ABSTRACT
Septins are cytoskeletal proteins implicated in cytokinesis and host-pathogen interactions. During macroautophagy/autophagy of Shigella flexneri, septins assemble into cage-like structures to entrap actin-polymerizing bacteria and restrict their dissemination. How septins assemble to entrap bacteria is not fully known. We discovered that mitochondria support septin cage assembly to promote autophagy of Shigella. Consistent with roles for the cytoskeleton in mitochondrial dynamics, we showed that DNM1L/DRP1 (dynamin 1 like) can interact with septins to enhance mitochondrial fission. Remarkably, Shigella fragment mitochondria and escape from septin cage entrapment in order to avoid autophagy. These results uncover a close relationship between mitochondria and septin assembly, and identify a new role for mitochondria in bacterial autophagy.
Shigella flexneri is a Gram-negative enteroinvasive bacterial pathogen used as a paradigm to study bacterial autophagy. Autophagy of Shigella relies on receptor proteins SQSTM1/p62 and CALCOCO2/NDP52 that recognize ubiquitinated substrates or damaged cellular membrane surrounding cytosolic bacteria, thereby recruiting autophagy-related (ATG) proteins for autophagosome formation. To escape from autophagy, Shigella express IcsA on the bacterial membrane to recruit WASL/N-WASP and activate the ARP2/3 complex, allowing the pathogen to form actin tails and spread from cell-to-cell. However, IcsA is recognized by ATG5, a protein necessary for autophagosome maturation. To counteract ATG5 recognition, Shigella secretes IcsB to camouflage IcsA and enable efficient actin tail polymerization.
Septins, a highly conserved family of GTP-binding proteins, are a unique component of the cytoskeleton. Septin subunits form hetero-oligomeric complexes that assemble into nonpolar filaments and rings. By associating with cellular membranes and actin filaments, septins are implicated in a variety of cellular processes including cytokinesis and host-pathogen interactions. In the case of host cell invasion by Shigella, septins form cage-like structures around actin-polymerizing bacteria to promote their targeting to autophagy. Whether septin-mediated autophagy influences Shigella proliferation was unknown. Using bacteria engineered to conditionally express fluorescent reporters dependent upon metabolic activity, we showed that host cells employ septin cages and SQSTM1-mediated autophagy to restrict bacterial proliferation.
Previous work has shown that septin cage formation is dependent on actin polymerization and autophagy markers SQSTM1, CALCOCO2, ATG5, ATG6, and ATG7. To identify novel host factors underpinning septin cage assembly, immunoprecipitation and mass spectrometry was performed on Shigella-infected HeLa cells expressing STREP-FLAG-tagged SEPT6. From this approach, 56 proteins associated with Shigella-septin cages were identified including SEPT2, SEPT6, SEPT7, SEPT9, and SEPT11 (i.e., septins expressed in HeLa cells), as well as autophagy markers SQSTM1 and LC3B. Unexpectedly, 21% of identified proteins were annotated as exclusively mitochondrial, suggesting a link between mitochondria and septin cage assembly. Consistent with this, ∼80% of septin cages entrapping cytosolic Shigella were closely associated with mitochondria. Correlative light electron microscopy and live-cell imaging indicated that mitochondria support septin cage assembly around cytosolic Shigella. To test this, we infected cells depleted of DNM1L, a cytosolic GTPase that mediates mitochondrial fission, or MFN1 (mitofusin 1), a transmembrane GTPase that mediates mitochondrial fusion. In DNM1L-depleted cells where mitochondria are elongated and more membrane is available for septin assembly, we observed a significant increase in the number of Shigella-septin cages. Conversely, in MFN1-depleted cells where mitochondria are fragmented and less membrane is available for septin assembly, we observed a significant reduction in the number of Shigella-septin cages. Together, these results clearly demonstrate a role for mitochondria in septin cage assembly.
Actin and non-muscle myosin II form the contractile ring during cytokinesis, and also enable DNM1L-mediated mitochondrial fission in a process called mitokinesis. Given that septins are associated with the contractile ring during cytokinesis and interact with mitochondria during septin cage assembly, we tested the role of septins in mitochondrial fission. In septin-depleted cells, the recruitment of DNM1L to mitochondrial membrane is significantly reduced and the length of mitochondria is significantly increased. In agreement with a role for septins in DNM1L-mediated mitochondrial fission, a variety of high-resolution microscopy techniques showed that septins localize to the sites of DNM1L-mediated mitochondrial fission and immunoprecipitation experiments revealed SEPT6-DNM1L interaction. Future experiments designed to study interactions between septins and other factors that promote mitochondrial fission, such as the endoplasmic reticulum (ER), will be of great interest to help determine a precise role for septins in this process.
During invasion of host cells, Shigella is recognized to locally induce mitochondrial fission for intracellular survival. Given the importance of mitochondria in the assembly of septin cages around Shigella, we tested if bacteria could fragment mitochondria to avoid septin cage entrapment. Indeed, mitochondria neighboring septin-cage entrapped Shigella are significantly longer than mitochondria neighboring intracellular Shigella lacking septin cages. Consistent with roles for the cytoskeleton in mitochondrial fission, Shigella-mediated mitochondrial fission is dependent on IcsA-mediated actin polymerization. Taken together, these results indicate that actin-polymerizing Shigella fragment mitochondria to counteract septin cage formation. It is not yet known if bacteria can employ septins to recruit DNM1L during IcsA-mediated actin polymerization, or if mitochondrial fission induced by actin-polymerizing Shigella relies on a mechanism independent of septins and DNM1L.
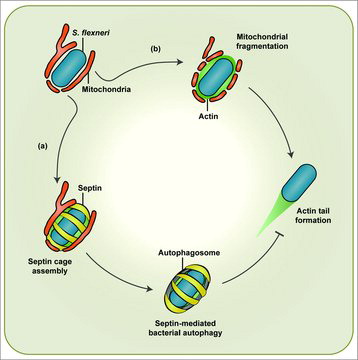
In summary, the use of Shigella to study bacterial autophagy has uncovered new links between mitochondria and septin assembly (). Interactions between mitochondria and the cytoskeleton have been increasingly recognized, and future investigation of mitochondria-cytoskeleton interactions in vitro and in vivo will provide a better understanding of fundamental processes underlying mitochondrial dynamics and autophagy.
Figure 1. Shigella-mitochondria interplay mediates septin cage or actin tail formation. In the cytosol mitochondria promote septin cage assembly around actin-polymerizing Shigella flexneri for clearance by autophagy (a). To counteract septin cage assembly S. flexneri fragments mitochondria in an IcsA-dependent manner (b). In this way, bacteria form actin tails for cell-to-cell spread.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
