?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The Argan tree (Argania spinosa (L.) (Skeels)) is a woody species endemic to Algeria (Tindouf region mostly) and Morocco. It is a plant species of oleo-agro-sylvo-pastoral and ecological importance as it contributes to the fight against desertification and climate change. Moreover, the seed is used for extracting oil, which has high dietic and cosmetic qualities. In this work, the fruit harvest period and Argania spinosa productivity are related to the phenological process of flowering and fruiting. Our results show that the experimental argan trees of Mostaganem bloom late to be pollinated in the spring and remain dormant throughout the summer, in contrast to the endemic Tindouf trees that bloom early in September, bear fruit and ripen in the same season. This explains why that the fruits of the Tindouf region are the richest compared to the fruits of the other two regions. Our study shows that the pulp always contains more than almonds, as the oil was extracted and analyzed, the pulp is the fraction richest in phenols, while almond is the poorest fraction (3.43 ± 0.72 mg/g, 1.34 ± 0.03 mg/g), the same thing goes for the total flavonoid content (9 ± 0.5 mg/g, 2.83 ± 0.14 mg/g). The greatest activity was recorded in the fruits of Tindouf. As for the components of the fruit, the highest activity was recorded in the pulps then the almonds and the lowest in the oil (502 ± 11.42 g, 216 ± 8.43 g, 174 ± 3.61 g), respectively. Concerning primary metabolites, the fruit is rich (the almond is rich in proteins, the pulp is rich in sugar and the oil is rich in lipids).
Introduction
Argania spinosa (L.) (Skeels) is a rare species with oleo-agro-sylvo-pastoral importance, it is characterized by great ecological plasticity, observed on the Saharan, semi-arid, and arid climate. Argan oil has been well known for its cardioprotective properties, and it is also used in the treatment of skin infections. Argan oil is principally composed of mono-unsaturated (up to 80%) and saturated (up to 20%) fatty acids. As minor components, it contains polyphenols, tocopherols, sterols, and triterpene alcohols (El Monfalouti, Citation2013). Chemistry and a few pharmacological aspects of argan oil have been studied; there are still no strong clinical data available that provide evidence of the efficacy of argan oil in humans. That argan oil constituents have pharmacological properties in vitro is not sufficient to ascertain the clinical potential of whole argan oil. More studies are necessary to determine its impact on human health. In Tindouf (Algerian area) and Morocco, the position of argan oil as a natural product, with strong consumer expectations resulting from traditional claims of activity that are insufficiently supported by scientific proof is shared by several other plant extracts or products. Such a trend is likely to continue in view of the strong current demand for food supplements. This demand justifies pharmacological studies on these products (Harhar et al., Citation2011; Hilali et al., Citation2005). Argan oil has high levels of oleic and linoleic acids and antioxidant compounds, and the impact of argan oil on cardiovascular disease (Derouiche et al., Citation2005). Minor compounds of argan oil, such as sterols, may be involved in its cholesterol-lowering effect (Khallouki et al., Citation2003). The antidiabetic effect of argan oil has been claimed for a long time in traditional medicine; however, the mechanism of regulation of the level of glucose in the blood remains unknown (Emonard et al., Citation1999). The antihypertensive effect of argan oil and its mechanism of action have been studied by Berrougui et al. (Citation2004).
Concerning tree productivity, flowering and fruiting, according to Benchettouh (Citation1999), Miloudi (Citation2006), Benaouf and Miloudi (Citation2017), Kechairi (Citation2018) there are two types of argan trees: Late argan trees bloom once a year in the spring in Mostaganem and Mascara, have worn two generations of fruit, the knotted fruits of the present season and the fruits knotted the past growing season. The length of the observed cycle is 16 months. And early argan trees that begin to bloom in the autumn in Tindouf, where the fall of ripe fruit begins their abscission in the spring of the following year.
Materials and Methods
Observations of the production and fruiting of the argan tree are made twice a month (on a sample of 10 trees observed) for 3 years (2018–2020), on twigs marked with small plates attached to the branches. In our case, we followed the trees according to the two East and West exposures.
Study Regions
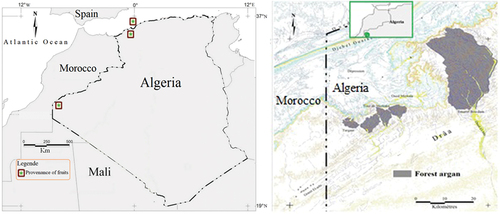
Mature fruits of Argania spinosa were collected from three origins (when fallen on the ground) : Argan grove of Tindouf in South-West Algeria (28 ° N, 8 ° W) from Djbel Ouarkziz to Hamada de Tindouf; Our Argan trees experimental of Mostaganem are located in the coast (Mediterranean area), its geographical location (35° 48. 170” N; 00° 03.994‘ W) and Mascara from Oggaz region (35 ° 34. 00 ’ N; 0 ° 16. 00 ” W), the two regions bioclimatically identified with a Mediterranean diet: a rainy period of five months ().
The harvest was carried out in June/July 2018. All the fruit samples were fresh, ripe, and healthy. After drying the fruits, the pulp and almond were finely grounded using a grinder and then screened through sieves, the powder stored at in a refrigerator to prepare the methanol extract.
Figure 2. Localisation of Argan fruit studies; Forest argan carte in Algerian Sahara (Kechairi and Abdoun, Citation2016).
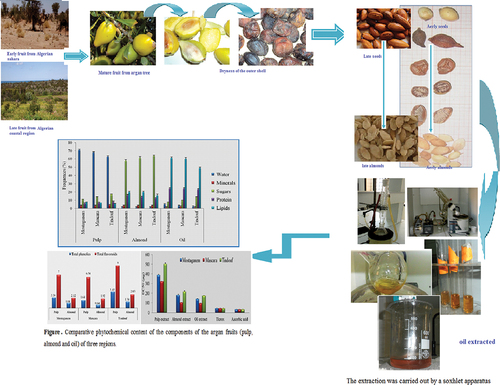
Argan Oil Preparation
The extraction was carried out by a soxhlet apparatus, according to the standard (Afnor, Citation1988) the technique consists in using a polar organic solvent (hexane). Twenty-five grams of almond seeds powder are placed in a cartridge. The volatile compounds were extracted by the solid-phase microextraction method (SPME).
Phytochemical Analysis
Determination of Water Content
The water content is determined according to method no. 934.06. 1990, recommended by (AOAC: The Association of Analytical Communities International, Citation1990). Five grams of sample were weighed in the fresh state, then placed in an oven set at 50°C overnight. The dry matter was removed from the oven after cooling in a desiccator, the dried sample was weighed. The water content was calculated according to the following formula:
Pf: Weight of the “fresh plant” sample. Ps: Weight of the “dry plant” sample. H%: Humidity rate expressed as a percentage.
Determination of Mineral Content
The mineral content was determined according to method no. 923.03.1990, recommended by A.O.A.C (AOAC International, Citation1990). This method involves calcining the sample at 550°C in a muffle furnace until a whitish ash of constant weight is obtained. Two grams of sample were weighed into crucibles and then placed in a muffle furnace set at 550°C for 5 h, until a whitish ash was obtained. The crucibles were weighed after cooling in a desiccator. Organic matter was calculated using the following formula:
Or : M%: Organic matter content Mi: Initial mass (mass of the crucible containing the sample in g) Mf: Final mass (mass of crucible and ash in g) P: Mass of the test portion
Determination of Lipid Content
The lipid content was determined according to the AOAC method (AOAC International AOAC: Official Methods of Analysis, Citation1990). Twenty-five grams of sample was added to the filter paper cartridges and then placed on the Soxhlet. 250 mL of hexane was poured into a flask. The flask was heated for 4 h. After removing the solvent by distillation, the flask was dried at a temperature of 70–80°C, then weighed after cooling in the desiccators. The fat content was determined according to the following formula:
P1: weight of the empty balloon (g) P2: weight of the balloon with the extracted oil (g) P3: weight of the test sample (g)
Determination of Protein Content
The protein content was determined according to the method of Kjeldahl no. 960.52. 2000, recommended by A.O.A.C (AOAC International AOAC: Official Methods of Analysis, Citation1990). This method is based on quantifying the nitrogen content, and then the protein content is calculated by multiplying the total nitrogen content N (%) by the coefficient 6.25. This method takes place in two stages: The first is the digestion phase which consists of a chemical attack with sulfuric acid to eliminate the organic matter. One gram of the sample was mixed with 1 g of Kjeldahl catalyst (copper potassium sulfate) and 15 ml of sulfuric acid, and the mixture was prepared in a mineralization flask by applying gradual heating. When the solution becomes clear, it was cooled and made up to 100 ml with distilled water. The second step is the distillation which consists in dissolving the mineral nitrogen in the form of ammonia. This step is carried out in a rotavapor by adding 20 ml of sodium hydroxide to 35% in the flask and 25% boric acid in a 250 ml flask. The ammonia was collected in a solution of boric acid. The last step is the titration, which was performed by adding a few drops of the TACHIRO indicator (mixture of methylene blue and methyl red) to the flask containing ammonia and boric acid. The excess ammonia was then determined with 0.05 N sulfuric acid by simple titration. The total nitrogen content was determined by the following formula:
N: Percentage of nitrogen (%) P: Percentage of protein (%) Co: Normality of sulfuric acid (0.05) V: Volume of sulfuric acid poured (mL) P: Weight of the test portion (g)
Determination of Sugar Content
The sugar content is calculated simply according to the following formula:
H: Moisture (%) (= water content) P: Protein (%) L: Fat (%) M: Mineral matter (%) (= ash content)
Dosage of sugars and proteins in the oil was determined from the vegetable extract of the almonds, the extraction was carried out by maceration. A 2g pea of powdered almonds was mixed with 5ml of 80% methanol, and put in labeled airtight bottles, then left for 24 hours in the dark. The homogeneous mixture and centrifuged at (1000 rpm for 15 min), the supernatant was obtained. Then 5 ml of methanol was added to the pellet and a second centrifugation was carried out at 100 rpm for 10 minutes and the two supernatants were mixed.
Preparation of Extract
Pulp and Almond
(20) g of pulp powder and almonds were defatted using petroleum ether (60–80°C) and then extracted with 200 ml of methanol/water (70/30, v/v) in an ultrasonic bath for 30 min. The solvent was then evaporated, and the extract was obtained with a yield of 13%. The extract was stored at 20°C until use.
Oil Argan
(1) g of oil was weighed and solubilized with 1 ml of n-hexane and 2 ml of methanol/water (v/v, 60/40) in a centrifuge tube, the whole vortexed for 2 min and centrifuge the total volume at 3000 rpm, the extraction was repeated three times on the supernatant to extract as much as possible, the residual part was washed with n-hexane and evaporated to dryness under reduced pressure at 35°C. The argan oil analyses were carried out according to the same operating conditions for the pulp and almonds.
Total Polyphenol and Flavonoid Determination
The total phenolics in the methanolic extract were determined by the Folin and Ciocalteu method (Folin and Ciocalteu, Citation1927). Gallic acid was used as the standard to generate a calibration curve, and measurements were carried out at 725 nm using a UV–vis spectrophotometer (Shimadzu UV 1650, Italy). The results were expressed as milligrams of gallic acid-equivalent per gram dry mass (mg GAE/g dry mass). Total flavonoids were estimated according to the method of Grand et al. Quercetin was used as the standard to generate the calibration curve, and measurements were carried out at 510 nm using a UV–vis spectrophotometer (Shimadzu UV 1650, Italy). Results were expressed as milligrams of quercetin-equivalent per gram dry mass (mg QAE/g dry mass).
Polyphenol Characterization by UPLC – ESI–QTOF – MS
Polyphenolic compounds were identified using an Acquity Ultra-Performance LC system (UPLC) coupled to a XEVO-G2-QTOF (quadrupole time of flight) mass spectrometer (Waters Corporation, Manchester, United Kingdom), equipped with an electrospray ionization (ESI) source operating in positive and negative modes, using the binary solvent manager software (Waters Corporation, UK). Chromatographic separation was carried out on a UPLC HSS T3, C18 column (1.8 μM, 2.1 × 100 mM, Waters Corporation) at 30 oC. The mobile phase comprised Solvent (A) (0.1% formic acid, v/v) and Solvent (B) (100% methanol). The gradient used was 0–5 min, 0–10% B; 5–10 min, 10–15% B; 10–15 min, 15–20% B; 15–20 min, 20–30% B; 20–30 min, 30–40% B; and 30–35 min, 40–0% B. The solvent flow rate was 0.4 mL/min. The injection volume was 2 μL. The analysis was performed at a wavelength of 280 nm, data depending on MS scanning from 50 to 1500 m/z. The analysis was performed in positive and negative ion modes using an electrospray source with a source temperature of 120°C and a desolvation temperature of 500°C, a capillary voltage of 1 kV, and a sampling cone voltage of 30 eV. Highly pure N2 (˃99.9995%) was used as the desolvation gas (flow rate, 10 L/h) and cone gas (flow rate, 1000 L/H). Data were acquired using the MassLynx software (version 4.1, Waters). The exact masses and MS fragmentation data were compared with those in the respect for phytochemicals and Mass Bank metabolomics data banks and in previous reports to identify phenolic compounds (Taleuzzaman et al., Citation2015).
Antioxidant Assay
The inhibitory activity of the DPPH radical (2–2-Diphenyl-1-picrylhydrazyl): the antioxidant power of the extracts tested was estimated by comparison with a synthetic antioxidant which is Torox and natural antioxidant Vitamin C (ascorbic acid). DPPH assay of the methanolic extract of Components of the fruit (pulp, almond, and oil) was carried out as described by Blois (Blois, Citation1958). Dried extract was weighed and reconstituted to known concentrations. The extract (0.3 ml) and a methanolic solution of DPPH (2.7 ml; 5 mg in 100 ml methanol) were mixed and incubated at room temperature for 1 h in the dark. The absorbance at 515 nm was then measured (Shimadzu UV 1650, Italy). A control solution was prepared with 0.3 ml methanol and 2.7 ml methanolic solution of DPPH. Ascorbic acid and Trolox were used as reference compounds. Radical scavenging activity is expressed as the percentage inhibition of DPPH (D) as in Eq x.
Statistical Analysis
Data are presented as means ± standard deviation (SD) and were statistically analyzed using analysis of variance (ANOVA). A value of p ≤ .05 was considered to indicate statistical significance. Analyses were carried out using Statistica software (version 6.0, StatSoft).
Results and Discussion
In Algeria, argan tree lives endemically in the far western desert in a harsh desert environment where it receives humidity from the Atlantic Ocean (Tindouf) ; the tree domesticated by seed in the northern regions of the Mediterranean, with arid and semi-arid climates where did the tree adapt well and its cultivation projects succeed and reach the stage of fruiting and oil extraction and marketing (Mostaganem and Oggaz). In Morocco, The tree grows in all arid, semi-arid environment and mountainous regions because it is a country facing the ocean. The map () showing all named locations
Floral Biology
In Algeria, according to Benchettouh (Citation1999), Miloudi (Citation2006), Kechairi (Citation2009), and Benaouf and Miloudi (Citation2017) there are two types of argan trees:
- Late argan trees bloom once a year in the spring in Stidia and Oggaz, have borne two generations of fruit, the knotted fruits of the present campaign and the knotted fruits of the past campaign in the process of ripening. The observed cycle length is 16 months.
- Early argan trees which begin to bloom in the autumn in Tindouf, where the fall of ripe fruits begins their abscission in the spring of the flowering year so the length of its cycle observed is 9 months.
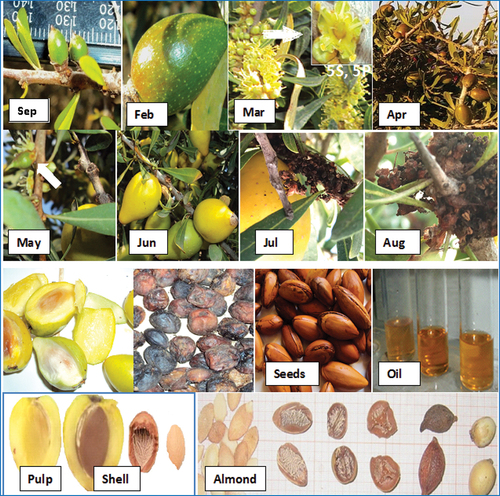
From our observations over several years, we have concluded that the Mostaganem argan tree is a forest fruit tree and is characterized by a very rare phenological case, as it blooms and then self-pollinates; and its flowers have the ability to resist and remain dormant all summer until the first rains of autumn to bear fruit and start to grow. All winter and spring it blossoms again to meet two generations on its branches. The fruits of the previous year and the flowers of the new year that pollinate throughout the spring to lose their petals and sepals and only keep their fertilized ovaries to go dormant. With the exception of a very small percentage of these flowers, they bear fruit immediately and directly (, May), but in their small size they are doomed to death and drop automatically in June as this is the period of spontaneous fruit drop ().
Table 1. Plant organs borne by argan tree.
Flowering
Period from March to May, we notice the appearance of yellow tips on the twigs; this is the end of the budding stage and the beginning of flowering. The majority of the flowers open marking the end of the flowering stage and the onset of dormancy, at the same time the fruits of the past year continue to grow and grow ().
Pollination
The pollination of the argan tree is especially entomophilous, the insects intervening. The transmission of the alleles depends, among other things, on the availability of the pollen, its fertility, its transport, and the receptivity of the stigma. Pollen fertility is variable according to trees and climatic years.
Fruit Production
The flowers fertilized in the spring remain dormant all summer, they will germinate with the first rains of September, the appearance of small fruits begins, the small fruits continue their growth in the next year from January to June; Period from June to July. We note that the density of last year’s fruits is high, especially in the part exposed to sunlight. As for the number of fruits, it varies from 1 to 5 on the branch and from 1 to 2 on the same node. The fruits gradually turn yellowish. Fruit drop is almost complete: This is the ripening stage.
The argan tree, a species of the family of Sapotaceae, presents variations of production according to the abiotic conditions of the natural environment. Thus, during the summer period when the temperature is high compared to the other seasons of the year, we note a significant density of fruits, from 1 to 5 on the branch and which become ripe at the end of the season. In a second step, we conclude that the argan of the Algerian tell (Mostaganem and Mascara) starts flowering late (in the spring) compared to the argan tree of Tindouf (southern Algeria) in the autumn (Kechairi, Citation2009).
Then, in comparison with our results on the argan tree in Algeria (West and Southwest Regions of Tindouf) with the results of Metro (Citation1953), Montoya Olivier (Citation1984), Bani-Aameur and Benlahbil (Citation1999), and Ferradous et al. (Citation1996) on the Moroccan argan, it seems very useful to point out that there is in general a similarity in the progression over time of the different phenological stages of the species. As a result, we observe that significant physiological changes in the argan tree occur mainly during two periods of the year; that is from June to September and from February to April during which the temperature is relatively optimal. The first period (June to September) is marked by the maturation of fruits that turn yellowish.
These trees bear fruit of different sizes. Flowering is maximal from March to the end of May, but flowers can appear at any season with a peak in the winter (near the coast) and in the spring. This last season is the most favorable period (Boudy, Citation1950). The young fruits resulting from this flowering remain incompletely developed until the first rains of the following autumn. In October, preexisting berries begin to grow (M’hirit, Citation1998).
Phytochemical Analysis
Primary Metabolites Determined
The results showed that the contents of the components of the fruits of the desert region are characterized by their richness in sugars, while their water content is weak compared to the fruits of the two regions close to the Mediterranean. However, the other parameters are practically the same in the different parts of the fruit at the level of the three regions (almond is rich in proteins, pulp is rich in sugar and oil is rich in lipids). Our results obtained regarding the content of the argan fruit for primary metabolites were almost identical to studies conducted on the Algerian argan by Djied in 2016 and my previous study that I conducted with Abiayyad in 2014. ().
Table 2. Comparative phytochemical content of the components of the argan fruits (pulp, almond, and oil) of three regions.
Total Polyphenol and Flavonoid Determination
The content of total polyphenols differs from part to part of argan fruit, we find that at the level of the Tindouf region, the pulp is the part richest in phenolics, while the almond is the poorest part (3.43 ± 0.72 mg/g, 1.34 ± 0.03 mg/g). We can therefore classify the polyphenol content at the fruit of the three regions: pulp > almond. What draws attention is that the fruits of the desert environment (Tindouf) are the richest in the content of phenolics.
The content of total flavonoids differs from part to part of the argan fruit and it was more than double compared to the phynolics. At the fruit of the Tindouf region which is the richest compared to the other two regions also where the pulp represents the richest part, compartmentally with the almond (9 ± 0.5 mg/g, 2.83 ± 0.14 mg/g).
Examination of the TOF-MS chromatogram in positive mode of the phenolics extract of argan oil from different regions reveals the presence of the same phenolics compounds such as: caffeic, vanillic, coumaric, and gallic acid ().
So the analyses proved the richness of the components of the argan fruit with very significant values of polyphenols and flavonoids in varying proportions, and the pulp is very rich compared to the folded almonds, which is a source of oil pressing ().
Table 3. Comparative of total phenolic and flavonoid contents of the components of the argan fruits (pulp and almond) of three regions, unit of measurement mg/g.
Determination of the Scavenger Effect of the DPPH Radical
Recently, polyphenols have received special attention. They are natural products of great value and endowed with many physiological properties that make them able to protect cells against damage caused by free radicals (El Monfalouti et al., Citation2010; Sies, Citation2010; Zheng et al., Citation2010). Polyphenols can also prevent degenerative diseases. They have other antioxidant properties (Gómez - Caravaca et al., Citation2006; Muanda et al., Citation2011).
Polyphenols in argan oil are known to have interesting therapeutic properties (Khallouki et al., Citation2003). Several phytochemical studies on several parts of the argan tree or on by-products have already been carried out with particular attention to polyphenols (Charrouf and Guillaume, Citation2007; Rojas et al., Citation2005).
Our study is interested in quantifying the total polyphenol content of the almond and argan pulp for three origins of argan fruits, the first was brought from the nature reserve of the argan grove of Tindouf in the south west of the Algerian Sahara, the other two were brought from Mascara and Mostaganem, which are located in the North West of the country (coastal region near the sea where the argan tree is well introduced).
The antioxidant activity of the various extracts of argan trees according to the DPPH radical was evaluated spectrophotometrically by following the reduction of this radical which is accompanied by its change from violet color to yellow color, measurable at 517 nm. The% trapping (= anti-radical activity) of the free radical DPPH shows the ability of the extract, at a given concentration, to reduce or not radicals. Our results are expressed as an extract concentration (mg/mL) capable of inhibiting 50% of the reaction, the data are summarized in , which represents the IC50 of argan extracts from different parts of fruit of three different regions (Tindouf, Mostaganem, and Mascara) with respect to the free radical DPPH. The anti-free radical activity of Tindouf extracts was greater compared to that of Mostaganem and Mascara. The anti-free radical activity of the other extracts does not show a significant difference when comparing the three regions studied. Taken together, extracts from the Tindouf region being the region with the most antioxidant activity.
Antioxidant Activity
The antioxidant potential of argan fruit extract was determined by a DPPH-scavenging assay. Argan fruit extracts showed concentration-dependent antiradical activity. The EC50 value of Trolox and ascorbic acid were (46.82 ± 0.93 μg and 31.86 ± 1.25 μg), respectively. Thus, argan extract and the commercial antioxidants Trolox and ascorbic acid exhibited significantly different DPPH radical-scavenging activities (p < .05). The largest activity was recorded in the fruits of the desert environment. As for the components of the fruit, the largest activity was recorded in the pulp and then almonds and the weakest in the oil (502 ± 11.42 μg, 216 ± 8.43 μg and 174 ± 3.61 μg), respectively ().
Figure 4. Comparative phytochemical content of the components of the argan fruits (pulp, almond and oil) of three regions.
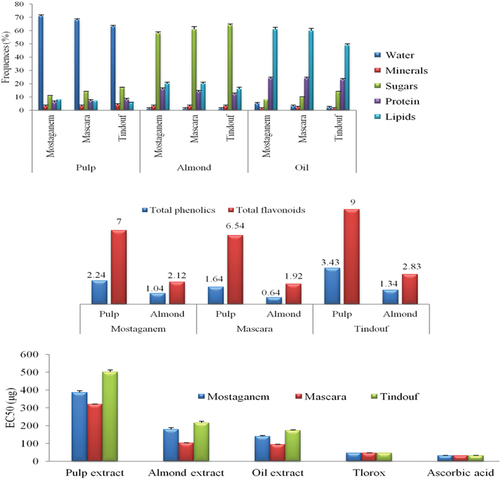
Table 4. EC50 values of the extract of the oil, almond, and pulp of argan fruit, Trolox, and Ascorbic acid.
The correlation test in the three regions indicates that the antioxidant activities in the methanolic extract are associated with the total contents of phenolic compounds and flavonoids; in the pulp, flavonoids have a highly positive and significant correlation with the content of total phenolic compounds (R = 0.99, p˂0.01), and the antioxidant activities are significantly and positively correlated with the content of total flavonoids and the contents of phenolic compounds (R = 0.83, p˂0.01). Similarly for almonds, the correlation is positive and significant of (R = 0.91, p˂0.01) and (R = 0.74, p˂0.01) respectively.
The results obtained show that the Tindouf region contains slightly more polyphenols than the other two. On the other hand, the argan kernel contains few polyphenols regardless of the origin, which reflects the low polyphenol content of argan oil. Moreover, if we compare our results with studies, such as those of El Monfalouti et al (Citation2010, Citation2013) and Badreddine (Citation2017) in Morocco; Dakiche (Citation2017), Djied (Citation2016) and Benaouf et al. (Citation2014) in Algeria.
This differentiation is well noted even in various parts of the fruit of the argan tree from one region to another. The latter may be the result of several factors: aridity and position are related to the bioclimatic stages (Saharan, arid, and semi-arid), pedological, geographical, pathological, genetic, and harvest periods.
Regarding the flowering and fruiting process of the tree, we found that the Tindouf argan tree in the Saharan stage (Kechairi, Citation2018). Unlike trees in the north, they bloom in late spring and then pollinate in early summer to remain dormant until the first fall of the fall rains to bear fruit and start growing for three seasons and fully mature at the same time with Tindouf trees, so their life cycle is longer. This explains the uneven difference in the richness of the content of the components of the fruit of the three regions.
Flavonoids are ubiquitous secondary metabolites of plants; they belong to the large group of polyphenols which are biologically and chemically very diverse. Several studies have already demonstrated the undeniable role of flavonoids as antioxidants. It is estimated that around 2% of the organic carbon photosynthesized by plants, or some 109 tonnes per year, is converted into flavonoids (Lhuillier et al., Citation2007). Flavonoids are considered important micronutrients since they can play antioxidant roles or have various biological properties (Milane, Citation2004). However, the nutritional quality and the systemic effects of flavonoids depend on their absorption from the digestive tract. Quercetin is regularly consumed by humans because it is the main flavonoid found in the diet (Tieppo et al., Citation2007). Their dietary intake is quite high compared to other dietary antioxidants like vitamins C and E (Fiorentino et al., Citation2007).
The results obtained show that the pulp is the part richest in flavonoids. In contrast, almonds contain minimal amounts of flavonoids compared to other parts of the argan fruit. As with phenolics, the Tindouf region contains more flavonoids. Indeed, these flavonoids play an important role in the protection of plants against various environmental attacks (Fahmi et al., Citation2013). In conclusion, the differences in the contents of phenolics and flavonoids obtained in the different parts of argan (almond, shell, and oil) in the three regions are largely due to the aforementioned environmental factors. The richness of the different parts in phenolics and flavonoids could be explained by the adaptation of the argan tree to Saharan, arid, and semi-arid climatic conditions, which increase the synthesis of phenolic compound to adapt to the climate. Plants have been shown to respond to environmental stimuli by synthesizing phenolic compounds that can protect them against various aggressions.
Conclusins
We conclude that the flowering and fruiting period, the climate and the origin affect the composition of fruit (primary metabolites: proteins, lipids, and sugars and secondary metabolites: phenolics, flavonoids, and the activity of antioxidants).
The pulp and almonds are very rich in proteins, fats, and sugars, and this is what allows this fruit to be their food source for ruminants like camels and goats in Tindouf and Adrar in the Algerian desert, where these animals digest the pulp and throw out the seeds with the waste, this facilitates the natural seeds germination processes of this tree. In addition to its lasting environmental benefits by resisting drought and climate change and adapting to any climate, our results have effectively shown that fruits from trees planted near the sea outside their original environment than we in Algeria have given the same wealth and therefore the same desired environmental benefits for humans and even animals.
Argan oil is rich in anti-oxidants and well positioned between nutrition and health. The phytochemical screening of the fruit has shown that the extracts prepared from this plant are rich in phenolics and flavonoids and that they are endowed with a good antioxidant power. Indeed, the argan oil extracted from this plant is extremely rich in fatty acids which gives it important antioxidant properties likely to attribute to this food a therapeutic interest in the context of the prevention of neurodegenerative deseases, Alzheimer, complications of menopause in women, inflammation, heart desease, bone fracturesfor example, in our region locally, it is used in alternative medicine to treat skin diseases, especially psoriasis and eczema, and is also suitable in the manufacture of cosmetic products (shampoo, toothpaste, and anti-wrinkle creams …).
Through our results, we discovered that the environment has a significant impact on the content and composition of fruits, when environmental conditions are harsher and drier as a form of adaptation and endurance; the tree produces greater quantities of polyphenolic compounds and antioxidants in order to protect itself and preserve its perenniality against any danger. Also, the flowering and fruiting (early and late fruit) also have an effect on the yield and significance of the fruits.
Highlights
A study of the productivity of the argan tree introduced in the north of Algeria (late fruit), through its flowering and fruiting, and compared to those endemic that grow in the southern desert (early fruit).
By comparing their content of primary metabolites (proteins, lipids, and sugars) and secondary metabolites (phenolics, flavonoids, and antioxidant activities).
Acknowledgments
The authors thank the SNV faculty at Mascara University and USTHB university for providing the necessary research facilities. Colleague Kechairi Reda, the people of Kada Kies and Mohamed Larbi.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Afnor. 1988. Compendium of French standards on fats, oilseeds, derived products. 4th ed. French Association of Standardization, Paris.
- AOAC International AOAC: Official Methods of Analysis. 1990 Vol. Volume 1.
- Badreddine, A. 2017. Preparation characterization of extracts of Argania spinosa and argan oil and evaluation of their neuroprotective effects invivo and invitro, Doctoral Thesis, Marocco university.
- Bani-Aameur, F., and S. Benlahbil. September 25 1999. Flowering and fruiting periods of argan (Argania spinosa (L. Skeels)). Conférence on Plant Taxonomy, Lisbonpp. 16–19.
- Benaouf, Z., F. Abiayyad, C. Jaradat, and G. Hamedi. 2014. The physicochemical characteristics of argan oil from two provenances in Algeria, Tindouf and Mostaganem. Master thesis, University of Mascara. 35–47.
- Benaouf, Z., and A. Miloudi .2017. Phenological study and contribution of mycorrhization on the growth of the argan tree (Argania spinosa (L.) Skeels) in Western Algeria., PhD thesis, USTHB university, Algeria, USTHB University.
- Benchettouh, A. 1999. Contribution to the study of mineralization, phenology and mycorrhization in the argan tree (Argania Spinosa (L) Skeels). Thesis Of Magister, University Of Mostaganem 12:45–52.
- Berrougui, M., C. Alvarez de Sotomayor, A. Perz-Guerrero, M. Attaib, E. Hmamouchi E, M.D. Marhuenda, Herrera, and M.D. Herrera. 2004. Argan (Argania spinosa) oil lowers blood pressure and improves endothelial dysfunction in spontaneously hypertensive rats. Br. J. Nutr. 92(6):921–929. doi: 10.1079/BJN20041293.
- Blois, M.S. 1958. Antioxidant determination by use of free radical stable. Nature 1958(181):199–1250.
- Boudy, P. 1950. Economie forestière nord-africaine (monographies et traitements des essences forestières. Tome II(1):Larousse. 382–416.
- Charrouf, Z., and D. Guillaume. 2007. Phenols and polyphenols from Argania spinosa. American J. Food Technol. 2(7):679–683. doi: 10.3923/ajft.2007.679.683.
- Dakiche, H. 2017. The argan tree: characterization of active ingredients and determination of biological and pharmacological activities, Doctoral Thesis, Algeria university.
- Derouiche, A., M. Cherki, A. Drissi, Y. Bamou, A.M. El Messal, A. Idrissi-Oudghiri, J.M. Lecerf, and A. Adlouni. 2005. Nutritional intervention study with argan oil in man: Effects on lipids and apolipoproteins. Ann. Nutr. Metab. 49(3):196–201. doi: 10.1159/000087072.
- Djied, S. 2016. Extraction, identification, and histolocalization of secondary metabolites in different organs of argan tree (Argania spinosa) Skeels of Algeria., PhD Thesis, Algeria university. 84–92.
- El Monfalouti, H. 2013. Contribution to the determination of photoprotector properties and anti-oxidants of argan derivatives: chemical and physiological studies, PhD Thesis, Marocco university
- El Monfalouti, H., D. Guillaume, C. Denhez, and Z. Charrouf. 2010. Therapeutic potential of argan oil- a review. J. Pharm. Pharmacol. 62(12):1669–1675. doi: 10.1111/j.2042-7158.2010.01190.x.
- Emonard, H., V. MARCQ, C. MIRAND, and W. HORNEBECK. 1999. Inhibition of gelatinase a by oleic acid. Ann. Ny. Acad. Sci. 878:647–649. doi: 10.1111/j.1749-6632.1999.tb07751.x.
- Fahmi, F., S. Tahrouch, and A. Hatimi. 2013. Geoclimatic influences on flavonoids contents of the leaves of the argan tree Influences géoclimatiques sur la composition en flavonoides des feuilles de l’arganier Argania spinosa. J. Mater. Environ. Sci 4(6):881–886.
- Ferradous, A., F. Bani-Aameur, and P. Dupuis .1996. Stationary climate, phenology and fructification of the argan tree (Argania spinosa L. Skeels), Proceedings of the Hassan II Agricultural and Veterinary Institute, Morocco, 17, 51–60.
- Fiorentino, A., B. D’Abrosca, S. Pacifico, A. Golino A, P. Monaco, P. Oriano, and P. Monaco. 2007. Reactive oxygen species scavenging activity of flavone glycosides from melilotus neapolitana. Molecules 12(2):263–270. doi: 10.3390/12020263.
- Folin, O., and V. Ciocalteu. 1927. On tyrosine and tryptophane determination in proteins. J. Biol. Chem. 73(2):627–650. doi: 10.1016/S0021-9258(18)84277-6.
- Gómez - Caravaca, A., M. Gómez- Romero, A. Arráez-Román, A. Segura-Carretero, and A. Fernández-Gutiérrez. 2006. Advances in the analysis of phenolic compound in products derived from bees. J. Pharm. And Biomed. Anal 41(4):1220–1234. doi: 10.1016/j.jpba.2006.03.002.
- Harhar, H., S. Gharby, B. Kartah, H. El Monfalouti, D. Guillaume, and Z. Charrouf. 2011. Influence of argan kernel roasting-time on virgin argan oil composition and oxidative stability. Plant Food For Human Nutri 66(2):163–168. doi: 10.1007/s11130-011-0220-x.
- Hilali, M., Z. Charrouf, A. Soulhi, L. Hachimi, and D. Guillaume. 2005. Influence of origin and extraction method on argan oil physicochemical characteristics and composition. J. Agric. Food Chem. 53(6):2081–2087. doi: 10.1021/jf040290t.
- Kechairi, R. 2009. Contribution to the ecological study of argan argania spinosa (L) skeels in the region of Tindouf (Algeria. Me. Magis. Uni. Of USTHB Algiers 11:14–17, 48.
- Kechairi, R. 2018. Study of the Tindouf argan grove: State of play, constraints and prospects for its development, PhD thesis, Telemcen university, Algeria.
- Kechairi, R., and F. Abdoun. 2016. État des lieux cartographiques de l’arganier Argania spinosa (L.) Skeels (Sapotaceae) en Afrique Nord-Occidentale (Algérie et Sahara Occidental). Int J Environ Stud 73(2):286–293. doi: 10.1080/00207233.2016.1148448.
- Khallouki, F., C. Younos, R. Soulimani, T. Oster, Z. Charrouf, B. Spiegelhalder, H. Bartsch, and R.W. Owen. 2003. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur. J. Cancer Prev. 12(1):67–75. doi: 10.1097/00008469-200302000-00011.
- Lhuillier, A., N. Fabre, F. Moyano, N. Martins, C. Claparols, I. Fourasté I, and C. Moulis. 2007. Comparison of flavonoid profiles of Agauria salicifolia (Ericaceae) by liquid chromatography-UV diode array detection–electrospray ionisation mass spectrometry. J. Chromatogr. A 1160(1–2):13–20. doi: 10.1016/j.chroma.2007.03.038.
- Metro, A. 1953. Observations préliminaires faites sur l’arganier à l’oued Cherrate et à Dar Askraoui en vue de la sélection généalogique. Ann. de la Recher. Fores. (Rebat). Rap. ann. 1952:101–115.
- M’hirit, O. 1998. The argan tree a multipurpose forest fruit tree, Pierre Mardaga Edit. Belgium. 41: 11–54.
- Milane, H. 2004.La quercétine et ses dérivés : molécules à caractère pro-oxydant ou capteurs de radicaux libres; études et applications thérapeutiques. Thèse de doctorat. Strasbourg.
- Miloudi, A. 2006. The physiological and biochemical responses of the argan tree (Argania spinosa L.Skeels) to natural abiotic factors. PhD Thesis, University of Oran ES SENIA. 1-2, 30–90.
- Montoya Olivier, J.M. 1984. El Argan, potential silvopastoral y de repoblacion en Espana. Ann. de l’institut Nation. d’I A SF. 16:141–152.
- Muanda, N., F.D. Koné, A. Dicko, R. Soulimani, and C. Younos. 2011. Phytochemical composition and antioxidant capacity of three Malian Medicinal plant parts. Evidence-Based Compl. and Alter. Med. 2011:1–8. doi: 10.1093/ecam/nep109.
- Rojas, L.B., S. Quideau, P. Pardon, and Z. Charrouf. 2005. Colorimetric evaluation of phenolic content and GC-MS characterization of phenolic composition of alimentary and cosmetic argan oil and press cake. J. Agric. Food Chem. 16(23):9122–9127. doi: 10.1021/jf051082j.
- Sies, H. 2010. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 501(1):2–5. doi: 10.1016/j.abb.2010.04.006.
- Taleuzzaman, M., S. Ali, and S. Gilani. 2015. Ultra performance liquid chromatography a review Austin. J. Anal. Pharm. Chem 2(n°6):1056.
- Tieppo, J., R. Vercelino, A.S. Dias, M.F. Silva Vaz, J.N. Picada, C.A. Marroni, N.P. Marroni, J.A.P. Henriques, and J.N. Picada. 2007. Evaluation of the protective effects of quercetin in the hepatopulmonary syndrome. Food Chem. Toxicol. 45(7):1140–1146. doi: 10.1016/j.fct.2006.12.020.
- Zheng, R., Y. Shi, Z. Jia, C. Zhao, Q. Zhang, and X. Tan. 2010. Fast repair of DNA radicals. Chem Soc Rev 39(8):2827–2834. doi: 10.1039/b924875g.