Abstract
Conventional cancer therapies often rely on genotoxic-stress, which kills tumor cells via caspase-dependent apoptosis or mitotic catastrophe. Metabolic stress, which exploits the different metabolic requirements between tumor and normal cells and selectively starves the tumor cells to death, emerges as a new therapeutic approach to circumvent conventional drug resistance in recent years. Arginine, a critical amino acid in the biosynthesis of proteins, nitric oxide, and polyamine, is considered a semi-essential amino acid, because of the intrinsic ability of normal cells to synthesize arginine from citrulline. Many tumor cells, including prostate cancer cells, have defects in this pathway, due to the selective, often epigenetic, suppression of the expression of ASS1 (argininosuccinate synthase 1). As a result, prostate cancer cells are addicted to external arginine, and can be selectively killed by arginine deprivation. For therapeutic applications, arginine-metabolizing enzymes such as arginase, or more recently ADI (arginine deiminase), have been employed. ADI-PEG20 (pegylated ADI) shows significant specificity toward tumor cells, with a very good safety profile and is undergoing phase III clinical trials for hepatocellular carcinoma. We have been studying the cell-killing mechanisms associated with ADI-treatment and found several unusual features. When CWR22Rv1 prostate cancer cells lacking ASS1 are treated with ADI-PEG20, the massive cell death is caspase-independent and delayed until 72 h post treatment. Autophagy, with regular-sized autophagosomes, is potently induced during the first 24 h and then attenuated. At 48 h afterwards, a second phase of autophagy with large autophagosomes follows. We showed previously that autophagy during the initial autophagic phase serves a protective role, since blockade of early autophagy hastens the cell death.
At later time points (96 and 120 h), autophagy appears to contribute to cell death. A fraction of autophagosomes and autolysosomes becomes enlarged with sizes up to 20X that of the regular ones (). Strikingly, these mega-autophagosomes often contain DAPI or DRAQ5-stained materials, suggesting the presence of DNA. Immunohistochemical studies reveal the presence of histones and XRCC6/KU70-KRCC5/KU80 as well, indicating that the cargo is not just naked DNA, but also includes chromatin. We thus referred to it as chromatin-autophagy or chromatophagy. This process is different from apoptosis and other selective forms of autophagy. Chromatophagy distinguishes itself from apoptosis in that (1) leaked DNA species are not encased by plasma membrane, (2) the nucleus is not fractured, (3) the cell and plasma membrane are alive and intact, and (4) cell morphology also appears to be normal and the cell is able to maintain proper adhesion. Piecemeal microautophagy of the nucleus (PMN) also differs from chromatophagy because PMN often occurs in lower eukaryotic cells and degradation of nuclear cargo is usually through nuclear-vacuole junctions and does not involve the formation of an autophagosome. The chromatophagy phenotype also varies from micronuclei (MN), as MN are fully enclosed by the nuclear membrane, are usually significantly smaller in size, and again their formation does not involve autophagosomes. Based on both high-resolution electron microscopy and fluorescence microscopy, chromatophagy is maintained via a double-membraned structure, derived from fusion between an autophagosome membrane and the nuclear membrane. Consistent with this notion is the detection of NUP98 (nucleoporin 98kDa), a nuclear outer membrane protein, on the membrane of chromatin-containing autophagosomes. Using genetic inhibitors (shRNAs targeting ATG5 and BECN1) and a pharmacological inhibitor (3-methyladenine), we showed that the nuclear DNA leakage phenotype depends on autophagosome formation, suggesting that autophagy plays an active role in “extracting” damaged chromatin from the nucleus. Based on the structure of LMNA (lamin A/C), the nucleus remains largely intact, except at the interface where autophagy occurs, and the lamin structure appears to be locally interrupted. In the latter regard, it is also different from nucleophagy.
Figure 1. Summary of chromatophagy inducer, mediators, and unique features. Prolonged arginine starvation of prostate cancer cells induces excessive autophagy, leading to giant aggregates of autophagosomes/autolysosomes. In addition, it causes mitochondrial dysfunction, and ROS generation, eventually resulting in nuclear DNA damage and membrane remodeling. Fusion of autophagosomes/autolysosomes with nuclear membranes allows broken chromatin to be captured directly by the fused autophagic vesicles. The immunofluorescence image shows leaked chromatin/DNA (blue) being captured by a giant autophagosome (green). Autophagosomes are visualized with GFP-LC3 (green) and lysosomes are labeled with LysoTracker (red).
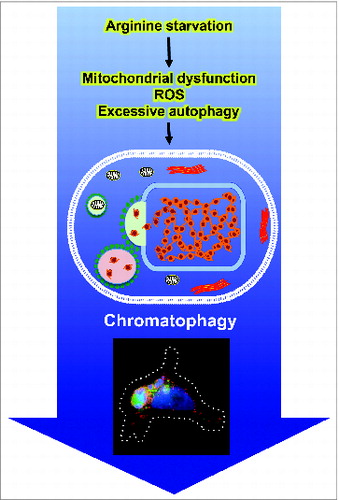
How was the leaked chromatin generated? Our studies showed that an elevated level of ROS (reactive oxygen species) is involved, and N-acetylcysteine (NAC), an ROS scavenger, attenuates this phenotype. The consequences of excessive ROS include nuclear DNA and membrane damage, which are both observed in chromatophagy. Based on the appearance of DNA damage markers such as γ-H2AFX and autophagy blockade studies, we found that the accumulation of γ-H2AFX is not affected by the autophagic process. Thus, arginine deprivation induces 2 parallel tracks leading to chromatophagy: one involves generation of ROS and DNA damage, and the other, excessive autophagy and nuclear membrane rupture. These processes, however, may not be completely independent, as ROS may modulate autophagy and vice versa. Previously, we showed that arginine deprivation activates AMPK and attenuates MTOR signals, which are likely to contribute to autophagy induction. A question then is how arginine deprivation induces ROS. At this juncture, we do not exactly know. What we observed is the general downregulation at the transcript level of nuclear genes involved in mitochondria functions including components of the respiratory complex. This results in the nearly complete impairment at late time points of electron transport and oxidative phosphorylation, a likely cause of ROS generation. A model depicts the sequence of events leading to chromatophagy during arginine starvation is shown in .
Chromatophagy can be induced by ADI as well as media lacking arginine, indicating it is caused by starvation of arginine, but not off-target effects of ADI. Chromatophagy is observed in other cell lines such as PC3 (prostate), UMUC3 (urinary bladder), and Mia (pancreas) and therefore, is not a peculiarity of CWR22Rv1 cells. In the case of Mia and UMUC3, on the one hand, chromatophagy occurs in the presence of caspase-dependent apoptosis, and thus these 2 processes are not mutually exclusive. On the other hand, not all autophagy-inducing agents (e.g., rapamycin) are capable of inducing chromatophagy, and not all cell lines respond to arginine deprivation with chromatophagy. In an earlier study, we showed that in breast cancer MB-MDA-231 cells, arginine deprivation similarly induces mitochondrial dysfunction and ROS generation, resulting in mitophagy, and DNA-leakage at later time points.
In summary, chromatophagy appears to be a new cell-killing mechanism induced by a combination of excessive autophagy and ROS. Agents that induce chromatophagy would synergize well with conventional therapies relying on caspase-dependent apoptosis, and may help overcome treatment resistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
