ABSTRACT
The strawberry (Fragaria ×ananassa Duch.) is a commercially important crop with high water requirements, making strategies that mitigate the influence of water deficits on plant growth necessary. This study aimed to evaluate the effects of shading on the vegetative growth of strawberry cv. Sweet Ann under a water deficit. The treatments consisted of the combination of two levels of shading (light intensity reduced by 47% vs. non-shaded plants) and two levels of water availability (water deficit vs. well-watered plants). The water deficit reduced the leaf water potential from −1.52 to −2.21 MPa, and diminished stomatal conductance, net photosynthetic rate (from 9.13 to 2.5 µmol m−2 s−1), maximum quantum efficiency of PSII photochemistry (from 0.79 to 0.67), and biomass accumulation, but increased the electrolyte leakage. The shading allowed the water-deficient plants to maintain water potential (−1.58 MPa) and photosystem II efficiency (0.79) and to increase water use efficiency (from 14.80 to 86.90 µmol CO2/mmol H2O), net photosynthetic rate (from 2.40 to 9.40 µmol m−2 s−1) and biomass of leaves, crowns, and roots, as compared to the non-shaded plants without a water limitation. These results suggest, for the first time in strawberry, that a reduction in incident light intensity attenuates the effects of stomatic and non-stomatic limitations caused by a water deficit during vegetative growth in strawberry.
Introduction
The strawberry (Fragaria ×ananassa Duch.) is a crop of high socio-economic value worldwide because of its organoleptic properties and contents of vitamins, minerals, and bioactive compounds such as antioxidants (Fierascu et al., Citation2020). Strawberry plants have high water requirements because of their superficial root system, large leaf area, and high water content in fruits (García-Tejero et al., Citation2018; Klamkowski and Treder, Citation2006) and are highly susceptible to water deficits during crop establishment (El-Farhan and Pritts, Citation1997; Grant et al., Citation2012), which impact plant growth, yield, and fruit quality (Liu et al., Citation2007).
In recent years, drought periods have increased in frequency and intensity worldwide (Leng and Hall, Citation2019; OECD, Citation2014). Additionally, in climate change scenarios, water availability is projected to decrease, and global water demand to increase (Mo et al., Citation2017; OECD, Citation2014), which will potentially cause unforeseen stress conditions in open field crops and even those grown under protected conditions.
Plants exhibit numerous physiological, morphological, biochemical, and molecular mechanisms to cope with water deficits (Farooq et al., Citation2009) with variable effects on plant growth. Stomatal closure, mediated by abscisic acid, is one of the first responses to low water availability (Martin-StPaul et al., Citation2017; Osakabe et al., Citation2014) since it limits leaf transpiration; however, it also reduces CO2 uptake and, therefore, diminishes photoassimilate production and plant growth (Galmés et al., Citation2013). In strawberry plants, reduced stomatal conductance, accompanied by significantly lower photosynthetic rates, has been reported as a typical response to drought (Martínez-Ferri et al., Citation2016).
As water stress increases, reactive oxygen species (ROS) are produced and may cause damage to proteins, nucleic acids, lipids, and chlorophylls (Munné-Bosch and Peñuelas, Citation2004). ROS accumulation causes lipid peroxidation in membranes and damage in plant photosystem II (PSII; Duan et al., Citation2013). In strawberry plants, water stress has resulted in excessive generation of ROS, which increase membrane permeability and reduce the maximum quantum efficiency of the PSII photochemistry (Fv/Fm; Gulen et al., Citation2018). Consequently, reduced photosynthetic rates slow down plant growth, change the pattern of assimilate distribution (Martínez-Ferri et al., Citation2016), and affect the number of leaves, leaf expansion rate, total plant biomass, and yield (Blanke and Cooke, Citation2004; Grant et al., Citation2012). Depending on the cultivar, reductions between 25–37% in plant growth have been reported (Klamkowski and Treder, Citation2008), and decreases of 33% in yield and 17% in fruit size have been recorded (El-Farhan and Pritts, Citation1997).
Most herbaceous species, including strawberry, do not have efficient mechanisms to mitigate the effects of water deficits (Martínez-Ferri et al., Citation2016). Therefore, it is paramount to evaluate strategies aimed to improve plant performance under water-limited conditions (Ghaderi et al., Citation2015). The use of shading nets in environments with high light intensity and low water availability might be an effective and inexpensive technique to alleviate water deficits in strawberry plants (Ahemd et al., Citation2016). Shading has been successfully applied in crop species including apricot (Prunus armeniaca) (Nicolás et al., Citation2005), kiwi (Actinidia deliciosa) (Montanaro et al., Citation2009), and olive (Olea europaea) (Sofo et al., Citation2009) to mitigate the effects of drought stress and has become a common practice to improve fruit quality and plant physiology in subtropical fruits (Mditshwa et al., Citation2019). Likewise, in strawberry plants, shading nets are increasingly used, even over plastic tunnels, to protect plants and fruits from excessive sunlight (Neri et al., Citation2012). The positive effect of shading on plant water status and physiology is attributed to reduced intensity of incident light and changes in microclimatic variables such as air temperature, relative humidity, and vapor pressure deficit (Mditshwa et al., Citation2019).
In strawberry plants, the effects of water stress and shading on physiological and yield responses have been independently studied (Casierra-Posada et al., Citation2012; Klamkowski et al., Citation2015; Tabatabaei et al., Citation2008). However, there are no reports on ajoint evaluation of the effects of water deficits and shading on strawberry physiology. The objective of this research was to evaluate the effect of shading on the vegetative growth of strawberry plants under a water deficit, as a potential strategy to mitigate water shortage effects on this crop.
Materials and Methods
Plant Material and Growth Conditions
This study was carried out under greenhouse conditions at the Faculty of Agricultural Sciences, Universidad Nacional de Colombia, Bogotá (Colombia) located at 2640 m a.s.l. Stolons of the neutral day strawberry (Fragaria ×ananassa) cv. Sweet Ann (Planasa, USA) with roots and crowns with a 12 and 2 cm length, respectively, were planted in 3 L plastic pots (one stolon per pot) containing a mixture of soil, blond peat, and burnt rice husk (16:1:1 w/w/w). According to the physicochemical analysis of the substrate (Table S1), and nutrient requirements of strawberries (Tagliavini et al., Citation2005), the plants were fertilized once a week with 320 ml of a nutrient solution composed of 0.42 g l−1 KNO3, 0.86 g l−1 Ca(NO3)2, 0.12 g l−1 NH4H2PO4, and micronutrients (Fe, B, Mn, Zn, and Cu). Fertilizer was distributed to avoid altering the water status of the plants according to the treatments. S and Mg were not included as part of the fertilization because of their initial contents in the substrate (Table S1). All plants were watered maintaining the substrate water capacity (SWC) from planting until the start of the treatments. To avoid early flower development, flower buds were removed from the plants during the experiment.
Air temperature and relative air humidity were recorded with a VP-3 sensor (Decagon Devices Inc., USA), and Photosynthetically Active Radiation (PAR) was registered with a PAR sensor (Vernier, USA) positioned 30 cm above the plants (Table S2); the sensors were coupled to a Data Logger Decagon EM50 (Decagon Devices Inc., USA).
Treatments
From planting, the experiment took 74 days and, when the plants had 3–4 expanded leaves (50 days after planting), the treatments were established in a 2 × 2 factorial design. Treatments consisted of the combination of two levels of shading and two levels of water availability: i) shading (S) with a 47% reduction in incident light (maximum and minimum diurnal PAR of 251.70 and 21.35 µmol m−2 s−1, respectively) or non-shaded (NS) conditions (maximum and minimum diurnal PAR of 476.07 and 40.28 µmol m−2 s−1, respectively) and ii) water deficit (WD) or well-watered (WW) plants. The combination of the factors resulted in four treatments: NS-WW, NS-WD, S-WW, and S-WD, and each treatment had four replicates consisting of one plant. The shading was provided with a commercially available polyethylene black net with 33% shading and a weight of 31 g .m−2, placed 1.5 m above the plants. Under shading, the average relative air humidity (RH) was 62%, and the average air temperature (T) was 21°C, while the non-shaded conditions had 59% RH and a T of 26°C.
Based on the substrate moisture, the WW plants were maintained at a capacity of 100% Volumetric Water Content (VWC). For the WD treatments, it has been reported that strawberry cultivars and wild strawberry (Fragaria virginiana) with a water deficit for 7–15 days was enough to obtain physiological responses (Gulen et al., Citation2018; O’Neill, Citation1983). However, it has been also shown that a progressive increase in the level of stress may be valuable for detecting additional changes in water relations and chlorophyll fluorescence (Liu et al., Citation2007; Razavi et al., Citation2008). Thus, the WD treatments had 50% VWC during the first 15 days and, subsequently, the water supply was suspended until reaching 25% VWC, which was maintained until the end of the experiment (74 days after planting), using water content measurements as described below.
Substrate Volumetric Water Content and Leaf Water Status
The substrate volumetric water content (SVWC) was measured daily between 9:00 and 10:00 am with a portable soil moisture sensor (ThetaProbe ML2x, Delta-T Devices, Cambridge, UK) at a 10 cm depth. The SVWC is the ratio between the volume of water present and the total volume of the sample and is expressed as m3 .m−3. The Leaf water potential (Ψlw) was measured in the first completely expanded leaf of the upper third of the plants at 5, 10, 17, and 24 days after treatment (DAT) at midday with a Scholander pressure chamber (PMS Instruments Model 615, CA, USA).
Leaf Gas Exchange, Photosynthetic Rate, and Water Use Efficiency
The gas exchange parameters were measured in the first completely expanded leaf of the upper third of the plants at 5, 10, 17, and 24 days after treatment (DAT). Net photosynthetic rate (Pn), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were registered using an LI-6200 photosynthesis measurement system (LI-COR Inc., Biosciences, USA) from 9:00 to 11:30 am with natural light (428.46–476.07 µmol m−2 s−1 for non-shaded plants and 224.30–251.77 µmol m−2 s−1 for shading treatment) and a CO2 concentration of 380 to 400 µL L−1. The intrinsic water use efficiency (WUEi) was calculated as the ratio of photosynthetic rate and stomatal conductance.
Relative Chlorophyll Content (SPAD)
The relative chlorophyll content (SPAD values) was determined using a portable chlorophyll SPAD-502 (Konica Minolta, Sakai, Osaka, Japan) making 10 measurements on the same leaves that were used to measure photosynthetic rate.
Chlorophyll a Fluorescence
The maximum quantum efficiency of PSII photochemistry (Fv/Fm) was measured in leaves that were dark-adapted for 30 min using a MINI-PAM modulated fluorometer (Walz®, GmbH Effeltrich, Germany); this measurement was performed on the same leaves used to measure the photosynthetic rate. The measurements were done at a steady state of 2,000 μmol m−2 s−1 and a saturating state of 5,000 μmol m−2 s−1 of actinic light for 0.80 s.
Membrane Permeability
Cell membrane permeability in leaves was evaluated with electrolyte leakage (EL) according to Gulen and Eris (Citation2004). Ten 5-mm-diameter leaf discs were placed in Falcon tubes with 10 ml of deionized water at 16°C. The electrical conductivity (EC) was determined with a conductometer (HI 9835 Hanna®, Spain). Electrolyte leakage values were expressed as a percentage in relation to the highest value using the equation EL = (EC1/EC2)*100, where EC1 = EC at 4 h, and EC2 = EC after heating for 15 min at 90°C.
Specific Leaf Area and Dry Mass Distribution
To determine the leaf area (LA) of each plant, the nondestructive estimation method was employed according to Grijalba et al. (Citation2015), who utilized the following equation in strawberry cv. Albion: y = 0.8316X1.9784, where y is the area of the leaflet (cm2), and X is the length (cm) of each leaflet measured from its base to the apex at the central rib. From the sum of individual leaflet areas, the total leaf area of each plant was obtained. Each plant was separated into roots, leaves, and crown at the end of the experiment (24 DAT), and the plant material was dried at 65°C until reaching a constant weight. Total dry weight and dry weights of the roots (RDW), shoots (SDW), crown (CDW), and leaves (LDW) were determined, where the shoot corresponded to crown plus leaves. The specific leaf area (SLA) was determined as the ratio of fresh-leaf area/dry mass.
Statistical Analysis
The experiment consisted of a full factorial experiment with two factors (Shading and water deficit), two levels per factor, and four treatments (combinations between the levels of each factor) as detailed above. A two-way ANOVA was carried out to determine the effects of the individual factors and treatments on the analyzed variables. The data are presented for factors and their combinations. Mean comparisons were done with a Tukey’s multiple range test (p < .05, 0.01, and 0.001). The statistical analysis was done with SAS v. 9.4 (Statistical Analysis System, USA).
Results and Discussion
The water deficit affected all physiological variables except Ci, SPAD values, SLA, RDW, and CDW, while the shading affected all variables except Ci and SLA (). Significant interactions between factors (water deficit and shading) were found for Ψlw,gs,Pn, WUEi, Fv/Fm, EL, SLA, RDW, SDW, CDW, LDW, and TDW and are described below.
Table 1. Analysis of variance (ANOVA) for physiological variables of leaf water potential (Ψlw), stomatal conductance (gs), intracellular CO2 concentration (Ci), photosynthetic rate (Pn), intrinsic water use efficiency (WUEi), SPAD values, maximum quantum efficiency of PSII photochemistry (Fv/Fm), electrolyte leakage percentage (EL), leaf area (LA), specific leaf area (SLA), shoot dry weight (SDW), root dry weight (RDW), crown dry weight (CDW), leaf dry weight (LDW), and total dry weight (TDW) of strawberry plants cv. Sweet Ann under shading and water deficit. Averages and significance are shown for each level and factor.
Substrate Volumetric Water Content and Leaf Water Status
Water shortage during 24 DAT negatively affected water status in the strawberry plants (). The VWC in the WW plants varied between 0.43 and 0.40 m3 m−3. For the WD plants, the VWC was 0.22–0.21 m3 m−3 (50% SVWC) until 15 DAT and decreased to 0.13–0.10 m3 m−3 (25% SVWC) at 20 DAT, maintaining these values up to the end of the experiment ().
Figure 1. Substrate volumetric water content (SVWC) during 0–30 DAT (a) and leaf water potential (Ψlw) at 24 DAT (b) of ‘Sweet Ann’ strawberry plants grown under non-shaded and shading conditions. DAT: days after treatment. Values are the means of four replicates, with error bars representing the standard error. Means denoted by the same letter do not significantly differ at p ≤ .01 according to the Tukey’s test.
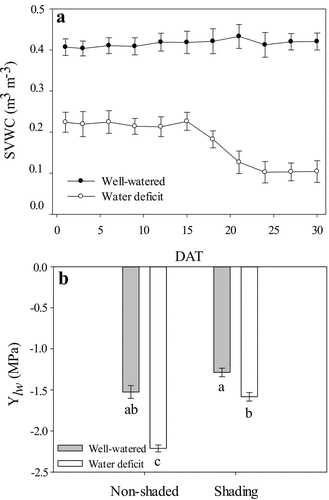
The individual factors (water deficit and shading) and the combination of these two factors had a significant effect on leaf water potential (Ψlw) in the strawberry plants (). In the NS-WD treatment, Ψlw was significantly lower (−2.21 MPa) than that observed in the NS-WW plants (−1.53 MPa; ). A decrease in Ψlw under water deficit has been reported as one of the main effects of this stress in strawberry (Grant et al., Citation2012, Citation2010; Klamkowski and Treder, Citation2008). The decrease in Ψlw could be an acclimatization response to cope with adverse conditions and an indirect measure of the severity of stress (Holmgren et al., Citation2012). Reduced Ψlw values might be a consequence of reduced osmotic potential, which is caused by the synthesis of compatible solutes facilitating water absorption during abiotic stress (Hasegawa et al., Citation2000). To counteract water deficits, strawberry plants employ osmotic adjustment, increase cell membrane stability, increase leaf thickness, and improve water use efficiency (Grant et al., Citation2012, Citation2010).
No significant differences in Ψlw were found between the S-WW and the S-WD plants, as compared to the NS-WW plants (). These data indicated that the shading had a positive effect on the water status of the water-stressed plants by avoiding a reduction in Ψlw despite the water limitation (). Awang and Atherton (Citation1994) and Ma et al. (Citation2015) reported for strawberry and other plants in the Rosaceae family that shading increased Ψlw in well-watered plants and those subjected to abiotic stress (water deficit or salinity), which, in turn, imparted greater tolerance to the adverse effects of stress.
Leaf Gas Exchange, Photosynthetic Rate and Water Use Efficiency
The shading had a significant effect on gs, which was significantly lower in the shaded plants (0.30 mol m−2 s−1) than in the non-shaded plants (0.36 mol m−2 s−1), independent of water availability (). For the water deficit factor, the gs was significantly reduced from 0.509 mol m−2 s−1 in the WW plants to 0.155 mol m−2 s−1 in the WD plants. As a result of the interaction of factors (), the gs was significantly lower in the NS-WD, S-WW, and S-WD treatments with respect to the NS-WW treatment (control), with the lowest values observed in the WD plants (0.17 and 0.14 mol m−2 s−1 in the NS and S treatments, respectively).
Figure 2. Stomatal conductance (gs) (a), net photosynthesis rate (Pn) (b) and intrinsic water-use efficiency (WUEi) (c) at 24 DAT of ‘Sweet Ann’ strawberry plants grown under non-shaded and shading conditions. DAT: days after treatment. Values are the means of four replicates, with error bars representing the standard error. Means denoted by the same letter do not significantly differ at p ≤ .01 according to the Tukey’s test.
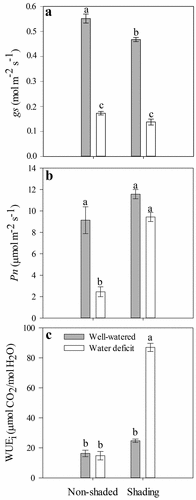
Reductions in gs in response to shading have been reported for various species, including the strawberry (Choi et al., Citation2016; Gerardin et al., Citation2018). In shaded tobacco plants, Gerardin et al. (Citation2018) attributed reduced gs values to signaling mediated by the intercellular CO2 (Ci) to optimize the concentration of CO2 in leaves. In this regard, Mott (Citation1988) indicated that, in the dark, the stomata of wheat did not respond to a free CO2 concentration, and their opening depended on Ci. Despite the reduction in gs caused by both the water deficit and shading, the Ci in strawberry leaves remained around 368 µmol mol−1, with no significant differences between the treatments (). Thus, the reduction in gs () can be attributed to the effect of light intensity on stomata functioning. This relationship between the light intensity and stomatal density was established in wild strawberry (Jurik et al., Citation1982) and herbaceous plants, such as spinach (Nguyen et al., Citation2019). On the other hand, the S plants had a significantly higher leaf area (304.89 ± 42.33 cm2) than the NS plants (227.60 ± 35.81 cm2; ), which might have affected stomatal density (Onwueme and Johnston, Citation2000; Qiu et al., Citation2018); low stomatal density in the S plants might have had a further effect on the gs.
The reduction in gs observed in the WD plants was a response that has previously been observed in many species as a strategy to prevent water loss through transpiration (Blanke and Cooke, Citation2004; Klamkowski and Treder, Citation2006). Furthermore, in strawberry plants grown under water deficit or waterlogging, a decrease in gs was observed without a reduction in Ci (Blanke and Cooke, Citation2004; Klamkowski and Treder, Citation2006) as reported here. This response can be attributed to the direct effects of water stress on non-stomatal components of photosynthesis (Yordanov et al., Citation2000).
Shading and water availability had a significant effect on Pn, which was significantly lower in the WD and NS plants (). In the interaction of factors, significantly lower Pn values were observed in the NS-WD plants (2.45 µmol m−2 s−1) with respect to the NS-WW plants (9.14 µmol m−2 s−1). The S-WD plants did not show significant differences in Pn with the S-WW (11.57 µmol m−2 s−1) and NS-WW (9.14 µmol m−2 s−1) treatments. This indicated that shading allowed the WD plants to maintain a photosynthetic activity equal to that of the WW plants () despite a significant reduction in gs in the WD-S treatment, as discussed above. Maintaining the photosynthetic activity will represent an advantage for water-stressed plants, with a potential impact on plant yield. The changes in microclimate might have a photoprotective effect on plants by decreasing PAR and air temperature and increasing relative humidity, thus, attenuating the negative effects of a water deficit (Ahemd et al., Citation2016; Li et al., Citation2014). This could explain the significantly higher Pn values observed in the S-WD plants, as compared to the NS-WD plants. In some strawberry varieties, a low light intensity produced a reduction in yield and fruit quality; however, 40% shading levels did not significantly reduce plant growth or net photosynthesis (Choi et al., Citation2014). Our results indicated that shading at 47% did not represent a limitation for plant growth. On the contrary, this level of shading could likely reduce the effects of water deficit on Pn during vegetative growth by avoiding photoinhibition and allowing plants to resist the negative effects of a water shortage. Future studies on the individual effect of different temperatures, RHs, and light intensities or wavelengths may be valuable to elucidate additional responses of strawberry plants under abiotic stress.
The intrinsic water use efficiency (WUEi) could be indicative of plant adaptation to water-limited environments (Medrano et al., Citation2015). The WUEi was significantly higher in the S-WD plants (86.90 µmol CO2/mol H2O) than in the other treatments (14.8–24.8 µmol CO2/mol H2O; ). In strawberry plants grown under a water deficit, an increase in WUEi was previously found as part of plant responses to contend with a water deficit (Grant et al., Citation2015).
The increase in WUEi in the S-WD plants was because Pn did not decrease in comparison to the NS-WD plants despite the low gs and similar Ci in both treatments (; ). In turn, this may have been due to the non-stomatal limitations in the NS-WD plants since high solar radiation under a water deficit can cause an imbalance between electron excitation and use in photosynthesis, resulting in photoinhibition (Choi et al., Citation2016). Moreover, a strong relationship exists between 13C isotope discrimination and WUEi (Kumar and Singh, Citation2009). Grant et al. (Citation2009) reported that strawberry plants with a low water deficit had a higher 13C/12C ratio associated with a higher WUEi and increased rate of photosynthesis. In our study, a lower gs, a statistically similar Ci, and higher Pn and WUEi in the S-WD plants may have been due to lower discrimination of 13C, which added to the effect of shading and might have maintained the photosynthetic activity of the S-WD plants. However, subsequent studies assessing discrimination of 13C under these conditions are necessary to verify this assumption.
Relative Chlorophyll Content (SPAD)
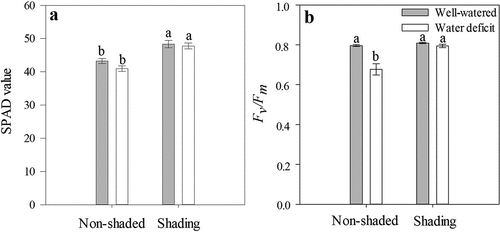
The S plants had higher SPAD values (47.80–48.30) than the NS plants (41.00–43.30), without influencing the water availability (; ). The increase in SPAD values might have contributed to maintaining a high Pn in the S-WD plants () because of a better absorbance of incident radiation (Zhang et al., Citation2015). Similar results were reported in various species subjected to shading, including strawberry (Luo et al., Citation2012; Mauro et al., Citation2011; Russo and Honermeier, Citation2017). At the same time, Roiloa and Retuerto (Citation2007) reported reduced levels of chlorophyll in Fragaria vesca leaves grown under low light intensity. In water-deficient plants, reduced contents of chlorophyll caused by ROS have been found (Roiloa and Retuerto, Citation2007); however, this effect was not observed in the present experiment.
Figure 3. SPAD values (a) and maximum quantum efficiency of PSII photochemistry (Fv/Fm) (b) at 24 DAT of ‘Sweet Ann’ strawberry plants grown under non-shaded and shading conditions. DAT: days after treatment. Values are the means of four replicates, with error bars representing the standard error. Means denoted by the same letter do not significantly differ at p ≤ .01 according to the Tukey’s test.
Chlorophyll a Fluorescence
In the NS-WD plants, Fv/Fm significantly decreased (0.68 ± 0.03) with respect to the NS-WW plants (0.80 ± 0.005), while no differences in Fv/Fm were observed between the S-WD and S-WW plants (0.79 ± 0.009 vs. 0.81 ± 0.003; ). Reduced Fv/Fm values in the NS-WD plants suggested the presence of non-stomatal limitations caused by damage in PSII (Baker, Citation2008), as reported in other plants subjected to severe or moderate water deficits (Grant et al., Citation2010). Fv/Fm values below 0.79 are commonly reported as indicators of PSII damage by different types of stress in strawberry plants (Choi et al., Citation2016; Na et al., Citation2014). In this regard, in strawberry plants exposed to abiotic stress such as high temperatures, Fv/Fm values over 0.72 indicated a lower PSII efficiency and were the main reason for reduced photosynthetic rates (Kadir and Sidhu, Citation2006). Similar results were previously found by Duan et al. (Citation2013) in shaded strawberry plants. In this way, shading might also prevent photoinhibition in water-deficient strawberry cv. Sweet Ann (Liu et al., Citation2007; Zeng et al., Citation2010). Additionally, Choi et al. (Citation2016) found a negative correlation between gs and Fv/Fm in strawberry leaves. In the present study, reduced gs values in shaded plants might be related to PSII efficiency, which allowed the S-WD plants to tolerate stress conditions and maintain their photosynthetic activity.
Membrane Permeability
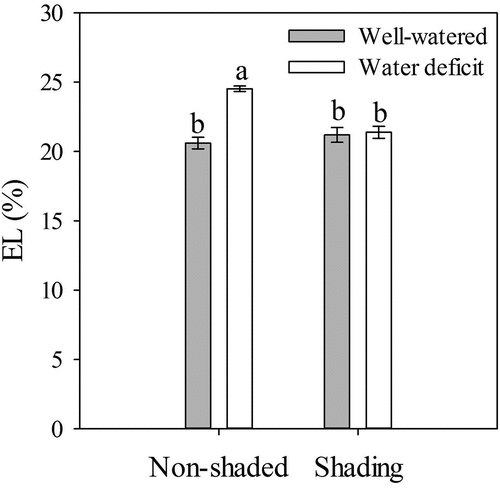
The EL was significantly affected by shading, water limitation, and the combination of both factors (). In the NS-WD plants, the EL (24.5%) was significantly higher than in the NS-WW treatment (20.6%), while no differences were observed in EL between the NS-WW, S-WD, and S-WW plants (). Higher EL values in the NS-WD treatment might indicate cell membrane damage resulting from high ROS activity that caused lipid peroxidation and loss of selective membrane permeability (McDonald and Archbold, Citation1998; Sun et al., Citation2015). These results suggest that a lower light intensity favored the membrane integrity by decreasing ROS production, attenuating the effects of water stress on strawberry plants.
Figure 4. Electrolyte leakage (EL) at 24 DAT of ‘Sweet Ann’ strawberry plants grown under non-shaded and shading conditions. DAT: days after treatment. Values are the means of four replicates, with error bars representing the standard error. Means denoted by the same letter do not significantly differ at p ≤ .01 according to the Tukey’s test.
Specific Leaf Area and Dry Mass Distribution
The water deficit and shading had a significant effect on the accumulation of dry mass in strawberry plants. For the SLA, a significant increase was observed only in the NS-WD treatment (218.5 cm2 g−1) when compared to the NS-WW plants (171.2 cm2 g−1), while the other treatments did not differ in SLA (). The root dry weight (RDW) was higher in the S-WD plants (2.3 g) with respect to the NS-WW and NS-WD plants (1.2–1.5 g), but without differences from the S-WW plants (). The shoot dry weight (SDW) was significantly lower in the NS-WD plants (1.70 g) than in the other treatments (2.70–3.10 g) that did not differ in this variable (). The S-WD plants accumulated the highest crown weight, CDW (1.40 g), without significant differences from the NS-WW plants, while the S-WW and NS-WD plants had the lowest CDW values (0.90–1.0 g), without differences from the NS-WW (1.1 g) (). The leaf dry weight (LDW) was significantly lower in the NS-WD (0.8 g) than the other treatments (1.40–1.70 g), which did not differ in this variable (). The highest total dry weights (TDW) were registered in the S-WW (4.80 g) and S-WD (4.90 g) when compared to the NS-WW treatment (4.20 g); on the contrary, the NS-WD plants had the lowest TDW (2.90 g; ).
Figure 5. Specific leaf area (SLA) (a), root dry weight (RDW) (b), shoot dry weight (SDW) (c), crown dry weight (CDW) (d), leaves dry weight (LDW) (e), and total dry weight (TDW) (f) at 24 DAT of ‘Sweet Ann’ strawberry plants grown under non-shaded and shading conditions. DAT: days after treatment. Values are the means of four replicates, with error bars representing the standard error. Means denoted by the same letter do not significantly differ at P ≤ .01 according to the Tukey’s test. For LDW differences are given at p ≤ .05.
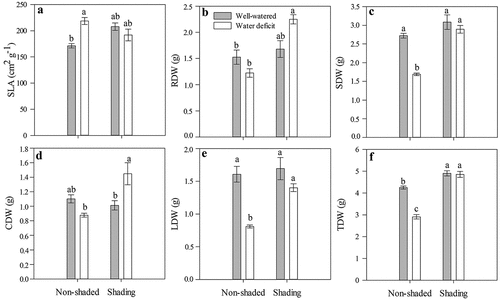
An increase in SLA in the NS-WD plants was apparently related to a reduced leaf thickness and was attributed to a reduction in leaf area and an imbalance between assimilation, distribution, and use of carbon during the water deficit (Álvarez et al., Citation2011). In fact, the WD plants had a significantly smaller leaf area (198.90 ± 15.11 cm2, p < .001) than the WW plants (338.29 ± 29.76 cm2; ) and a reduced Pn in the NS-WD treatment (). Leaf area reduction is a typical response to water deficits to reduce transpiration area and is accompanied by reduced carbon assimilation from stomatal and non-stomatal limitations. These responses restricted plant growth and were observed in the lower weight of the NS-WD plants ().
Another factor that could influence the accumulation of biomass under water stress is the increase in the use of carbohydrates for maintaining respiration in existing organs because of the drastic decrease in photosynthetic rates (Sánchez-Blanco et al., Citation2009). However, further studies would be necessary to assess the respiration of strawberry plants under a water deficit and shading.
In the S-WD plants, shading favored a better carbon balance by maintaining and increasing dry weight in all organs than in the NS-WD plants (). A higher accumulation of biomass was related to a greater Pn, WUEi, and Fv/Fm in the S-WD treatment than in the NS-WD plants, which indicated attenuation of the effects of the water deficit in the shaded plants and agreed with previous reports on the effects of low radiation under stress conditions (Montanaro et al., Citation2009).
The highest accumulation of photoassimilates in roots and crowns of the S-WD plants () may represent an advantage for plant establishment under water deficits or during recovery after a water deficit. In strawberry plants, favorable effects on the development of vegetative and reproductive buds have been reported depending on the amount and type of carbohydrates accumulated in crowns (Kirschbaum et al., Citation2010). Although yield was not evaluated, the increase of above-ground plant biomass in different organs accompanied by a greater water use efficiency has been implicated in increases in crop yields (Koester et al., Citation2016; Lobell et al., Citation2014) and can represent an advantage for strawberry production under stress conditions. Biomass production is a function of plant efficiency to intercept and convert sunlight into photoassimilates and, along with other parameters, is commonly used to evaluate abiotic stress tolerance in strawberry cultivars (Ferreira et al., Citation2019). As described above, shading alleviated the detrimental effects of water deficit on plant physiology. Therefore, we speculate that higher biomass accumulation will allow a higher translocation of photoassimilates from leaves to fruits later in the production cycle, maintaining yields. Nevertheless, evaluations considering fruit yield and quality are still needed.
Conclusions
The reduction of incident light by 47% generated a microclimate that mitigated the effect of stomatic and non-stomatic limitations in the strawberry plants cv. Sweet Ann under a water deficit. By reducing photosynthetically active radiation, shading induced a better water balance and a higher water use efficiency. Shading improved photosynthetic performance and increased biomass accumulation in the water-deficient plants. In the present study, the use of shading nets proved to be an effective and promising alternative to manage water stress during vegetative growth. Our results suggested that shading can be applied to alleviate the negative impacts of water shortages on plant physiology and maintain plant development. Future research assessing its potential and effects during strawberry production could lead to its use as a common practice in this crop.
Author’s Contribution
H.A. Cordoba-Novoa, M.M. Pérez-Trujillo, and B.E. Cruz designed and performed the experiments and analyzed the data. L.P. Moreno, S. Magnitskiy, and N. Flórez guided the research and provided technical support for designing and conducting the experiments. H.A. Cordoba-Novoa, S. Magnitskiy, and L.P. Moreno wrote the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (25 KB)Acknowledgments
The authors acknowledge the help of the Facultad de Ciencias Agrarias, Universidad Nacional de Colombia (Bogotá) for the partial financing of this project. The gratitude is extended to the Universidad Militar Nueva Granada (Cajicá) for the loan of the equipment employed in the research.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15538362.2022.2114056
References
- Ahemd, H.A., A.A. Al-Faraj, and A.M. Abdel-Ghany. 2016. Shading greenhouses to improve the microclimate, energy and water saving in hot regions: A review. Sci. Hortic. 201:36–45. doi:10.1016/j.scienta.2016.01.030.
- Álvarez, S., A. Navarro, E. Nicolás, and M.J. Sánchez-Blanco. 2011. Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in callistemon plants during drought conditions. Sci. Hortic. 129(2):306–312. doi: 10.1016/j.scienta.2011.03.031.
- Awang, Y.B., and J.G. Atherton. 1994. Salinity and shading effects on leaf water relations and ionic composition of strawberry plants grown on rockwool. J. Hortic. Sci. 69(2):377–383. doi: 10.1080/14620316.1994.11516467.
- Baker, N.R. 2008. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59(1):89–113. doi: 10.1146/annurev.arplant.59.032607.092759.
- Blanke, M.M., and D.T. Cooke. 2004. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 42(2):153–160. doi: 10.1023/B:GROW.0000017489.21970.d4.
- Casierra-Posada, F., J.E. Peña-Olmos, and C. Ulrichs. 2012. Basic growth analysis in strawberry plants (Fragaria sp.) exposed to different radiation environments. Agron. Colomb. 30(1):25–33.
- Choi, H.G., B.Y. Moon, and N.J. Kang. 2016. Correlation between strawberry (Fragaria ×ananassa Duch.) productivity and photosynthesis-related parameters under various growth conditions. Front. Plant Sci. 7:1607. doi:10.3389/fpls.2016.01607.
- Choi, H.G., B.Y. Moon, N.J. Kang, J.K. Kwon, K. Bekhzod, K.S. Park, and S.Y. Lee. 2014. Yield loss and quality degradation of strawberry fruits cultivated under the deficient insolation conditions by shading. Hortic. Environ. Biotechnol. 55(4):263–270. doi: 10.1007/s13580-014-0039-0.
- Duan, R., M. Huang, Z. Wang, Z. Zhang, and W. Fan. 2013. Effects of shading stress and light recovery on the photosynthesis characteristic and chlorophyll fluorescence characteristic of Fragaria ananassa Duch. cv. Toyonoka. Adv. J. Food Sci. Technol. 5(6):787–792. doi: 10.19026/ajfst.5.3164.
- El-Farhan, A., and M. Pritts. 1997. Water requirements and water stress in strawberry. Adv. in Strawberry Res. 16(1):5–12.
- Farooq, M., A. Wahid, N. Kobayashi, D. Fujita, and S.M.A. Basra. 2009. Plant drought stress: Effects, mechanisms, and management. Agron. Sustain. Dev. 29:185–212. doi:10.1051/agro:2008021.
- Ferreira, J.F.S., X. Liu, and D.L. Suarez. 2019. Fruit yield and survival of five commercial strawberry cultivars under field cultivation and salinity Stress. Sci. Hortic. 243:401–410. doi:10.1016/j.scienta.2018.07.016.
- Fierascu, R.C., G. Temocico, I. Fierascu, A. Ortan, and N.E. Babeanu. 2020. Fragaria genus: Chemical composition and biological activities. Molecules 25(3):498. doi: 10.3390/molecules25030498.
- Galmés, J., J.M. Ochogavía, J. Gago, E.J. Roldán, J. Cifre, and M.À. Conesa. 2013. Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: Anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ. 36(5):920–935. doi: 10.1111/pce.12022.
- García-Tejero, I.F., D. López-Borrallo, L. Miranda, J.J. Medina, J. Arriaga, J.L. Muriel-Fernández, and E. Martínez-Ferri. 2018. Estimating strawberry crop coefficients under plastic tunnels in Southern Spain by using drainage lysimeters. Sci. Hortic. 231:233–240. doi:10.1016/j.scienta.2017.12.020.
- Gerardin, T., C. Douthe, J. Flexas, and O. Brendel. 2018. Shade and drought growth conditions strongly impact dynamic responses of stomata to variations in irradiance in Nicotiana Tabacum. Environ. Exp. Bot. 153:188–197. bot.2018.05.019 doi:10.1016/j.envexp.
- Ghaderi, N., S. Normohammadi, and T. Javadi. 2015. Morpho-physiological responses of strawberry (Fragaria ×ananassa) to exogenous salicylic acid application under drought stress. J Agric. Sci. Techol. 17(1):167–178.
- Grant, O., C. James, P. Dodds, and N. Šurbanovski. 2009. Carbon isotope composition indicates improved photosynthesis water use efficiency of strawberry plant under deficit irrigation. Acta Hortic. 889:397–402. doi:10.17660/ActaHortic.2011.889.49.
- Grant, O.M., M.J. Davies, A.W. Johnson, and D.W. Simpson. 2012. Physiological and growth responses to water deficits in cultivated strawberry (Fragaria ×ananassa) and in one of its progenitors, Fragaria chiloensis. Environ. Exp. Bot. 83:23–32. doi:10.1016/j.envexpbot.2012.04.004.
- Grant, O.M., A.W. Johnson, M.J. Davies, C.M. James, and D.W. Simpson. 2010. Physiological and morphological diversity of cultivated strawberry (Fragaria ×ananassa) in response to water deficit. Environ. Exp. Bot. 68(3):264–272. doi: 10.1016/j.envexpbot.2010.01.008.
- Grant, O.M., Ł. Tronina, J.I. García-Plazaola, R. Esteban, J.S. Pereira, and M. Manuela Chaves. 2015. Resilience of a semi-deciduous shrub, Cistus salvifolius, to severe summer drought and heat stress. Funct. Plant Biol. 42(2):219–228. doi: 10.1071/FP14081.
- Grijalba, C.M., M.M. Pérez-Trujillo, D. Ruiz, and A.M. Ferrucho. 2015. Strawberry yields with high-tunnel and open-field cultivations and the relationship with vegetative and reproductive plant characteristics. Agron. Colomb. 33(2):147–154. doi: 10.15446/agron.colomb.v33n2.52000.
- Gulen, H., and A. Eris. 2004. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 166(3):739–744. doi: 10.1016/j.plantsci.2003.11.014.
- Gulen, H., M. Kesici, C. Cetinkaya, and S. Ergin. 2018. Proline and antioxidant enzyme activities in some strawberry cultivars under drought and recovery. Not. Bot. Horti. Agrobot. Cluj. Napoca. 46(2):570–578. doi: 10.15835/nbha46211077.
- Hasegawa, P.M., R.A. Bressan, J.-K. Zhu, and H. Bohnert. 2000. Plant cellular and molecular responses to high salinity. Plant Mol. Biol. 51(4):63–99.
- Holmgren, M., L. Gómez-Aparicio, J.L. Quero, and F. Valladares. 2012. Non-linear effects of drought under shade: Reconciling physiological and ecological models in plant communities. Oecologia 169(2):293–305. doi: 10.1007/s00442-011-2196-5.
- Jurik, T.W., J.F. Chabot, and B.F. Chabot. 1982. Effects of light and nutrients on leaf size, CO2 exchange, and anatomy in wild strawberry (Fragaria virginiana). Plant Physiol. 70(4):1044–1048. doi: 10.1104/pp.70.4.1044.
- Kadir, S., and G. Sidhu. 2006. Strawberry (Fragaria ×ananassa Duch.) growth and productivity as affected by temperature. Hort. Sci. 41(6):1423–1430.
- Kirschbaum, D.S., K.D. Larson, S.A. Weinbaum, and T.M. Dejong. 2010. Relationships of carbohydrate and nitrogen content with strawberry transplant vigor and fruiting pattern in annual production systems. Amer. J. Plant Sci. Biotechol. 4( special issue):98–103.
- Klamkowski, K., and W. Treder. 2006. Morphological and physiological responses of strawberry plants to water stress. Agric. Conspec. Sci. 71(4):159–165.
- Klamkowski, K., and W. Treder. 2008. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. J. Fruit Ornam. Plant Res. 16:179–188.
- Klamkowski, K., W. Treder, and K. Wójcik. 2015. Effects of long-term water stress on leaf gas exchange, growth and yield of three strawberry cultivars. Acta Sci. Pol. Hortorum Cultus. 14(6):55–65.
- Koester, R.P., B.M. Nohl, B.W. Diers, and E.A. Ainsworth. 2016. Has photosynthetic capacity increased with 80 years of soybean breeding? An examination of historical soybean cultivars. Plant Cell and Environ. 39(5):1058–1067. doi: 10.1111/pce.12675.
- Kumar, S., and B. Singh. 2009. Effect of water stress on carbon isotope discrimination and rubisco activity in bread and durum wheat genotypes. Physiol. Mol. Biol. Plants 15(3):281–286. doi: 10.1007/s12298-009-0032-8.
- Leng, G., and J. Hall. 2019. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 654:811–821. doi:10.1016/j.scitotenv.2018.10.434.
- Li, T., L.N. Liu, C.D. Jiang, Y.J. Liu, and L. Shi. 2014. Effects of mutual shading on the regulation of photosynthesis in field-grown sorghum. J. Photochem. Photobiol. B 137:31–38. doi:10.1016/j.jphotobiol.2014.04.022.
- Liu, F., S. Savić, C.R. Jensen, A. Shahnazari, S.E. Jacobsen, R. Stikić, and M.N. Andersen. 2007. Water relations and yield of lysimeter-grown strawberries under limited irrigation. Sci. Hortic. 111(2):128–132. doi: 10.1016/j.scienta.2006.10.006.
- Lobell, D.B., M.J. Roberts, W. Schlenker, N. Braun, B.B. Little, R.M. Rejesus, and G.L. Hammer. 2014. Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Sci. 344(6183):516–519. doi: 10.1126/science.1251423.
- Luo, H., L. Feng, M. Li, and L. Zhang. 2012. Effects of shading on the chlorophyll fluorescence parameters of Fragaria vesca cv. ‘Selva’ and ‘Midlight. J. Hebei Agric. Univ. 35(5):25–28.
- Ma, P., T.H. Bai, X.Q. Wang, and F.W. Ma. 2015. Effects of light intensity on photosynthesis and photoprotective mechanisms in apple under progressive drought. J. Integr. Agric. 14(9):1755–1766. doi: 10.1016/S2095-3119(15)61148-0.
- Martínez-Ferri, E., C. Soria, M.T. Ariza, J.J. Medina, L. Miranda, P. Domíguez, and J.L. Muriel. 2016. Water relations, growth and physiological response of seven strawberry cultivars (Fragaria ×ananassa Duch.) to different water availability. Agric. Water Manage. 164:73–82. doi:10.1016/j.agwat.2015.08.014.
- Martin-StPaul, N., S. Delzon, and H. Cochard. 2017. Plant resistance to drought depends on timely stomatal closure. Ecol. Letters 20(11):1437–1447. doi: 10.1111/ele.12851.
- Mauro, R.P., A. Occhipinti, A.M.G. Longo, and G. Mauromicale. 2011. Effects of shading on chlorophyll content, chlorophyll fluorescence and photosynthesis of subterranean clover. J. Agron. Crop. Sci. 197(1):57–66. doi: 10.1111/j.1439-037X.2010.00436.x.
- McDonald, S., and D. Archbold. 1998. Membrane competence among and within Fragaria species varies in response to dehydration stress. J. Am. Soc. Hortic. Sci. 123(5):808–813. doi: 10.21273/JASHS.123.5.808.
- Mditshwa, A., L. Magwaza, and S. Tesfay. 2019. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci. Hortic. 256(1):108556. doi: 10.1016/j.scienta.2019.108556.
- Medrano, H., M. Tomás, S. Martorell, J. Flexas, E. Hernández, J. Rosselló, A. Pou, J. Escalona, and J. Bota. 2015. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 3(3):220–228. doi: 10.1016/j.cj.2015.04.002.
- Mo, X., S. Hu, Z. Lin, S. Liu, and J. Xia. 2017. Impacts of climate change on agricultural water resources and adaptation on the north China plain. Adv. Clim. Chang. 8(2):93–98. doi: 10.1016/j.accre.2017.05.007.
- Montanaro, G., B. Dichio, and C. Xiloyannis. 2009. Shade mitigates photoinhibition and enhances water use efficiency in kiwifruit under drought. Photosynthetica 47(3):363–371. doi: 10.1007/s11099-009-0057-9.
- Mott, K.A. 1988. Do stomata respond to co2 concentrations other than intercellular? Plant Physiol. 86(1):200–203. doi: 10.1104/pp.86.1.200.
- Munné-Bosch, S., and J. Peñuelas. 2004. Drought-induced oxidative stress in strawberry tree (Arbutus unedo l.) growing in Mediterranean field conditions. Plant Sci. 166(4):1105–1110. doi: 10.1016/j.plantsci.2003.12.034.
- Na, Y., H.J. Jeong, S. Lee, H.G. Choi, S. Kim, and I.R. Rho. 2014. Wild and cultivated strawberry species chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 55(4):280–286. doi: 10.1007/s13580-014-0006-9.
- Neri, D., G. Baruzzi, F. Massetani, and W. Faedi. 2012. Strawberry production in forced and protected culture in Europe as a response to climate change. Can. J. Plant Sci. 92(6):1021–1036. doi: 10.4141/CJPS2011-276.
- Nguyen, T.P.D., T.T.H. Tran, and Q.T. Nguyen. 2019. Effects of light intensity on the growth, photosynthesis and leaf microstructure of hydroponic cultivated spinach (Spinacia oleracea l.) under a combination of red and blue LEDs in house. Int. J. Agric. Technol. 15(1):75–90.
- Nicolás, E., A. Torrecillas, J. Dell Amico, and J.J. Alarcón. 2005. Sap flow, gas exchange, and hydraulic conductance of young apricot trees growing under a shading net and different water supplies. J. Plant Physiol. 162(4):439–447. doi: 10.1016/j.jplph.2004.05.014.
- O’Neill, S.D. 1983. Role of osmotic potential gradients during water stress and leaf senescence in Fragaria virginiana. Plant Physiol. 72(4):931–937. doi: 10.1104/pp.72.4.931.
- OECD. 2014. Climate change, water and agriculture: Towards resilient systems. OECD Studies on Water. OECD Publishing, doi:10.1787/9789264209138-en.
- Onwueme, I.C., and M. Johnston. 2000. Influence of shade on stomatal density, leaf size and other leaf characteristics in the major tropical root crops, tannia, sweet potato, yam, cassava and taro. Exp. Agric. 36(4):509–516. doi: 10.1017/S0014479700001071.
- Osakabe, Y., K. Osakabe, K. Shinozaki, and L.-S.P. Tran. 2014. Response of plants to water. Front. Plant Sci. 5:86. doi:10.3389/fpls.2014.00086/abstract.
- Qiu, T., Y. Wu, Z. Shen, Y. Wu, D. Lu, and J. He. 2018. Effects of shading on leaf physiology and morphology in the “Yinhong” grape plants. Rev. Bras. Frutic. 40(5):1–10. doi: 10.1590/0100-29452018037.
- Razavi, F., B. Pollet, K. Steppe, and M.C. Van Labeke. 2008. Chlorophyll fluorescence as a tool for evaluation of drought stress in strawberry. Photosynthetica 46(4):631–633. doi: 10.1007/s11099-008-0108-7.
- Roiloa, S.R., and R. Rutuerto. 2007. Responses of the clonal Fragaria vesca to microtopographic heterogeneity under different water and light conditions. Environ. Exp. Bot. 61:1–9. doi:10.1016/j.envexpbot.2007.02.006.
- Russo, M., and B. Honermeier. 2017. Effect of shading on leaf yield, plant parameters, and essential oil content of lemon balm (Melissa officinalis l.). J. Appl. Res. Med. Aromat. Plants 7:27–34. doi:10.1016/j.jarmap.2017.04.003.
- Sánchez-Blanco, M.J., S. Álvarez, A. Navarro, and S. Bañón. 2009. Changes in leaf water relations, gas exchange, growth and flowering quality in potted geranium plants irrigated with different water regimes. J. Plant Physiol. 166(5):467–476. doi: 10.1016/j.jplph.2008.06.015.
- Sofo, A., B. Dichio, G. Montanaro, and C. Xiloyannis. 2009. Shade effect on photosynthesis and photoinhibition in olive during drought and rewatering. Agric. Water Manage. 96(8):1201–1206. doi: 10.1016/j.agwat.2009.03.004.
- Sun, C.H., X.H. Li, Y.L. Hu, P. Zhao, T. Xu, J. Sun, and X.L. Gao. 2015. Proline, sugars, and antioxidant enzymes respond to drought stress in the leaves of strawberry plants. Korean J. Hortic. Sci. Technol. 33(5):625–632. doi: 10.7235/hort.2015.15054.
- Tabatabaei, S.J., M. Yusefi, and J. Hajiloo. 2008. Effects of shading and no3:nh4 ratio on the yield, quality and N metabolism in strawberry. Sci. Hortic. 116(3):264–272. doi: 10.1016/j.scienta.2007.12.008.
- Tagliavini, M., E. Baldi, P. Lucchi, M. Antonelli, G. Sorrenti, G. Baruzzi, and W. Faedi. 2005. Dynamics of nutrients uptake by strawberry plants (Fragaria ×ananassa Dutch.) grown in soil and soilless culture. Europ. J. Agron. 23:5–25. doi:10.1016/j.eja.2004.09.002.
- Yordanov, I.T., V. Velikova, and T. Tsonev. 2000. Plant responses to drought and stress. Photosynthetica 38(1):171–186. doi: 10.1023/A:1007201411474.
- Zeng, X., X. Feng, F. Xiang, Z. Song, J. Wu, R. Wu, and Y. Gu. 2010. Effects of shading on photosynthesis of strawberry. J. Hebei Agric. Univ. 49(11):2811–2814. doi: 10.14088/j.cnki.0439-8114.2010.11.054.
- Zhang, Y.J., F. Yan, H. Gao, Y.Z. Xu, Y.Y. Guo, E.J. Wang, Y.H. Li, and Z.K. Xie. 2015. Chlorophyll content, leaf gas exchange and growth of oriental lily as affected by shading. Rus. J. Plant Physiol. 62(3):334–339. doi: 10.1134/S1021443715030206.
