ABSTRACT
The present study focused on the examination of the seed and pollen morphology of nine native Chinese species of the genus Sorbus L. using Light Microscope (LM) and Scanning Electron Microscope (SEM). The crucial features investigated for seeds include color, shape, epidermal cell size, and seeds coat ornamentation. On the other hand, the pollen grains are analyzed based on quantitative traits as well as the following quantitative traits: polar and equatorial view, shape, apertures and exine ornamentation. Pollens of the studied taxa were monad and trizonocolporate. The most important pollen features include exine ornamentation based on traits of striae and grooves (length and width) and perforations (number and diameter). The variability of the observed morphological features for both seeds and pollen provide important characteristics to differentiate the studied Sorbus species.
Introduction
The genus Sorbus L. belongs to the subfamily Maloideae, family Rosaceae, which comprises approximately 250 species (Phipps et al., Citation1990). Sorbus is widely distributed throughout the temperate regions of the Northern Hemisphere, with over 90 species found in China alone (Phipps et al., Citation1990). The Maloideae represent a significant cluster of arboreal taxa comprising nearly 1000 species and 20–30 genera, depending on the specific circumscription employed (Phipps et al., Citation1990). Pome fruits are the most important edible temperate fruit species in this subfamily, with Sorbus species being valued for their relatively small, but vitamin-rich fruits that are used for juices, jams, and jellies (Sottile et al., Citation2017; Yü and Lu, Citation1974). Some Sorbus species, such as S. aucuparia L. and S. domestica L., are commonly cultivated fruit crops worldwide (Sarv et al., Citation2021; Sottile et al., Citation2017). In addition, certain species, including S. aucuparia (Bozhuyuk, Citation2021), S. domestica (Sottile et al., Citation2017), S. rufopilosa Schneid. (Oh et al., Citation2016), and S. tianschanica Rupr. (Wang et al., Citation2016), have been traditionally used for their medicinal properties, such as diuretic, laxative, anti-inflammatory, and vasoprotective effects, as well as for the treatment of various gastrointestinal and respiratory tract-related disorders. Sorbus species are also valued as ornamental plants due to their brightly colored fruits, which range in color from crimson and scarlet to orange, pink, yellow, and pure white (Lu and Spongberg, Citation2003; McAllister, Citation2005).
Species of the genus Sorbus are commonly categorized into six subgenera or sections, namely Aria, Chamaemespilus, Micromeles, Torminalis, Sorbus, and Cormus, according to Phipps et al. (Citation1990). However, the genus Sorbus exhibits a high degree of morphological variability, and recent molecular evidence has suggested that the genus in its broad sense is polyphyletic and should be split into multiple genera, as proposed by Campbell et al. (Citation2007), Potter et al. (Citation2007), and Lo and Donoghue (Citation2012). Taxonomically, Sorbus represents one of the most challenging genera in the family Rosaceae, mainly due to its complexity resulting from polyploidy or genome doubling, often accompanied by gametophytic apomixis and hybridization, as reported by Nelson-Jones et al. (Citation2002) and Campbell et al. (Citation2007).
Seeds and pollen morphology have been widely recognized as important characteristics for taxonomic identification in the family Rosaceae and other Angiosperm families (Barser et al., Citation2021; Eide, Citation1981; Faegri and Iversen, Citation1992; Moore et al., Citation1991; Reitsma, Citation1966; Teppner, Citation1965; Ullah et al., Citation2022). In Sorbus species, seeds form and seeds coat structure have been shown to be useful characteristics for identification (Aldasoro et al., Citation1998, Citation2004; Bednorz et al., Citation2006; Bednorz, Citation2007; Dowidar et al., Citation2003). In fact, the importance of seeds coat exine sculpture in the taxonomy of Sorbus has been confirmed by Maciejewska-Rutkowska and Bednorz (Citation2004). Palynomorphological characteristics are also considered to be important for taxonomic classification and delimitation within the family Rosaceae, according to many palynologists (Eide, Citation1981; Hebda and Chinnappa, Citation1990; Song et al., Citation2016; Ueda and Tomita, Citation1989; Ullah et al., Citation2022; Zhou et al., Citation2000). Exine sculpture is particularly important, with the number and width of striae and grooves, as well as the number and size of perforations being the most significant traits (Bednorz and Fujiki, Citation2003; Bednorz et al., Citation2005; Lechowicz et al., Citation2021; Wrońska-Pilarek et al., Citation2022; Yang et al., Citation2019). Additionally, other important characters for identification within the family Rosaceae include the length of polar axis (P), pollen shape (P/E ratio), endoaperture, and operculum structure (Eide, Citation1981; Hebda and Chinnappa, Citation1990; Chung et al., Citation2010; Pathak et al., Citation2019; Wrońska-Pilarek et al., Citation2022). However, only a limited number of studies have been conducted on the pollen morphology of Sorbus species (Bednorz and Fujiki, Citation2003; Bednorz et al., Citation2005; Eide, Citation1981; Hebda and Chinnappa, Citation1990; Yang et al., Citation2019).
The purpose of this study is to investigate the pollen morphology and seeds coat micromorphology of nine native Chinese Sorbus species, in order to provide insight into the intrageneric relationships of the genus. Pollen and seeds morphology were analyzed using light and scanning electron microscopy. This research aims to contribute to a better understanding of the taxonomic relationships within the Sorbus genus, which may ultimately aid in the identification and conservation of these important plant species.
Material and Methods
Plant Collection and Sampling Sites
The specimens were collected from different localities in China or collected from herbarium species preserved at Nanjing Forestry University Herbarium (NF). A list of taxa and full voucher data is provided in . A total of 258 seed samples were studied in detail, and 270 pollen samples were examined. In this paper, the taxonomic classification of the studied taxa from the genus Sorbus was adopted after Phipps et al. (Citation1990).
Table 1. Sorbus taxa collected from China.
Seeds Morphological Characters
To observe seed macro-morphology, the mature seeds were cleaned in an ultrasonic bath and air-dried. Measurements and optical observations of 30 seeds for each species were recorded. Morphological studies were conducted by using a dissecting microscope.
For seed micromorphology, materials used were obtained from the mature fruit of the plants. Dried seeds were mounted directly on double-sided adhesive cellotape fixed to SEM stubs to critical-point and dried in CO2 before mounting. The mounted specimens were coated with gold in a sputter coater and examined using a scanning electron microscope. Seeds coat terminology follows that of Wilhelm (Citation1981) and, Whiffin and Tomb (Citation1972).
Investigation of Pollen Taxonomic Characteristics Through Light and Scanning Electron Microscopy
From each plant, several randomly selected flowers were collected. The measurements were based on 30 fully developed pollen grains per individual specimen, as recommended by Wrońska-Pilarek et al. (Citation2015), and the measured characters are provided in . Pollen grains were mounted in glycerin jelly after acetolysis procedure (Erdtman, Citation1952) with modifications for LM, and the examined characters were measured by the Leica light microscope, model DM 750 (Japan), with the aid of a 100× eyepiece.
Table 2. Seed morphological characteristics of nine species in Sorbus L.
For SEM, after acetolysis, the specimens were mounted on stubs of 12.5 mm diameter and then coated in a sputter coater with approximately 25 nm of gold – palladium, using the SEM model JEOL JSM-IT 300 (JEOL, Ltd., Tokyo, Japan). Exine sculpture elements were measured in an area of 25 μm2 following the methods of Ueda and Tomita (Citation1989). Normally, 3,000 to 10,000 magnifications were used for pollen morphological studies. The pollen terminology, in general, followed Gabrielian (Citation1978), Bednorz et al. (Citation2005) and Hesse et al. (Citation2009) for describing exine ornamentation. Both qualitative and quantitative characteristics of the pollen were studied in detail. For the measurement, ImageJ software was used.
Statistical Analysis
One-way analysis of variance (ANOVA) was applied to find out the differences in mean values among the taxa for assessing the variability. The Tukey’s post hoc test was used to test differences among means when the F-test was significant (p < .05). The mean, minimum, maximum and standard deviation values of the properties examined were determined. Principal Component Analysis (PCA) was conducted on quantitative variables.
The P/E ratio was calculated for each taxon based on the polar to equatorial diameter of the same pollen, using the formula P/E ratio = P/E × 100, where P is the polar axis and E is the equatorial diameter of the same pollen.
Results
The pollen and seed morphological characters for nine species of Sorbus are summarized in and SEM and LM images are shown in .
Figure 1. Shapes of seed in Sorbus. S. alnifolia(a), S. amabilis(b), S. folgneri(c), S. koehneana(d), S. megalocarpa(e), S. meliosmifolia(f), S. prattii(g), S. sargentiana(h), S. scalaris(i). Scale bars−1 mm.

Figure 2. Seed micrographs of Sorbus (SEM). S. alnifolia (a, d), S. amabilis (b, e), S. folgneri (c, f), S. koehneana (g, j), S. megalocarpa (h, k), S. meliosmifolia (i, l), S. prattii (m, p), S. sargentiana (n, q), S. scalaris (o, r). Scale bars−50 μm.
Figure 3. Pollen grains in equatorial view of Sorbus (SEM). S. alnifolia (a), S. amabilis (b), S. folgneri (c), S. koehneana (d), S. megalocarpa (e), S. meliosmifolia (f), S. prattii (g), S. sargentiana (h), S. scalaris (i). Scale bars−10 μm.
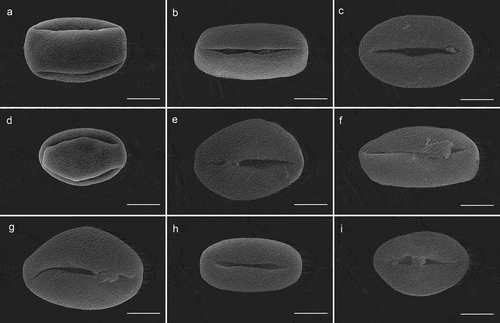
Figure 4. Pollen grains in polar view of Sorbus (SEM). S. alnifolia(a), S. amabilis(b), S. folgneri(c), S. koehneana(d), S. megalocarpa(e), S. meliosmifolia(f), S. prattii(g), S. sargentiana(h), S. scalaris(i). Scale bars−5 μm.
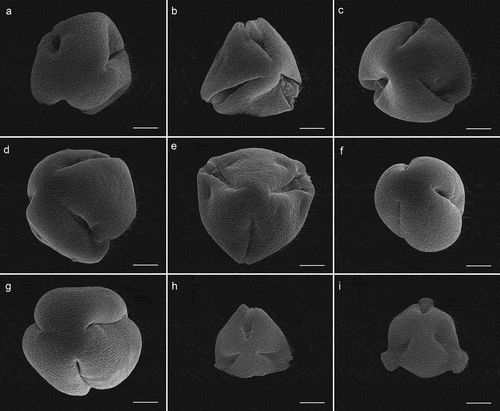
Figure 5. Pollen micrographs of Sorbus (SEM). S. alnifolia(a), S. amabilis(b), S. folgneri(c), S. koehneana(d), S. megalocarpa(e), S. meliosmifolia(f), S. prattii(g), S. sargentiana(h), S. scalaris(i). Scale bars−1 μm.
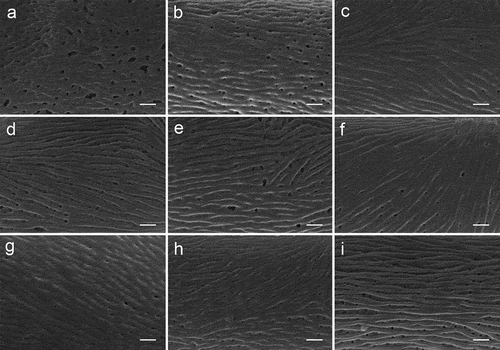
Table 3. Quantitative pollen features of analyzed taxa.
Seed Characters
The results of SEM and LM of the seed morphology of nine species of Sorbus are shown in and .
The morphological characteristics of the seeds including size, shape, color and surface sculpture of the seeds coat are summarized in . The size of mature seeds ranges from 2.42 × 1.33 × 0.92 mm (length × width × thickness) to 4.83 × 2.63 × 1.59 mm, with Sorbus sargentiana having the smallest seeds and S. megalocarpa having the largest. Sorbus seeds exhibit a wide range of outline shapes, including wide ovate, wide obovate, ovate, obovate, oblong, and narrow obovate, with the apex generally being roundish or obtuse. However, S. alnifolia, S. prattii, and S. sargentiana have slightly subacute or acute apices. And the base is often beak-like in S. alnifolia, S. prattii, and S. sargentiana, acute in S. amabilis and S. koehneana, or obtuse in S. folgneri, S. megalocarpa, S. meliosmifolia, and S. scalaris. Their color varies from light brown in S. alnifolia and S. amabilis, reddish brown in S. megalocarpa, S. sargentiana and S. scalaris, brown in S. folgneri, S. koehneana and S. meliosmifolia, to dark brown in S. prattii ().
The epidermal cells vary from polygonal to irregular, isodiametric or elongated in one direction. The anticlinal cell walls are straight or curved and the cell surface can be concave, flat or slightly convex, exhibiting different secondary sculptures such as smooth, unequally thickened, or curved (). The size of epidermal cells varies from 11.62 × 14.2 μm (S. koehneana) to 59.81 × 28.62 μm (S. meliosmifolia). Based on the differences in cuticular sculptures, two micromorphological types can be distinguished: ribbed-reticulate and light ribbed-reticulate.
Type I: Ribbed-Reticulate
The epidermal cells are polygonal, isodiametric, elongated, or irregularly shaped and possess highly raised anticlinal walls that form a reticulate ornamentation. The periclinal walls are concave and smooth. The testa cell dimensions range from 11.62 × 14.2 μm to 28.37 × 26.08 μm. The net ribbed due to the underneath layer, which is composed of sclerenchymatic fibers. Based on the shape of the epidermal cells, three subtypes can be distinguished.
Type IA: This subtype is characterized by polygonal to isodiametric or irregularly shaped epidermal cells that are approximately 13–20 μm in diameter, with unequally thickened lateral walls. This seeds coat morphology is typical of S. alnifolia ()) and S. folgneri (, f)), the latter of which exhibits hair-like depositions of epicuticular wax on the seed surface.
Type IB: This subtype is characterized by polygonal or isodiametric epidermal cells that are about 11–30 μm in diameter and possess equally thickened walls. This seeds coat morphology is typical of S. amabilis ()), S. sargentiana ()), S. scalaris ()) and S. koehneana ()). S. koehneana can be distinguished by its rough surface. Both S. sargentiana and S. scalaris possess epicuticular wax such as crusts and granules.
Type IC: This subtype is characterized by polygonal, elongated epidermal cells that are approximately 11–14 μm in diameter, with raised and equally thickened anticlinal walls. This seeds coat morphology is typical of S. prattii ()).
Type II: Light Ribbed-Reticulate
This type is characterized by a net-like surface consisting of isodiametric or elongated cells, mostly elongated longitudinally to the seed axis and measuring approximately 28–60 μm in diameter. The outer walls of the cells are slightly concave or almost flat. The lateral walls are weakly thickened, although well visible. The ribs are rather obscure as well as the depressions among them, which are weakly marked and not numerous. This seeds coat morphology is typical of S. megalocarpa and S. meliosmifolia, with the former exhibiting distinctly thicker anticlinal walls than the latter.
In summary, the seeds coat morphology of the studied species exhibits a range of distinct characteristics, including epidermal cell shape, thickness of anticlinal walls, and the presence of epicuticular wax and sclerenchymatic fibers, allowing for differentiation between the various subtypes.
Pollen Characters
The results of LM and SEM observations of the pollen morphology of nine species of Sorbus are shown in . The morphological observations of the quantitative and qualitative features are summarized in .
Figure 6. Equatorial and polar views of pollen grains under a Light Microscopy micrograph (LM). S. alnifolia (a, d), S. amabilis (b, e), S. folgneri (c, f), S. koehneana (g, j), S. megalocarpa (h, k), S. meliosmifolia (i, l), S. prattii (m, p), S. sargentiana (n, q), S. scalaris (o, r). Scale bars−10 μm.
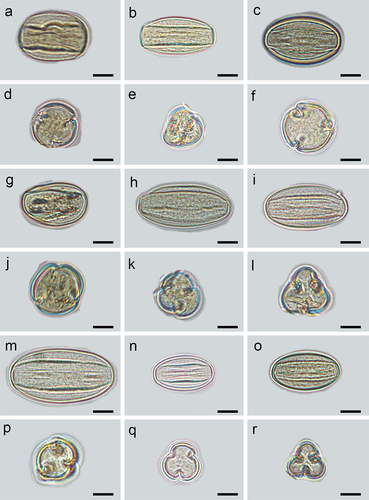
Table 4. Qualitative pollen features of analyzed taxa.
The analyzed pollen grains are monad exhibiting radial symmetry and isopolar (). Based on Erdtman’s size classification system (Erdtman, Citation1952), all the examined pollen grains were of medium size, with the average length of the polar axis (P) and equatorial axis (E) diameter being 36.08 μm (25.21–50.88 μm) and 20.19 μm (13.57–27.58 μm), respectively. S. prattii had the largest pollen grains, with a P × E of 45.54 × 24.34 μm, while S. sargentiana had the smallest, with a P × E of 31.54 × 16.69 μm. The pollen outline of polar view was mostly circular, with some occurrences of triangular obtuse shape, while in equatorial view, it was elliptic (). The P/E ratio ranges from 1.39 in S. alnifolia to 2.05 in S. amabilis and S. megalocarpa. Different pollen shapes were observed, including prolate and perprolate.
It can be concluded that all investigated species possess trizonocolporate pollen grains with long, deep, and tapered ectocolpi with regulate or granulate aperture membranes. The outline of ectocolpi was narrowly elliptic with a distinct margin that was slightly recurvate inwards. The mean length of the colpi ranged between 23.77 μm and 41.67 μm, and the ectoaperture studied was more than 3/4 the length of the polar axis. The three ectocolpi in the equatorial zone are regularly arranged, with equal distance between them. The endopori are located in the middle of ectocolpi, rather poorly marked.
The exine ornamentation of the pollen grains was usually striate-perforate, with striae, grooves, and perforations (). Striae were cylindrical straight, or forked, and of varying length, width, and height. They usually run parallel to the polar axis, but frequently formed fingerprint-like loops. Among the striae were distinct deep grooves, which form a linear pattern in most cases. The grooves were usually narrower than the striae, except for S. prattii and S. amabilis. Circular or elliptic perforations were observed at the bottom of the striae and were of different diameters, ranging from small to large (from 0.1 μm to 0.5 μm). However, S. alnifolia () has foveolae with a diameter of more than 1 μm, while S. prattii () has less visible perforations.
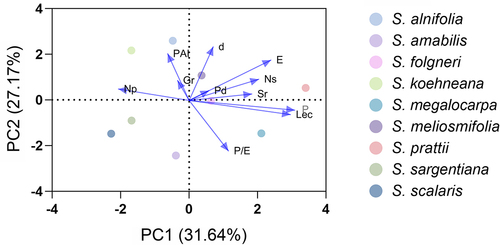
The results of ANOVA indicated that the main effects of the species were significant for all the quantitative traits (). The mean values and standard deviations for the observed traits indicated high variability among the tested samples, for which significant differences were found in terms of all the analyzed morphological traits. The most variable biometric traits were E and P, while lower variability was observed in Sr and Gr. The ranking of variability in the observed traits is as follows: P > E > P/E > LeC > d > Pd > Ns > PAI > Sr > Gr. The collected pollen quantitative data were explored using PCA to reveal the relationships among the taxa (). The first three PCAs explained 80.06% of the total variance of the analyzed data. In contrast, the eigenvalue was between 2.34–3.48 (). The PCA plot showed a clear separation in the space among the samples. The first two plots of principal components have been observed (). Taxon variation along PC1 designates dissimilarity with P, LeC, Np, E and Ns. PC2 provides information in dissimilarity in d, P/E ratio and PAI. This PCA analysis showed that the studied species showed dissimilarity in some characters.
Table 5. Principal component analysis of quantitative pollen features.
Discussion
In Rosaceae, seed morphology holds significant importance for phylogenetic studies and species delimitation (Chung et al., Citation2010; Dowidar et al., Citation2003; Potter et al., Citation2007; Ullah et al., Citation2021). Both seeds coat morphological and anatomical features are systematically useful (Elisens, Citation1985; Juan et al., Citation2000). Considering the importance of seed characters in Sorbus taxonomy, the anatomic structure of the seeds coat has been underlined by Gabrielian (Citation1978). Additionally, several studies have indicated that Sorbus displays substantial variation in seed shape, size and sculpturing, which provides valuable taxonomic information (Aldasoro et al., Citation1998, Citation2004; Bednorz et al., Citation2006; Bednorz, Citation2007; Dowidar et al., Citation2003; Maciejewska-Rutkowska and Bednorz, Citation2004). This study demonstrates that seed morphology, especially its microsculpture serves as reliable diagnostic features for distinguishing different species within the genus Sorbus. Ribbed-reticulate ornamentation is present in all studied taxa and has previously been reported for Polish Sorbus (Maciejewska-Rutkowska and Bednorz, Citation2004). Surface characteristics of cuticular sculptures, as well as cell shapes, can be used as diagnostic features for simple and pinnately leaved species. For example, S. megalocarpa and S. meliosmifolia, two of the simple-leaved species, can be distinguished by their light ribbed-reticulate ornamentation, whereas S. alnifolia and S. folgneri, the other two simple-leaved species, have seeds that differ in having polygonal to irregular cells.
Despite the importance of pollen grains for the identification of plant species, palynological studies on the genus Sorbus L. are scarce, especially in North America and China. Most research papers on the palynological studies of the genus Sorbus L. come from Europe (Bednorz and Fujiki, Citation2003; Bednorz et al., Citation2005; Boyd and Dickson, Citation1987; Stachurskaa et al., Citation1973). According to previous studies, the diagnostic features of Sorbus pollen grains were exine ornamentation (e.g., striae and groove length, width and direction, and perforation number and diameter) and the P and P/E ratio, as well as aperture structure (Bednorz and Fujiki, Citation2003; Bednorz et al., Citation2005; Eide, Citation1981; Hebda and Chinnappa, Citation1990; Moore et al., Citation1991; Yang et al., Citation2019).
We found the pollen shape was of little diagnostic value, as all studied taxa had elongated polar axis and had overlap in the shape of the grains, consist with the opinion of Bednorz et al. (Citation2005). However, in our study and in Yang’s et al. (Citation2019), the pollen grains of Chinese Sorbus species were found to be more prolate compared to those of Polish Sorbus species, which had a similar equatorial axis (E) diameter but smaller polar axis (P) length. (Bednorz and Fujiki, Citation2003; Bednorz et al., Citation2005). Generally, pollen size is less reliable (Moore et al., Citation1991). Nevertheless, it is still possible to ascertain some regularities, comparing our results with the data of Yang’s observation (Yang et al., Citation2019), that pollen grains of S. koehneana were usually smaller than the grains of other observed species. Besides, the studied pollen grains of Chinese Sorbus species were found to be medium-sized, with a polar axis diameter ranging from 25.21–40.41 μm. This result is consistent with the findings of Yang et al. (Citation2019). In contrast, the Polish Sorbus exhibited smaller pollen grains, with a polar axis diameter ranging from 22.91–34.9 μm (Bednorz and Fujiki, Citation2003; Bednorz et al., Citation2005). The significant differences observed between Chinese and Polish Sorbus species may be attributed to the higher temperatures and solar radiation in the Chinese provinces where our samples were collected (Hubei, Sichuan, and Yunnan). It has been reported that plants in environments with higher temperatures and potential evapotranspiration (PET) tend to produce less numerous but larger pollen grains (Ejsmond et al., Citation2011).
Two types of surface ornamentation, striate-perforate and foveolate, were recorded. These findings are in agreement with the research conducted by Yang et al. (Citation2019). However, the striae observed were wider compared to Polish Sorbus species, and the width of the groove was also larger. The perforations were also more visible than in Polish Sorbus species. Previous studies reported the number of apertures to be three, and rarely four. While in our study, only three-zonocolporate pollen grains were observed.
Conclusions
In this research, we examined the seed and pollen morphology of nine native Sorbus species in China. Our findings indicate significant variations in micro-morphological characters of both pollen and seeds. Pollen traits, including exine ornamentation such as striae and grooves (length and width), and perforations (number and diameter), have been demonstrated to be taxonomically significant. Similarly, seed characteristics, such as size, shape, and seed testa sculpture, are also useful for taxonomy. This study contributes additional information to the understanding of the taxonomy of Sorbus taxa in China.
Acknowledgments
The authors express their gratitude to Dahai Zhu and Tailun Hu for their assistance in collecting samples from wild. The research is part of the Master’s work of the principal author. This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Jiangsu Province, China (PAPD).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aldasoro, J.J., C. Aedo, F.M. Garmendia, F.P. Hoz, and C. Navarro. 2004. Revision of Sorbus subgenera Aria and Torminaria (Rosaceae - Maloideae). Syst. Bot. Monographs 69:1–148. doi: 10.2307/25027918.
- Aldasoro, J.J., C. Aedo, C. Navarro, and F.M. Garmendia. 1998. The Genus Sorbus (Maloideae, Rosaceae) in Europe and in North Africa: morphological analysis and systematics. Syst. Bot. 23:189–212. doi: 10.2307/2419588.
- Barser, B., M. Sagiroglu, G. Dogan, and H. Duman. 2021. Morphology of pollen in Ferula genus (Apiaceae). PhytoKeys 179:111–128. doi: 10.3897/phytokeys.179.66312.
- Bednorz, L. 2007. Morphological variability of fruits and seeds of Sorbus torminalis in Poland. Dendrobiology 57:3–14.
- Bednorz, L., and T. Fujiki. 2003. Pollen morphology of some European Sorbus species. Rocz. AR Pozn. CCCLIV, Bot 6:3–7.
- Bednorz, L., I. Maciejewska-Rutkowska, D. Wroińska-Pilarek, and T. Fujiki. 2005. Pollen morphology of the Polish species of the genus Sorbus L. Acta Soc. Bot. Pol 74(4):315–322. doi: 10.5586/asbp.2005.040.
- Bednorz, L., R. Walkowiak, I. Maciejewska-Rutkowska, and K. Moliński. 2006. Seed variability of the Polish species of the genus Sorbus (Rosaceae). Dendrobiology 55:3–9.
- Boyd, W.E., and J.H. Dickson. 1987. The pollen morphology of four Sorbus species, with special reference to two Scottish endemic species. S. Arranensis Hedl. and S. Pseudofennica E.F. Warb. Pollen Et Spores 29:59–71.
- Bozhuyuk, M.R. 2021. Morphological and biochemical diversity in fruits of rowanberry (Sorbus aucuparia L.) Genotypes. Erwerbs-Obstbau 63:431–435. doi: 10.1007/s10341-021-00603-4.
- Campbell, C., R. Evans, D. Morgan, T. Dickinson, and M. Arsenault. 2007. Phylogeny of subtribe pyrinae (formerly the maloideae, Rosaceae): Limited resolution of a complex evolutionary history. Plant Syst. Evol. 266:119–145. doi: 10.1007/s00606-007-0545-y.
- Chung, K.S., W.J. Elisens, and J.J. Skvarla. 2010. Pollen morphology and its phylogenetic significance in tribe Sangui-sorbeae (Rosaceae). Plant Syst. Evol. 285:139–148. doi: 10.1007/s00606-009-02629.
- Dowidar, A.E., M. Loutfy, E.A. Kamel, A.M. Ahamed, and H.H.L. Hafez. 2003. Studies on the Rosaceae I-Seed and/or achene macro and micromorphology. Pak. J. Biol. Sci. 6(20):1778–1791. doi: 10.3923/pjbs.2003.1778.1791.
- Eide, F. 1981. Key for Northwest European Rosaceae pollen. Grana 20:101–118. doi: 10.1080/00173138109427651.
- Ejsmond, M.J., D. Wrońska-Pilarek, A. Ejsmond, D. Dragosz-Kluska, J. Kozowski, P. Kołaczek, and J. Kozłowski. 2011. Does climate affect pollen morphology? Optimal size and shape of pollen grains under various desiccation intensity. Ecosphere 2(10):art 117. doi: 10.1890/ES11-00147.1.
- Elisens, M.J. 1985. The systematic significance of seed coat anatomy among new world species of tribe antirrhineae(Scrophulariaceae). Syst. Bot. 10(3):282–299. doi: 10.2307/2418592.
- Erdtman, G. 1952. Pollen morphology and plant taxonomy: angiosperms. Almqvist and Wiksell, Stockholm.
- Faegri, K., and J. Iversen. 1992. Textbook of pollen analysis. 3rd ed. Hafner Press, New York.
- Gabrielian, E. 1978. Sorbus L. in Western Asia and the himalayas. AN ArmSSR, Yerevan, Botanicheski Institut.
- Hebda, R.J., and C.C. Chinnappa. 1990. Studies on pollen morphology of Rosaceae in Canada. Canada. Rev. Paleobot. Palynol 64:103–108. doi: 10.1016/0034-6667(90)90123-Z.
- Hesse, M., R. Buchner, A. Froschradivo, H. Halbritter, R. Zetter, M. Weber, and S. Ulrich. 2009. Pollen terminology: An illustrated handbook. SpringerWein, New York.
- Juan, R., J. Pastor, and I. Fernández. 2000. SEM and light microscope observations on fruit and seeds in Scrophulariaceae from Southwest Spain and their Systematic Significance. Ann. Bot. 2:323–338. doi: 10.1006/anbo.2000.1188.
- Lechowicz, K., J. Bocianowski, and D. Wrońska-Pilarek. 2021. Pollen morphology and variability of species from the genus Rubus L. (Rosaceae) alien and invasive in Poland. J. Plant Taxon. Geogr 76(1):109–121. doi: 10.36253/jopt-10355.
- Lo, E.Y.Y., and M.J. Donoghue. 2012. Expanded phylogenetic and dating analyses of the apples and their relatives (Pyreae, Rosaceae). Mol. Phylogenet. Evol. 63:230–324. doi: 10.1016/j.ympev.2011.10.005.
- Lu, L.T., and S.A. Spongberg. 2003. Sorbus L, In: pp. 144–170. In: Z.Y. Wu, P.H. Raven, and D.Y. Hong (eds.). Flora of China 9. Science Press, Beijing & Missouri Botanical Garden Press, Saint Louis.
- Maciejewska-Rutkowska, I., and L. Bednorz. 2004. SEM and stereoscope microscope observations on the seeds of the Polish species of the genus Sorbus L. (Rosaceae). Acta Soc. Bot. Pol. 73(4):315–322. doi: 10.5586/asbp.2004.037.
- McAllister, H. 2005. The genus Sorbus - mountain ash and other rowans. Royal Botanic Gardens, Kew.
- Moore, P.D., J.A. Webb, and M.E. Collinson. 1991. Pollen analysis. Blackwell, London.
- Nelson-Jones, E., D. Briggs, and A. Smith. 2002. The origin of intermediate species of the genus Sorbus. Theor. Appl. Genet. 105:953–963. doi: 10.1007/s00122-002-0957-6.
- Oh, Y.N., S. Jin, H.J. Park, H.J. Kown, and B.W. Kim. 2016. Sorbus rufopilosa extract exhibits antioxidant and anticancer activities by inducing cell cycle arrest and apoptosis in human colon adenocarcinoma HT29 cells. J. Cancer Prev. 21(4):249–256. doi: 10.15430/JCP.2016.21.4.249.
- Pathak, M.L., M. Idrees, B. Xu, and X.F. Gao. 2019. Pollen morphology of subfamily Maloideae (Rosaceae) with special focus on the genus Photinia. Phytotaxa 404:171–189. doi: 10.11646/phytotaxa.404.5.1.
- Phipps, J.B., K. Robertson, P.G. Smith, and J.R. Rohrer. 1990. A checklist of the subfamily Maloideae (Rosaceae). Can. J. Bot. 68:2209–2269. doi: 10.1139/b90-288.
- Potter, D., T. Eriksson, R.C. Evans, S. Oh, J.E.E. Smedmark, D.R. Morgan, M. Kerr, K.R. Robertson, M. Arsenault, T.A. Dickinson, et al. 2007. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 266:5–43. doi: 10.1007/S00606-007-0539-9.
- Reitsma, T.J. 1966. Pollen morphology of some European Rosaceae. Acta Bot. Neerl 15:290–379. doi: 10.1111/j.1438-8677.1966.tb00234.x.
- Sarv, V., P.R. Venskutonis, R. Rätsep, A. Aluvee, R. Kazernaviˇciute, and R. Bhat. 2021. Antioxidants characterization of the fruit, juice, and pomace of sweet rowanberry (Sorbus aucuparia L.) Cultivated in Estonia. Antioxidants 10:1779. doi: 10.3390/antiox10111779.
- Song, J.H., H.K. Moon, and S.P. Hong. 2016. Pollen morphology of the tribe Sorbarieae (Rosaceae). Plant Syst. Evol. 302:853–869. doi: 10.1007/s00606-016-1303-9.
- Sottile, F., M.D. Signore, N.R. Giuggioli, and C. Peano. 2017. The potential of the Sorb (Sorbus domestica L.) as a minor fruit species in the Mediterranean areas: Description and quality traits of underutilized accessions. Prog. Nutr. 19(1):41–48. doi: 10.23751/pn.v19i1-S.5054.
- Stachurskaa, A., A. Sadowska, and T. Kuszell. 1973. Sorbus aucuparia L, Jąrząb pospolity (jąrzebina), Sorbus domestica L., Jąrząb domowy, Sorbus aria (L.) Cr, Jąrząb maczny, Sorbus chamaemespilus (L.) Cr, Jąrząb nieszypułkowy, Sorbus torminalis (L.) Cr., Jąrząb brekinia (brzęk), (Rosaceae). Kartoteka palynologiczna roślin polskich. Zeszyty Przyrod. Opol. Tow. Przyj. Nauk. 13:tab. 204–208. (in Polish with English summary).
- Teppner, H. 1965. Zur Kenntnis der Gattung Waldsteinia L. Schliüssel zum Bestimmen von Rosaceen Polleeinschlies-slich ählicher Pollenformen aus andere Familien. Phyton 34:224–238.
- Ueda, Y., and H. Tomita. 1989. Morphometric analysis of pollen exine patterns in roses. Engei Gakkai Zasshi 58(1):211–220. doi: 10.2503/jjshs.58.211.
- Ullah, F., Y. Gao, R.F. Jiao, and X.F. Gao. 2021. Comparative taxonomic variation in fruits and seeds’ surface morphology among populations of alpine Rosa sericea complex (Rosaceae). Microsc. Res. Tech. doi: 10.1002/jemt.23788.
- Ullah, F., Y. Gao, Z. Wajid, and X.F. Gao. 2022. Pollen morphology of rosa sericea complex and their taxonomic contribution. Diversity 14(9):705. doi: 10.3390/d14090705.
- Wang, S.K., X.G. Li, J. Yang, L. Wang, H.B. Xue, Y.L. Su, and L. Wang. 2016. Current situation and perspective of pear breeding for last two decades in China mainland. J. Fruit Sci 33(S1): 10–23.
- Whiffin, T., and A.S. Tomb. 1972. The systematic significance of seed morphology in the Neotropical capsular-fruited Melastomataceae. Am. J. Bot. 59(4):411–422. tb10112.x. doi: 10.1002/j.1537-2197.1972.tb10112.x.
- Wilhelm, B. 1981. Epidermal and seed surface characters of plants: Systematic applicability and some evolutionary aspects. Nord. J. Bot. 1(3):345–355. doi: 10.1111/j.1756-1051.1981.tb00704.x.
- Wrońska-Pilarek, D., A.M. Jagodziński, J. Bocianowski, and M. Janyszek. 2015. The optimal sample size in pollen morphological studies using the example of Rosa canina L. Palynology 39(1):56–75. doi: 10.1080/01916122.2014.933748.
- Wrońska-Pilarek, D., M. Sowelo, W. Antkowiak, J. Bocianowski, and K. Lechowicz. 2022. Pollen morphology and variability of native and alien, including invasive, species of the genus Spiraea L. (Rosaceae) in Poland. PLos One 17(8):e0273743. doi: 10.1371/journal.pone.0273743.
- Yang, L.H., Y.H. Wu, X. Pei, X.L. Guan, and J. Zhen. 2019. Pollen morphological characteristics and cluster analysis on some species in Sorbus L. null 28(3):84–90.
- Yü, T.T., and L.D. Lu.1974. Sorbus L. In: pp. 283–344. In: ed. T.T. Yü. Flora Reipublicae Popularis Sinicae. Vol. 36. Science Press, Beijing.
- Zhou, L.H., Z.X. Wei, and Z.Y. Wu. 2000. Pollen morphology of maloideae of China (Rosaceae). Acta Bot. Yunnanica 22:47–52.