11%) was observed in 0.2% TTC (Triphenyl Tetrazolium Chloride) and lowest (84.35 ± 4.12%) in 1% acetocarmine stain for G. imberti (Kandhasamy et al.,Citation2017). However, viability in G. celebica was 68% using TTC viability assay (Sutthinon et al., Citation2018). Pollen viability of G. gummigutta was reported to be high at evening hours and 23.6 ± 6.4% pollen grains were viable at 7.00 pm, after 13 hours most of the pollen grains lost its viability. The fertility of G. gummigutta pollen grains was 94.1 ± 0.66% on the day of anthesis at 5.00 pm, and then fertility slightly decreases (Aswathi, Aswani, and Sabu Citation2018).
Sexual Reproduction in G. indica
In earlier studies of reproduction and fruit set in G. indica, it was observed that male plants produced fertile pollen grains. When transferred to the sigmatic surfaces, they germinated profusely, traversed the stylar canal reaching to the female gametophyte. The embryological events were recorded by hand cut as well as paraffin block vertical sections of ovary. However, embryo formation was not noticed in any section. It was then concluded that the seeds of G. indica were formed by parthenogenesis without development of embryo or endosperm. However, molecular fingerprinting studies indicated that progeny bands were polymorphic indicating occurrence of sexual reproduction (Dike et al., Citation2020). A systematic study was undertaken to evaluate the occurrence of sexual reproduction.
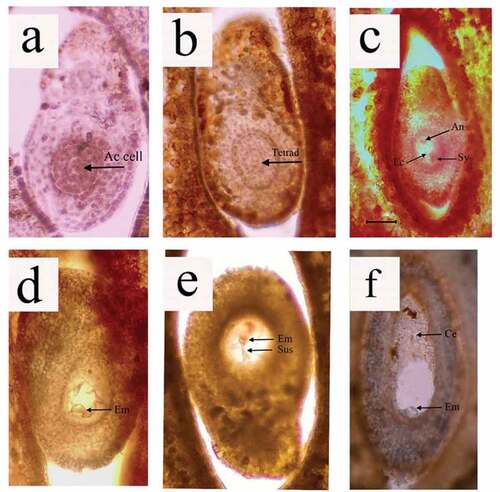
V.S. of flowers showed presence of ovule which was anatropous and crassinucellate. In pre-anthesis events, at day − 5 (5 days before anthesis) in the ovule, the megaspore mother cell was detected in the nucellus surrounded by integument (). This megaspore mother cell gave rise to a linear tetrad which could be seen at Day − 3 evening sections (). By the time of anthesis, the ovule is ready with fully developed female gametophyte. Structures such as synergids, egg cell, and antipodal cells were observed in female gametophyte (). Embryo formation took place between 8 and 24 h after anthesis. In flowers collected at 8 h after anthesis, a well developer globular embryo was observed () in about 61.64% of all type of female flowers. In some of the flowers collected 24 h after anthesis, even embryo with suspensor was noticed. (). On Day 2, i.e. 48 h after anthesis, cellularization has progressed from chalazal end and filled most of embryo sac and further development of embryo are not observed (). An embryo as globular structure is seen to be hidden at micropylar end near radicular pole of seed. Similar type of globular embryo at micropylar end was also noted in G. prainariaa by (Rohani et al., Citation2021).
Figure 10. Embryogenesis in G. indica, Ac cell: Archesporial cell, An: Antipodal cells, Ec: Egg cell, Sy: Synergids, Em: Embryo, Sus: Suspensor, Ce: Cellularization.
As flower develops into young fruit, a vascular strand can be seen, which traversed through the middle of the seed and connected to the micropylar and chalazal ends (). This is visible in seed section as well (). It was referred to as “embryonic axis” by Teo in G. mangostana (Teo, Citation1992). In mature seeds also, the embryo is seen to be hidden at radicular end of seed in this embryonic axis (, d).
Figure 11. (a) Vascular strand connecting micropylar and chalazal ends in young fruit, (b) vascular strand in seed section, (c) section of seed showing rudimentary cotyledons and (d) section showing hidden embryo.
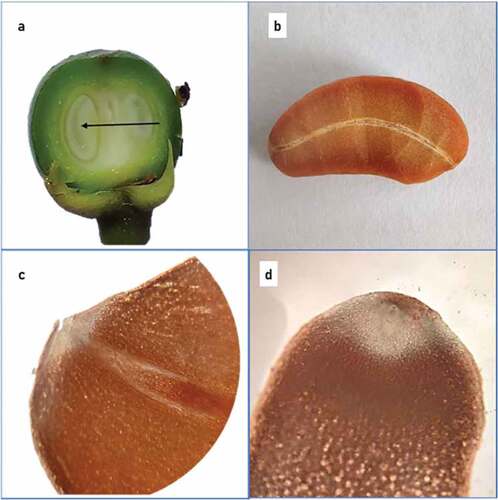
In fully developed seed in G. indica, normal embryonic structures like cotyledons, radicle, and plumule were not observed (Noor et al., Citation2016). Most of the seed was a mass of undifferentiated tissue of cortical parenchyma cells full of storage materials. Morphology and structure of seed did not show any structure akin to plumule or a radicle. The absence of well-defined endosperm, cotyledons, and embryonic axis or any structure remotely similar to it, indicated that it was not a true seed.
The seed germination was quite unique. The plumule and radicle appear to be emerging from the two different ends of seeds regardless of species, this confers unique Garcinia type germination to the genera. At the time of germination, the procambial tissue increases in length and divides to provide vasculature to emerging plumule and radicle developing from both radicular and germular poles. The presence of polyembryonic seeds in G. hombroniana showing separate embryos each with separate procambium has been well described with photographs by Noor et al. (Citation2016).
Regeneration of multiple seedlings from whole seed and seed halves or segments further indicated the apomictic (agamospermous) nature of seed (Malik et al., Citation2005). Thus, in G. indica, though the embryo sac appears to be developed before anthesis and even the embryo development does occur, the embryo is derived gametophytic apomixis where megaspore mother cell fails to undergo meiosis; instead, it undergoes mitotic division to produce an embryo sac. Our earlier breeding experiments conducted by also suggests the possibility of apomixis in G. indica. In above study, to check the possibility of sexual reproduction or apomixis in G. indica, the seeds raised progeny produced from natural as well as artificial pollination was subjected to molecular fingerprinting. For plant F159 when allowed to pollinate naturally only 12.5% progeny was produced by sexual reproduction. But in the same plant the artificial pollination resulted in 42.85% of sexual progeny (Dike et al., Citation2020).
Furthermore, it is essential to study the various floral morphotypes in female flower, the nature of functional stamens or staminodes, pollen viability, ability of fruit formation under different fertilization regimens, and their relationship with the formation of sexual and apomictic progeny.
World-wide thousands of plant species are being included under endangered species and facing risk of extinction. The study of reproductive biology of these threatened, endangered plants may give clue about pollination failure, pollinator abundance or limitations, poor seed production, etc. that will help in conservation of species.
Acknowledgments
The authors are thankful to K.E. T’s V. G. Vaze College (Autonomous) of Arts, Science and Commerce, Mulund, Mumbai for providing laboratory facilities; Dr. S. V. Sawardekar, Department of Agronomy, Dr. B. S. K. K. V., Dapoli, Dist. Ratnagiri for Climate data; and Mr. Sachin Gorivale for on-field assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aswathi, P., K. Aswani, and M. Sabu. 2018. Reproductive biology of malabar tamarind (Garcinia Gummi-Gutta (L.) Rob.: An endemic, medicinal and spice plant from Western Ghats. Int. J. Plant Reprod. Biol. 10(1):65–68. doi: 10.14787/ijprb.2018.
- Awachare, C.M., and K.K. Upreti. 2020. Phenological growth stages in mangosteen (Garcinia Mangostana L.) according to the extended BBCH scale. Ann. Appl. Biol. 176(1):16–25. doi: 10.1111/aab.12552.
- Bawa, K.S., and J.H. Beach. 1981. Evolution of sexual systems in flowering plants. Ann. Mo. Bot. Gard. 68(2):254. doi: 10.2307/2398798.
- Dike, M., and M. Deodhar. 2019. Sexual reproduction in garcinia indica with special reference to various floral types, pollen structure, pollen viability and pollen germination on stigmatic surface. Acta Hortic. 1241(1241):299–306. doi: 10.17660/ActaHortic.2019.1241.42.
- Dike, M.S., S.K. Malik, S.V. Sawardekar, and M.A. Deodhar. 2020. Study of the mode of reproduction and fruit development in garcinia indica. Int. J. Fruit Sci. 20(1):20–38. doi: 10.1080/15538362.2018.1563022.
- Forrest, J.R.K. 2014. Plant size, sexual selection, and the evolution of protandry in dioecious plants. Am. Nat. 184(3):338–351. doi: 10.1086/677295.
- George S.T, A.K. Baby Latha, K. Lyla Mathew, and C.K. Geetha 1992. Pattern of flowering and flower development in Kodampuli (Garcinia cambogia Desr). Indian Cocoa, Arecanut & Spices J. 16: 68–70.
- Joseph, K.S., and H.N. Murthy. 2015. Sexual system of garcinia indica Choisy: Geographic variation in trioecy and sexual dimorphism in floral traits. Plant Syst. Evol. 301(3):1065–1071. doi: 10.1007/s00606-014-1120-y.
- Kandasamy, R., K. Puttaramaiah, S. Ramnath, and S. Venkataramegowda. 2017. Studies on Pollen Biology and Stigma Receptivity of Garcinia imberti Bourd. (Clusiaceae) - A Critically Endangered Tree of Western Ghats, Kerala (July 2017). The International Journal of Plant Reproductive Biology 9(2):109–114. https://ssrn.com/abstract=3861830 .
- Kandhasamy, Dr., Rajkumar and Puttaramaiah, Keshavanarayan and Ramnath, Shubharani, and Venkataramegowda, Sivaram, Studies on Pollen Biology and Stigma Receptivity of Garcinia imberti Bourd. (Clusiaceae) - A Critically Endangered Tree of Western Ghats, Kerala (July 2017). The International Journal of Plant Reproductive Biology 9(2) Jul., 2017, pp.109–114, Available at SSRN: https://ssrn.com/abstract=3861830
- Karnik AR (1978). Studies on flowering and fruiting in Kokum (Garcinia indica Choisy). [ M. Sc. Thesis, Konkan Agricultural University] Dapoli, Maharashtra, India, pp. 38–42. M. Sc. Thesis
- Leal, D.O., C.R. Benevides, R.C.P. Silva, L.D.R. Santiago-Fernandes, B. Sá-Haiad, and H.A. Lima. 2013. Garcinia brasiliensis: Insights into reproductive phenology and sexual system in a neotropical environment. Plant Syst. Evol. 299(8):1577–1585. doi: 10.1007/s00606-013-0833-7.
- Malik, S.K., R. Chaudhury, and Z. Abraham. 2005. Seed morphology and germination characteristics in three garcinia species. Seed Sci. Technol. 33(3):595–604. doi: 10.15258/sst.2005.33.3.07.
- Manikandan, G. 2016. Reproductive biology of garcinia imberti bourd and G travancorica bedd: Endemic and endangered tree species from Agasthyamalai biosphere reserve [The Gandhigram Rural Institute]. INFLIBNET Centre. http://hdl.handle.net/10603/186396
- Noor, N.M., W.M. Aizat, K. Hussin, and E.R. Rohani. 2016. Seed characteristics and germination properties of four garcinia (Clusiaceae) fruit species. Fruits 71(4):199–207. doi: 10.1051/fruits/2016008.
- Nuanjunkong, N., and U. Meesawat. 2008. Anatomical changes during pollen development of apomictic mangosteen (Garcinia Mangostana L .). Proceedings of the 7th IMT-GT UNINET and the 3rd International PSU-UNS Conferences on Bioscience, p. 21–23. https://www.researchgate.net/publication/273441708.
- Pangsuban, S., N. Bamroongrugsa, K. Kanchanapoom, and C. Nualsri. 2007. An evaluation of the sexual system of garcinia atroviridis L. (Clusiaceae), based on reproductive features. Songklanakarin J. Sci. Technol. 29(6):1457–1468. https://www.researchgate.net/publication/286334210.
- Richards, A. J. 2003. Apomixis in flowering plants: An overview. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358(1434), 1085–1093.
- Rohani, E.R., A.F. Norfadzilah, M.M. Clyde, and N.M. Noor. 2021. Insights into the mode of reproduction of garcinia prainiana. Fruits 76(1):22–29. doi: 10.17660/TH2021/76.1.3.
- Shameer, P.S., K.B. Rameshkumar, and N. Mohanan. 2016. Diversity of garcinia species in the Western Ghats: Phytochemical perspective. In: K.B. Rameshkumar (ed.). Diversity of garcinia species in the Western Ghats: Phytochemical perspective. Jawaharlal Nehru Tropical Botanic Garden and Research Institute, 1–8, Palode, Thiruvananthapuram 695 562, Kerala, India. doi: 10.25173/978-81-924674-5-0.
- Sutthinon, P., L. Samuels, and U. Meesawat. 2018. Male functionality in garcinia celebica l., a Candidate ancestor species of mangosteen (g. Mangostana L.). Botany. 96(10). doi: 10.1139/cjb-2018-0079.
- Sweeney, P.W. 2008. Phylogeny and floral diversity in the genus garcinia (Clusiaceae) and relatives. Int. J. Plant Sci. 169(9):1288–1303. doi: 10.1086/591990.
- Teo, C. K. (1990). In vitro culture of the mangosteen seed. Recent Advances in Horticultural Science in the Tropics 292, 81–86.