ABSTRACT
This study aimed to evaluate the combined effects of 1-methylcyclopropene (1-MCP) and modified atmosphere (MA) storage on volatile compound production and quality parameters of durian fruit cv. Monthong during storage. Whole durian fruit harvested at the commercial maturity (75–80%) was fumigated with 0–1,000 nL L−1 of 1-MCP for 12 hours. The fruits were then kept in MA chamber fitted with a silicone membrane window at 15°C up to 4 weeks. Volatile aroma compounds including 3 sulfur compounds, 1 ester, 5 alcohols, and 1 ketone were monitored during durian fruit storage. The production of diethyl disulfide, 3,5-dimethyl-1,2,4-trithiolane (2 isomers) and ethyl-2-methyl butanoate was strongly suppressed by 1-MCP/MA treatment. Husk and pulp color changes, pulp softening, respiration rate, production of ethylene, and fermentative metabolites were effectively retarded with 1-MCP/MA technique. 1-MCP treatment at 500 nL L−1 for 12 h under MA condition could extend the storage life of Monthong durian fruit up to 3 weeks without any negative effect. Therefore, this combined technique could be applied to maintain quality and control the ripening process of Monthong durian fruit.
Introduction
Durian (Durio zibethinus Murray), regarded as ‘the King of Fruit,’ is one of the most important fruit crops widely grown in Southeast Asia, including Thailand. Over 200 cultivars of durian in Thailand have been reported. Among them, ‘Monthong’ has been recognized as the most famous cultivar in both domestic and export market (Aschariyaphotha et al., Citation2021; Niponsak et al., Citation2015). Durian fruit is well known for its unique and exotic taste, flavor, and aroma. Ripe fruit has soft and creamy yellowish pulp (aril), sweet taste, as well as strong aroma. These characteristics may be desirable to some consumers, whereas it may be undesirable and offensive to others (Siriphanich, Citation2011). The characteristic flavor of durian is due to the presence of fat, sugar, amino acids, and volatile compounds such as esters and sulfur compounds, as well as alcohols (Aziz and Jalil, Citation2019; Pinsorn et al., Citation2018). The unique aroma can be described as a combination of sweet, fruity, sulfury, alcohol, nutty, and green aroma arising from volatile sulfur compounds, esters, alcohols, aldehydes, and ketones (Belgis et al., Citation2017; Niponsak et al., Citation2015; Voon et al., Citation2007). Sulfur compounds and esters were reported as two predominant groups of volatile compounds found in Malaysian durian cultivars (Chin et al., Citation2007; Voon et al., Citation2007; Yi et al., Citation2020). Diethyl disulfide, diethyl trisulfide, ethyl propionate, ethyl 2-methyl butanoate, and propyl 2-methyl butanoate were the five most abundant volatile compounds detected from fully ripe arils of seven Malaysian durian clones (Yi et al., Citation2020). With regard to Monthong durian, the most popular Thai durian cultivar, various sulfur compounds, including dimethyl sulfide, diethyl disulfide, diethyl trisulfide, dithiolane, 3,5-Dimethyl-1,2,4-trithiolane, and 3-methyl-thiozolidine, were detected from the fully ripe fruits (Aschariyaphotha et al., Citation2021; Laohakunjit et al., Citation2007). Moreover, 19 esters, having ethyl octanoate, ethyl hexanoate, methyl octanoate and ethyl-2-methyl butanoate as the compounds with high odor activity value (OAV), were found in the ripe arils of Monthong durian (Aschariyaphotha et al., Citation2021). Similarly to other climacteric fruits, synthesis of volatile compounds in durian pulp greatly enhanced during ripening. Ripening-induced changes in the volatile profiles during storage of minimally processed durian, the fleshly arils in the packaging that did not inhibit aerobic respiration, were reported (Niponsak et al., Citation2015). However, study on the changes in the predominant volatile compounds during the postharvest storage of whole durian fruit, especially with an application of delayed ripening technology, is still scarce.
The ethylene antagonistic 1-methylcyclopropene (1-MCP) has been extensively studied for its beneficial effects on fresh fruit and vegetables. This compound competitively binds to ethylene receptors, leading to inhibition of the ethylene action, thus altering subsequent ethylene-dependent processes, especially ripening (Watkins, Citation2006). The extent of 1-MCP effects depends on cultivars, developmental stage, time, and multiple applications. In case of Monthong durian, treatment with 1-MCP was shown to maintain pulp firmness, provided slight effect on soluble solids content but did not induce undesirable effects on husk and pulp color (Amornputti et al., Citation2014). 1-MCP may be used alone or coupled with other postharvest technology, including modified atmosphere (MA) storage. MA can be established by altering the gas composition (CO2 and O2) inside the air-restricted container or packaging. This is normally done by lowering O2 and elevating CO2 concentrations. After the gas composition reaching equilibrium, detrimental effects to the fruit should not occur (Kader, Citation2002). MA storage can be achieved by using a thin sheet of silicone membrane attached as a single window to a chamber. By this way, exchange of the air between interior and exterior part of the chamber can be obtained. This technique has been successfully applied to extend storage life of Cavendish banana (Chauhan et al., Citation2006; Steward et al., Citation2005) and Agrocybe chaxingu mushroom (Li et al., Citation2007). Considering the beneficial effects of both 1-MCP and MA on fresh fruit, a combination of both treatments may have synergistic effects to preserve quality and extend the storage life of durian fruit. However, research studies regarding effect of 1-MCP in combination with MA storage on the storage life of durian fruit are still limited. In this study, the coupled 1-MCP/MA technique was applied during prolonged storage of durian fruit. Due to its popularity, Monthong cultivar was selected as a representative of Thai durian in the present study. MA storage chamber fitted with silicone membrane window was used. Changes in characteristic volatile compounds as well as quality of durian fruit were determined over 4 weeks of storage period at 15°C.
Materials and Methods
Plant Material
Durian fruits (D. zibethinus Murray cv. Monthong) harvested at the commercial maturity (75–80% maturity) were obtained from a commercial orchard in Chanthaburi province. Fruits were transported to the Postharvest Laboratory, Faculty of Agriculture, Ubon Ratchathani University within the same day of harvest. To reduce variation among the samples, fruits with uniformity in size, shape, color, maturity level, and free of physical and biological damages were selected as experimental units in this study. Each durian fruit used in this study weighed approximately 3 kg and contained four arils. Single durian fruit was considered as an experimental unit (experimental replicate), and a total of 51 fruits were used for the experiment. Note that three fruits were used for collecting the data at the initial stage, and the remaining 48 fruits were used to monitor the changes in the fruit quality during storage.
Sample Preparation
Durian fruits were dipped into 200 μL L−1 NaOCl solution for 2–3 min for surface disinfection and air-dried. Fruits were then fumigated with 1-MCP (0.19% 1-MCP tablet, BioLene Co., Ltd., China) at 0, 100, 500, and 1,000 nL L−1 for 12 h at ambient temperature. Fumigation chamber was a tightly sealed chamber (450 L) equipped with a small fan for adequate air circulation inside the chamber. Each treatment consisted in 12 fruits for monitoring quality changes at four sampling dates. After treatment, individual fruit was separately kept in an air-tight acrylic chamber (15 L). Top lid of the chamber was fitted with a single wire-mesh window, where a thin sheet of silicone membrane (9 x 9 cm) was attached. This membrane sheet was made by casting a piece of thin fabric with a mixture of liquid silicone rubber and curing agent (Sil Model Co. Ltd., Thailand). The silicone membrane was cured at ambient temperature for 24–48 h before use. The thickness of the membrane sheet measured by a thickness gauge (model 7327, Mitutoyo, Japan) was approximately 170 μm. Other sides of the chamber were completely sealed. Therefore, gas exchange between interior and exterior part of the chamber was regulated through the silicone membrane, and subsequently generated appropriate MA inside the chamber.
The fruit samples were stored at 15°C up to 30 days. Relative humidity inside the MA chamber was approximately 91 ± 2%. Sampling was done weekly to evaluate husk (peel) and pulp (aril) color, pulp firmness, total soluble solids (TSS), titratable acidity (TA), whole fruit respiration and ethylene production, and biweekly for determination of fermentative metabolites and volatile compounds. In addition, changes of O2 and CO2 levels within each MA chamber were monitored every 2 days by an atmosphere analyzer (MAPtest 3050, Hitech Instruments, UK) throughout the experimental period. The experiment was done in triplicate.
Determination of Some Physical and Chemical Properties of Durian Samples
Husk and pulp color was measured with a chroma meter (CR 300, Konica Minolta, Japan). Color parameters were expressed in CIE L* a* b* system and calculated hue angle. Color reading of the peel was taken twice on the opposite side at the equatorial part of each fruit (two technical replicates per single fruit). Pulp color was read twice on the opposite side at the equatorial part of individual aril of each durian fruit (eight technical replicates per single fruit).
Pulp firmness of every aril inside durian fruit (four arils per fruit) was measured with a firmness tester (Effegi, Italy) fitted with a 5-mm cylindrical plunger. Two readings of each aril were recorded at the central part on the opposite side of the aril (eight technical replicates per single fruit).
For TSS and TA determination, mixed durian pulp sample from every aril of the fruit was blended with water (1:1 w/v) using a homogenizer (PT2100, Polytron, Switzerland). The slurry was then filtered through two layers of cheese cloth before subjected to TSS and TA determination. TSS (%) was measured with a hand-held refractometer (Atago, Japan), while TA (%) was measured by titration using 0.1 N NaOH and reported as malic acid equivalent. Dilution effect was taken into consideration for calculation of both values.
Determination of Respiration and Ethylene Production
Respiration and ethylene production of the whole fruit were determined by using a closed system procedure. Each durian fruit was placed in an air-tight container for 30 min. One milliliter of gas sample was then withdrawn with a 2.5 mL gas-tight syringe. The gas samples were analyzed with a gas chromatography (GC) (model 2410, Shimadzu, Japan) equipped with packed column (2.0 m × 3 mm Shincarbon, Porapak Q, Shimudzu, Japan) and flamed ionized detector. Helium gas, set at 50 mL min−1 constant flow rate, was used as carrier gas. The injection port temperature was set at 180°C, and column temperature was kept isothermally at 150°C. Standard gas containing 5% CO2 and 10 μL L−1 ethylene was used as an external standard. Retention time and area under GC peak were automatically obtained from GC Solution software (Shimadzu, Japan). Concentrations of CO2 and ethylene of the fruit sample were calculated from the area under each peak comparing to that of the gas standard.
Determination of Acetaldehyde, Ethanol and Ethyl Acetate
Acetaldehyde, ethanol and ethyl acetate, the fermentative metabolites developed under MA storage, were determined by a headspace-GC technique. Durian slurry was prepared with the same method used for TSS and TA determination. Five grams of the slurry were transferred into a 10-mL vial containing 1 g of NaCl (5:1). The vial was hermetically sealed immediately, and subsequently incubated in a water bath at 60°C for 30 min. One milliliter of the headspace gas sample was taken with a 2.5 mL gas-tight syringe. The gas sample was injected into GC for analysis, using similar apparatus and instrumental conditions as previously described. Identification of the fermentative compounds of the samples was done by comparing their retention times with the standards. Calibration curve of the peak area against the concentration (0–50 μL L−1) of each external standard was constructed for quantification of the compounds.
Determination of Volatile Aroma Compounds
Volatile aroma compounds were determined by solid-phase microextraction/gas chromatography–mass spectrometry (SPME/GC-MS) method. Durian slurry-NaCl mixture (5:1) was prepared with the same procedure as previously described. Ten grams of the mixture were weighed into a 20-mL vial. After spiking with 5 μL of 2-methyl-3-heptanone in methanol (0.18 ng g−1) as an internal standard, the sample vial was sealed with a PTFE/silicone septum secured by an aluminum cap. The vial was subsequently incubated in a silicone oil bath at 40°C for 20 min. Headspace volatile sample was then adsorbed for 30 min using a 1 cm 50/30 μm DVB/CarboxenTM/PDMS Stable FlexTM SPME fiber (Supelco, PA, USA). The SPME fiber was inserted into the splitless injection port of the GC–MS instrument for 5 min. The trapped volatile compounds were analyzed using the Agilent 6890 Plus GC/HP 5973 MSD, equipped with HP-FFAP capillary column (25 m × 0.32 mm i.d. × 0.50 μm film thickness; Hewlett-Packard, CA, USA). Ultra-high purity helium gas was used as carrier gas set at 1.6 mL min−1 constant flow rate. The injection port temperature was set at 220°C. Initial column temperature was 40°C upon injection, held at this temperature for 5 min, increased to 60°C at 15°C min−1 and held for 2 min, and finally increased to 180°C at 15°C min−1. Mass spectrometer conditions were listed as follows: MSD capillary direct-interface temperature was 250°C. Ionization energy was 70 eV. Mass range was 20–550 a.m.u. Electron multiplier (EM) voltage was obtained from autotune, and scan rate was 4.33 scan s−1.
Each volatile compound was identified on the basis of its Kovats retention index (RI) and mass spectrum using Wiley 275 L mass spectral database (Hewlett-Packard, CA, USA). Retention times of n-alkanes (C10 – C19) analyzed with HP-FFAP column were used to calculate Kovats retention indices. Peak integration was performed with HP Chemstation software (Hewlett-Packard, CA, USA). Semi-quantitative data of each compound were reported as area ratio of the compound and the internal standard.
Data Analysis
All experiments were performed in triplicate. The data were subjected to statistical analysis by using ANOVA procedure and mean separation and comparison were done by least significant difference (LSD) method at 95% level (P ≤ .05) using statistical program (SPSS statistical program v.15).
Results
Gas Composition inside the Modified Atmosphere Chamber
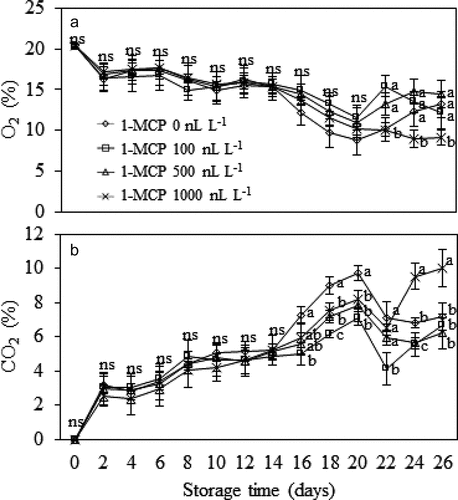
Changes in concentrations of O2 and CO2 inside the MA chamber are depicted in . These changes resulted from durian fruit respiration and restriction of air exchange through the silicone membrane window. It was found that levels of O2 inside the chamber steadily declined while CO2 levels increased gradually overtime during the first 2 weeks of storage. There was no significant effect of 1-MCP treatments on O2 and CO2 concentrations until the later stage of storage. The treatment with 1,000 nL L−1 1-MCP resulted in lowest O2 and highest CO2 levels at the end of storage period (24–26 days) (P ≤ .05) ().
Figure 1. Concentrations of O2 (A) and CO2 (B) inside the MA chamber containing Monthong durian fruit treated with different 1-MCP concentration and kept at 15°C up to 4 weeks. Vertical bars represent standard deviation of means from triplicate experiments. Mean values followed by different letter at similar storage time are significantly different (P ≤ .05); ns is not significantly different (P > .05).
Volatile Aroma Compounds of Durian Fruit
Identification of volatile aroma compounds in Monthong durian pulp is shown in . Odor characteristics of those volatile compounds are shown in . Four major groups of predominant volatile compounds, which were sulfur compound, ester, alcohol and ketone, were detected from the pulp samples. Production of sulfur compounds and ester, two predominant groups of volatile compounds in Monthong durian pulp, was greatly influenced by 1-MCP/MA technique. Diethyl disulfide was not detected in all treated fruits until the 4th week. In contrast, the 2 isomers of 3,5-dimethyl-1,2,4-trithiolane were detected at low concentration at the beginning of the experiment, and increased at longer storage time. Production of ethyl-2-methyl butanoate, the only ester presented in the 1-MCP/MA-treated samples, was also suppressed until the 4th week when accumulation of this volatile compound was prominent. At the 4th week, non-MCP-treated fruit had highest concentration of 3,5-dimethyl-1,2,4-trithiolane (both isomers) and ethyl-2-methyl butanoate, followed by the fruit treated with 100, 500 and 1,000 nL L−1 1-MCP, respectively (P ≤ .05).
Table 1. Area ratio of some volatile compounds in Monthong durian pulp treated with 1-MCP at different concentrations and kept in the MA chamber at 15°C up to 4 weeks.a.
Table 2. Odor characteristics of some volatile compounds isolated from Monthong durian pulp.
Relative amount of other volatile compounds presented in Monthong durian pulp were also affected by 1-MCP/MA treatment (). For the alcohols, accumulation of 3-methyl-1-butanol was relatively high in durian fruit from the beginning of storage. Its level in non-MCP-treated fruit was higher at the 4th week (P ≤ .05). However, relative amount of 3-methyl-1-butanol tended to fluctuate during storage. In case of 1-hexanol, its concentration in the non-MCP-treated fruit increased during storage. However, for the 1-MCP treated fruits, 1-hexanol gradually decreased as storage time increased. Note that other alcohols such as 1-octen-3-ol, 1-heptanol and 2-ethyl-1-hexanol were also detected at the 2nd and the 4th week at very low concentration. 1-MCP treatment did not affect the accumulation of these volatile compounds, as their levels remained relatively unchanged during storage. As for the volatile ketone, 3-hydroxy-2-butanone, relatively high level of this compound was observed in durian pulp at the beginning of the storage. This ketone dropped sharply at the 2nd week, and then increased at the 4th week. Fruit treated with 500 and 1,000 nL L−1 1-MCP showed greater level of 3-hydroxy-2-butanone at 4th week (P ≤ .05).
Physical and Chemical Properties of Durian Fruit
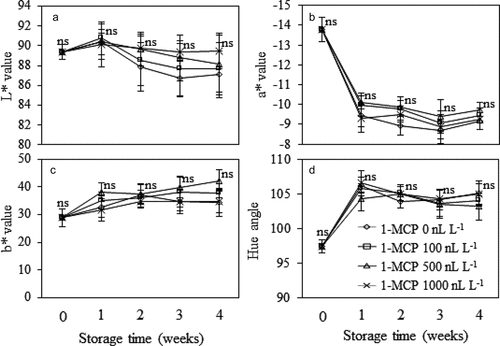
Husk color of Mongthong durian fruit was greatly affected by 1-MCP/MA treatment, especially toward the end of the storage period (). Treatment with 1,000 nL L−1 of 1-MCP effectively retarded husk color changes. At the 4th week, the fruit treated with 1,000 nL L−1 1-MCP had most intense green and darkest husk color, as shown by lowest L* and a* values and highest hue angle (P ≤ .05). Other 1-MCP treatments (100 and 500 nL L−1) also provided slightly better husk color of the fruit than non-MCP treated fruit. However, higher concentration of 1-MCP did not provide greater effects on retarding the changes in L*, a* and b* of the pulp samples (P > .05) ().
Figure 2. Color parameters, L* (A), a* (B), and hue angle (C) of the husk of Monthong durian fruit treated with different 1-MCP concentration and kept in MA chamber at 15°C up to 4 weeks. Vertical bars represent standard deviation of means from triplicate experiments. Mean values followed by different letter at similar storage time are significantly different (P ≤ .05); ns is not significantly different (P > .05).
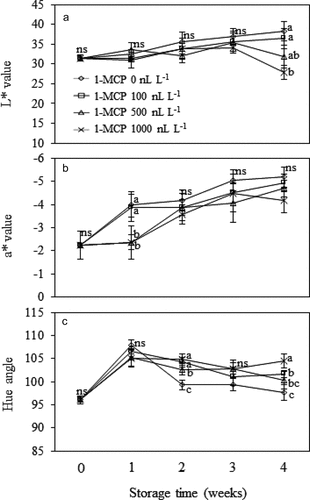
Figure 3. Color parameters, L* (A), a* (B), b* (C) and hue angle (D) of the pulp of Monthong durian fruit treated with different 1-MCP concentration and kept in MA chamber at 15°C up to 4 weeks. Vertical bars represent standard deviation of means from triplicate experiments. Mean values followed by ‘ns’ at similar storage time are not significantly different (P > .05).
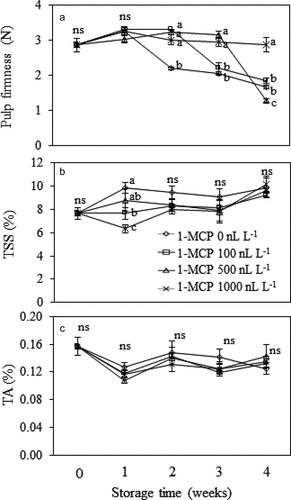
Changes in the firmness of Monthong durian pulp during storage was retarded with 1-MCP/MA technique ()). Significant drop of the pulp firmness was observed in non-MCP-treated fruit, and its firmness was lowest on the 2nd week of storage (P ≤ .05). For the fruit treated with 100 and 500 nL L−1 1-MCP, pulp firmness was maintained until the 2nd and the 3rd weeks of storage, respectively, and greatly decreased afterward. Treatment with 1,000 nL L−1 of 1-MCP resulted in highest retention of the pulp firmness throughout 4 weeks of storage (P ≤ .05). Regarding TSS and TA, although TSS of the durian fruit treated with higher 1-MCP concentrations seemed to be lower than the non-MCP treated fruit at the 2nd week, the values were not different at the end of storage (P > .05) ()). In addition, only slight changes in TA during storage were found, and the values were similar among the treatments (P > .05) ()).
Figure 4. Firmness (A), total soluble solid (TSS, B) and titratable acidity (TA, C) of Monthong durian fruit pulp treated with different 1-MCP concentration and kept in MA chamber at 15°C up to 4 weeks. Vertical bars represent standard deviation of means from triplicate experiments. Mean values followed by different letter at similar storage time are significantly different (P ≤ .05); ns is not significantly different (P > .05).
Respiration, Production of Ethylene, Acetaldehyde, Ethanol and Ethyl Acetate of Durian Fruit
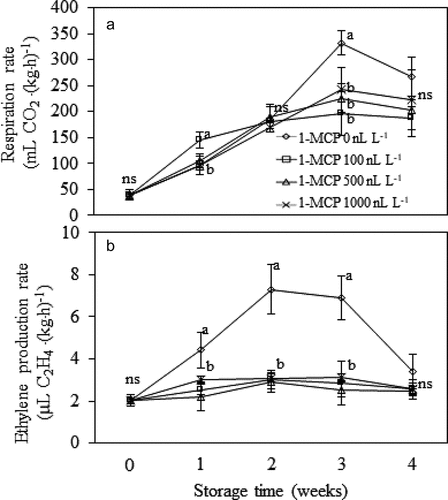
Rate of respiration and ethylene production was suppressed by 1-MCP/MA treatment ()). In comparison with non-MCP treated fruits, the treated samples exhibited lower respiration rate after the 3rd week (P ≤ .05) ()). Likewise, ethylene production of durian fruit was greatly inhibited by all 1-MCP treatments ()). The non-MCP treated fruit had higher ethylene production (P ≤ .05), showed climacteric rise after the 1st week and gradually decreased afterward ()).
Figure 5. Respiration rate (A) and ethylene production rate (B) of Monthong durian fruit treated with different 1-MCP concentration and kept in MA chamber at 15°C up to 4 weeks. Vertical bars represent standard deviation of means from triplicate experiments. Mean values followed by different letter at similar storage time are significantly different (P ≤ .05); ns is not significantly different (P > .05).
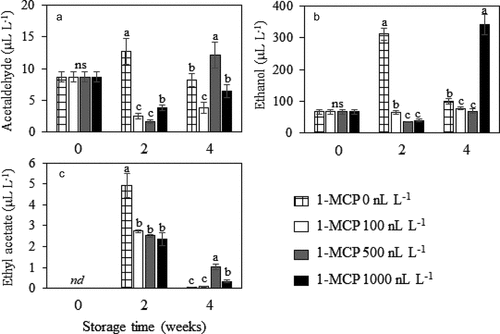
Production of acetaldehyde, ethanol, and ethyl acetate was influenced by 1-MCP treatment (). For non-MCP-treated fruits, the level of acetaldehyde, ethanol and ethyl acetate was several folds higher than that of the MCP-treated fruits at the 2nd week (P ≤ .05), then declined afterward. However, at the 4th week, durian fruit treated with 500 nL L−1 1-MCP produced highest amount of acetaldehyde ()) and ethyl acetate ()) while those treated with 1000 nL L−1 1-MCP produced highest amount of ethanol ()). Note that ethyl acetate was accumulated to its highest concentration at the 2nd week, and greatly decreased at the 4th week for all treatments ()).
Figure 6. Concentration of acetaldehyde (A) ethanol (B) and ethyl acetate (C) in Monthong durian fruit treated with different 1-MCP concentration and kept in MA chamber at 15°C up to 4 weeks. Vertical bars represent standard deviation of means from triplicate experiments. Mean values followed by different letter at similar storage time are significantly different (P ≤ .05); ns is not significantly different (P > .05); nd = not detected.
Discussion
MA storage at low temperature (15°C) combined with 1-MCP treatment could effectively maintain quality of Monthong durian fruit during long-term storage. In this study, O2 level was higher than 10% while CO2 level remained below 10% in all treatments at the 4th week. Under these conditions, off-odor/off-flavor development, which could occur during regular MA storage, was not expected (Kader, Citation2002). This was supported by the changes in fermentative metabolites, acetaldehyde, ethanol, and ethyl acetate, during Monthong durian storage. For the 1-MCP-treated fruit, concentration of those metabolites was either lower or similar to that of the non-MCP-treated fruit during the first two weeks, but the value greatly increased at the 4th week. Thus, it could be stated that 1-MCP/MA treated fruit could be kept at least 3 weeks without detrimental effects. During this period, synthesis of some desirable aroma compounds was also suppressed in the 1-MCP/MA treated fruit.
It was evidenced that some types of volatile aroma compounds found in Monthong durian fruit were much less than those previously reported (Aschariyaphotha et al., Citation2021; Laohakunjit et al., Citation2007). In those previous studies, the fruit at its fully ripening stage was used for the volatile compound identification. Initial husk dehiscence, an indicator of full ripening stage for durian fruit (Aschariyaphotha et al., Citation2021; Thongkum et al., Citation2018), was not detected in all durian samples during 4-week storage in this study. Lower degree of ripeness could result in less degree of volatile compound production in the fruit samples. However, for the predominant groups of the volatile compounds, sulfur compounds and ester reported in this study could be sufficient to indicate ripening level and aroma quality of the durian fruit. According to a previous study on the storage of minimally processed Monthong durian, diethyl disulfide and two isomers of 3,5-diethyl-1,2,4-trithiolane strongly correlated with some physicochemical qualities, including TSS, TA, and aril texture. Therefore, these volatile compounds were proposed as chemical ripeness markers of this durian cultivar (Niponsak et al., Citation2015). Genomic and transcriptomic analysis also revealed the potential association between volatile sulfur compounds and ripening process of durian fruit (Teh et al., Citation2017). Despite of their low OAVs, these sulfur compounds could provide sulfury/pungent odor in fully ripe Monthong durian (Aschariyaphotha et al., Citation2021; Laohakunjit et al., Citation2007; Niponsak et al., Citation2015). In contrast, based on its high OAV, ethyl-2-methyl butanoate was reported as one of the active volatile esters, providing fruity note in fully ripe arils of Monthong durian (Aschariyaphotha et al., Citation2021).
For the volatile alcohols and ketones, 1-hexanol, 2-ethyl-1-hexanol, and 3-hydroxy-2-butanone were previously detected in Monthong durian (Niponsak et al., Citation2015), whereas 3-methyl-1-butanol, 1-hexanol, 1-heptanol, and 3-hydroxy-2-butanone were identified in Malaysian and Indonesian durian (Belgis et al., Citation2017; Chin et al., Citation2007; Voon et al., Citation2007). According to our study, the sweet, herbaceous, earthy 1-octen-3-ol was firstly identified in unripe Monthong durian fruit. However, unlike the sulfur compounds and esters, significant correlation between these volatile alcohols/ketone and sensory description of fully ripe durian was not reported. Therefore, these volatile compounds might not largely influence the characteristic aroma of ripe durian flesh, and might not serve as effective ripeness markers.
The combined 1-MCP/MA treatments clearly retarded the production of predominant volatile compounds, diethyl disulfide, two isomers of 3,5-diethyl-1,2,4-trithiolane, and ethyl-2-methyl butanoate, indicating a delayed ripening of Monthong durian fruit. Biosynthesis of diethyl disulfide and ethyl-2-methyl butanoate was strongly suppressed by all 1-MCP/MA treatments during the first two weeks of storage. Moreover, dose-dependent suppression by 1-MCP on the production of both 3,5-diethyl-1,2,4-trithiolane isomers and ethyl-2-methyl butanoate was evidenced at the 4th week of MA storage. The combined 1-MCP/MA storage might directly affect the biosynthesis of volatile sulfur compounds via altering methionine γ-lyase activity, the key enzyme associated with the production of volatile sulfur compounds in durian during ripening (Teh et al., Citation2017), or provided an indirect effect via suppressing the ethylene production. In case of the volatile esters, 1-MCP/MA was shown to reduce respiration rate, which could result in reduced synthesis of acyl-CoA substrates required for volatile ester synthesis (Ortiz et al., Citation2010). This combined treatment might also interfere with the activity of alcohol o-acyltransferase, an important enzyme required for ester biosynthesis during ripening of many fruits (Wang et al., Citation2022). The 1-MCP and controlled atmosphere (CA) storage was shown to suppressed the biosynthesis of methyl esters, ethyl esters, and acetate esters in Tardibelle peach fruit during storage at 20°C for 7 days. This could result from the reduced biosynthesis of alcohols and acyl-CoA precursors, as well as the reduced activity of alcohol o-acyltransferase that catalyzed the esterification reaction (Ortiz et al., Citation2010).
Considering other quality parameters, treatment with 1-MCP could slightly suppress changes in husk color of durian fruit, especially at the later stage of MA storage. Although the effect of 1-MCP on TSS and TA was not clearly seen among the treatments, its effect on pulp firmness was prominent. Firmness of durian fruit pulp treated with 1,000 nL L−1 1-MCP remained high and relatively unchanged throughout the storage period. Those treated with 100 and 500 nL L−1 1-MCP could maintain their firmness for 2 and 3 weeks, respectively. Nevertheless, non-MCP-treated fruit kept in MA showed the pulp softening after 1 week of storage. The firmness results strongly indicated the synergistic effect of 1-MCP/MA treatment on retarding the ripening process of Monthong durian fruit.
Suppression effects of 1-MCP on both respiration and ethylene production have been reported in many fruits (Blankenship and Dole, Citation2003). In this study, 1-MCP at low concentration (100 nL L−1) in combination with MA storage was sufficient to suppress respiration rate and ethylene production. Ethylene production of durian fruit could show a high sensitive to 1-MCP treatment, thereby delaying ripening and preserving the quality of the fruit. 1-MCP could retard the ethylene production in durian fruit by suppressing aminocyclopropanecarboxylate oxidase activity (Amornputti et al., Citation2016). Lower respiration rate and ethylene production of 1-MCP/MA-treated fruit also affected the production of volatile aroma compounds, as previously discussed for the biosynthesis of volatile esters during Monthong durian storage. In addition, based on genomic and transcriptomic approaches, novel metabolic pathway representing the association between durian odor and fruit ripening was introduced. In that model, methionine was a precursor for the production of volatile sulfur compounds and ethylene via methionine γ-lyase and aminocyclopropane-1-carboxylic acid synthase, respectively (Teh et al., Citation2017). Lower concentration of ethylene, as induced by 1-MCP, could be partially related to the delayed production of diethyl disulfide and 3,5-diethyl-1,2,4-trithiolane during MA storage of Monthong durian fruit in this study.
Accumulation of fermentative metabolites, acetaldehyde, ethanol, and ethyl acetate inside the plant tissue was associated with CA and MA storage. The extent of accumulation depends on type and physiology of the tissue, CA/MA conditions and other environmental factors, especially temperature (Kader, Citation2002). In this study, non-MCP-treated fruit contained higher acetaldehyde, ethanol and ethyl acetate during storage. Thus, 1-MCP could reduce the accumulation of fermentative metabolites under MA storage of Monthong durian fruit. This could be related to the respiration rate reduction. Lower respiration rate induced by 1-MCP could result in reduced amount of pyruvate, a precursor for acetaldehyde, ethanol, and ethyl acetate production. This could eventually suppress the production of those fermentative metabolites, and reduced off-odor/off-flavor development in durian fruit during long-term MA and possibly CA storage.
Together, the results indicated the synergistic effects of 1-MCP/MA treatment on suppressing respiratory rate and ethylene production, leading to retardation of ripening-related processes, including production of volatile aroma compounds and fermentative metabolites, as well as quality characteristics of husk and pulp of Monthong durian fruit. Considering the changes in fermentative metabolites and pulp firmness, 1-MCP treatment at 500 nL L−1 for 12 h under MA storage at 15°C was sufficient to extend the storage life of Monthong durian fruit up to 3 weeks. During that period, overall quality of the durian fruit could be maintained, especially the pulp firmness, with limited production of both volatile aroma compounds and undesirable fermentative metabolites. Treatment with 1-MCP at higher concentration (i.e. 1,000 nL L−1) might cause undesirable effects on fruit ripening phenomenon. Maninang et al. (Citation2011) applied 50 μL L−1 1-MCP on Monthong durian fruits at 20°C for 6 h. The treated fruits were kept at 15°C (95% RH) for 16 days, then transferred to ambient condition until dehiscence (full table ripeness). Results showed that the production of volatile sulfur compounds, including diethyl disulfide and two isomers of 3,5-diethyl-1,2,4-trithiolane was delayed. Detectable amount of those compounds were found at the 3rd day after dehiscence. Although volatile esters were detected upon dehiscence, fewer types of volatile esters were found at lower concentration, in comparison with the untreated fruit. Amornputti et al. (Citation2014) treated Monthong durian fruits with 1-MCP at 500–2000 nL L−1 for 6 h or 12 h at 25°C, stored at 15°C, and subsequently transferred to 25°C with an application of ethephon to induce ripening. They reported that pulp of the fruit treated with 1000 nL L−1 or 2000 nL L−1 1-MCP did not soften properly. Based on those adverse effects and the results from the present study, 500 nL L−1 1-MCP combined with MA was the recommended treatment for storage life extension of Monthong durian fruit. Future study on sensory evaluation and consumer acceptance test of the fully ripe Monthong durian after prolonged storage under this recommended condition should be conducted. This can help verify the commercial application of 1-MCP/MA technique on Monthong durian fruit, especially for the export market.
Conclusions
Results from this study indicated that MA alone could not effectively extend the storage life and maintain optimum quality of Monthong durian fruit. A combined treatment of 1-MCP and MA storage at 15°C effectively retarded the biosynthesis of volatile aroma compounds and changes in other quality characteristics of Monthong durian fruit. 1-MCP/MA treatment greatly suppressed the production of some predominant volatile sulfur compounds and esters, as well as fermentative metabolites. The combined treatment also helped maintain husk color, firmness, TSS and TA of the durian pulp during storage. Those changes could result from reduced respiration rate and ethylene production, both effects induced by 1-MCP treatment and MA storage. In addition, 1-MCP treatment at 500 nL L−1 for 12 h under MA condition was sufficient to extend the storage life of Monthong durian fruit up to 3 weeks without any negative effect. Therefore, a combination of 1-MCP treatment and MA storage could be a promising technique to help control the ripening process of Monthong durian fruit. This technique may be adapted as the post-harvest handling of exported durian fruit especially if long period of distribution is required.
Acknowledgments
Financial support from the Postharvest Technology Innovation Center, Ministry of Higher Education, Science, Research and Innovation, Bangkok, Thailand, is gratefully acknowledged. Gratitude is also extended to the Faculty of Agriculture, Ubonratchathani University for their support.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Amornputti, S., S. Ketsa, and W.G. van Doorn. 2014. Effect of 1-Methylcyclopropene (1-MCP) on storage life of durian fruit. Postharvest Biol. Technol. 97:111–114. doi: 10.1016/j.postharvbio.2014.06.011.
- Amornputti, S., S. Ketsa, and W.G. van Doorn. 2016. 1-Methylcyclopropene (1-MCP) inhibits ethylene production of durian fruit which is correlated with a decrease in ACC oxidase activity in the peel. Postharvest Biol. Technol. 114:69–75. doi: 10.1016/j.postharvbio.2015.11.020.
- Aschariyaphotha, W., C. Wongs-Aree, K. Bodhipadma, and S. Noichinda. 2021. Fruit volatile fingerprints characterized among four commercial cultivars of Thai durian (Durio zibethinus). J Food Qual. 2021:1–12. Article ID 1383927 doi: 10.1155/2021/1383927.
- Aziz, N.A.A., and A.M.M. Jalil. 2019. Bioactive compounds, nutritional value, and potential health benefits of indigenous durian (Durio Zibethinus Murr.): A review. Foods. 8:1–18. doi: 10.3390/foods8030096.
- Bailly, S., V. Jerkovic, J. Marchand-Brynaert, and S. Collin. 2006. Aroma extraction dilution analysis of Sauternes wines. Key role of polyfunctional thiols. J. Agric. Food Chem. 54:7227–7234. doi: 10.1021/jf060814k.
- Belgis, M., C.H. Wijaya, A. Apriyantono, B. Kusbiantoro, and N.D. Yuliana. 2017. Volatiles and aroma characterization of several lai (Durio kutejensis)and durian (Durio zibethinus) cultivars grown in Indonesia. Sci. Hortic. 220:291–298. doi: 10.1016/j.scienta.2017.03.041.
- Blankenship, S.M., and J.M. Dole. 2003. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 28:1–15. doi: 10.1016/S0925-5214(02)00246-6.
- Burdock, G. A. 2010. Fenaroli's Handbook of Flavor Ingredients (6th ed.), 2159. New York: CRC Press.
- Chauhan, O.P., P.S. Raju, D.K. Dasgupta, and A.S. Bawa. 2006. Passive modified atmosphere packaging of banana (cv. Cavendish) using silicone membrane. Am J Food Technol. 1:129–138. doi: 10.3923/ajft.2006.129.138.
- Chin, S.T., S.A.H. Nazimah, S.Y. Quek, Y.B. Che Man, R. Abdul Rahman, and D. Mat Hashim. 2007. Analysis of volatile compounds from Malaysian durians (Durio zibethinus) using headspace SPME coupled to fast GC–MS. J Food Compos Anal. 20:31–44. doi: 10.1016/j.jfca.2006.04.011.
- Iraqi, R., C. Vermeulen, A. Benzekri, A. Bouseta, and S. Collin. 2005. Screening for key odorants in Moroccan green olives by gas chromatography-olfactometry/aroma extract dilution analysis. J. Agric. Food Chem. 53:1179–1184. doi: 10.1021/jf040349w.
- Kader, A.A. 2002. Modified atmospheres during transport and storage. In: A.A. Kader, (ed.). Postharvest Technology of Horticultural Crops. Publication 3311, University of California Agriculture and Natural Resources, 135–144, CA, USA.
- Laohakunjit, N., O. Kerdchoechuen, F.B. Mattal, J.L. Silva, and W.E. Holmes. 2007. Postharvest survey of volatile compounds in five tropical fruit using headspace-solid phase microextraction (HS-SPME). HortScience. 42:309–314. doi: 10.21273/HORTSCI.42.2.309.
- Li, T., M. Zhang, and S. Wang. 2007. Effects of modified atmosphere packaging with a silicone gum film as a window for gas exchange on Agrocybe chaxingu storage. Postharvest Biol. Technol. 43:343–350. doi: 10.1016/j.postharvbio.2006.10.006.
- Maninang, J.S., C. Wongs-Aree, S. Kanlayanarat, S. Sugaya, and H. Gamma. 2011. Influence of maturity and postharvest treatment on the volatile profile and physiological properties of the durian (Durio zibethinus Murray) fruit. Int Food Res J. 18:1067–1075.
- Niponsak, A., N. Laohakunjit, and O. Kerdchoechuen. 2015. Contribution to volatile fingerprinting and physico-chemical qualities of minimally processed durian cv. Monthong during storage: Identification of a novel chemical ripeness marker. Food Bioproc Technol 8:1229–1243.
- Ortiz, A., J. Graell, M.L. López, G. Echeverría, and I. Lara. 2010. Volatile ester-synthesising capacity in ‘Tardibelle’ peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chem. 123:698–704. doi: 10.1016/j.foodchem.2010.05.037.
- Peng, J.S.M. 2019. Volatile esters and sulfur compounds in durian & a suggested approach to enhancing economic value of durians. Malays. J. Sustain. Agric. 3:5–15. doi: 10.26480/mjsa.02.2019.05.15.
- Pinsorn, P., A. Oikawa, M. Watanbe, R. Sasaki, P. Ngamchuachit, R. Hoefgen, and S. Sirikantaramas. 2018. Metabolic variation in the pulps of two durian cultivars: Unravelling the metabolites that contribute to the flavor. Food Chem. 268:118–125. doi: 10.1016/j.foodchem.2018.06.066.
- Siriphanich, J. 2011. Durian (Durio ziberthinus Merr.). In: E.M. Yahia (ed.). Postharvest Biology and Technology of Tropical and Subtropical Fruit: Cocona to Mango. Woodhead Publishing 80–144 , Cambridge.
- Steward, O.J., G.S.V. Raghavan, D.G. Kerith, and Y. Gariépy. 2005. MA storage of Cavendish bananas using silicone membrane and diffusion channel systems. Postharvest Biol. Technol. 35:309–317. doi: 10.1016/j.postharvbio.2004.10.003.
- Teh, B.T., K. Lim, C.H. Yong, C.C.Y. Ng, S.R. Rao, V. Rajasegaran, W.K. Lim, C.K. Ong, K. Chan, V.K.Y. Cheng, et al. 2017. The draft genome of tropical fruit durian (Durio zibethinus). Nat. Genet. 49:1633–1641. doi: 10.1038/ng.3972.
- Thongkum, M., P.M. McAtee, R.J. Schaffer, A.C. Allan, and S. Ketsa. 2018. Characterization and differential expression of ethylene receptor genes during fruit development and dehiscence of durian (Durio zibethinus). Sci. Hortic. 240:623–630. doi: 10.1016/j.scienta.2018.06.052.
- Voon, Y.Y., N.S.A. Hamid, G. Rusul, A. Osman, and S.Y. Quek. 2007. Characterisation of Malaysian durian (Durio zibethinus Murr.) cultivars: Relationship of physicochemical and flavour properties with sensory properties. Food Chem. 103:1217–1227. doi: 10.1016/j.foodchem.2006.10.038.
- Wang, L., K. Chen, M. Zhang, M. Ye, and X. Qiao. 2022. Catalytic function, mechanism, and application of plant acyltransferases. Crit. Rev. Biotechnol. 42:125–144. doi: 10.1080/07388551.2021.1931015.
- Watkins, C.B. 2006. The use of 1-methylcyclopropene (1-MCP) on fruit and vegetables. Biotechnol. Adv. 24:389–409. doi: 10.1016/j.biotechadv.2006.01.005.
- Yi, T.X., A. Misran, C.K. Whye, L.D.J. Daim, P. Ding, and M.S.P. Dek. 2020. Postharvest quality indices of different durian clones at ripening stage and their volatile organic compounds. Sci. Hortic. 264:1–13. doi:10.1016/j.scienta.2019.109169. Article ID 109169.
