ABSTRACT
Lysosomes serve as the degradation hubs for macroautophagic/autophagic and endocytic components, thus maintaining cellular homeostasis essential for neuronal survival and function. LAMP1 (lysosomal associated membrane protein 1) and LAMP2 are distributed among autophagic and endolysosomal organelles. Despite widespread distribution, LAMP1 is routinely used as a lysosome marker and LAMP1-positive organelles are often referred to as lysosomal compartments. By applying immuno-electron microscopy (iTEM) and confocal imaging combined with Airyscan microscopy, we expand on the limited literature to provide a comprehensive and quantitative analysis of LAMP1 distribution in various autophagic and endolysosomal organelles in neurons. Our study demonstrates that a significant portion of LAMP1-labeled organelles lack major lysosomal hydrolases. BSA-gold pulse-chase assay further shows heterogeneous degradative capacities of LAMP1-labled organelles. In addition, LAMP1 intensity is not a sensitive readout to assess lysosomal deficits in familial amyotrophic lateral sclerosis-linked motor neurons in vivo. Our study thus calls for caution when interpreting LAMP1-labeled organelles in the nervous system where LAMP1 intensity, trafficking, and distribution do not necessarily represent degradative lysosomes or autolysosomes under physiological and pathological conditions.
Abbreviations: ALS: amyotrophic lateral sclerosis; BSA: bovine serum albumin; DRG: dorsal root ganglion; IGF2R/CI-M6PR: insulin like growth factor 2 receptor; iTEM: immuno-transmission electron microscopy; LAMP1/2: lysosomal associated membrane protein 1/2; P80: postnatal day 80; sMNs: spinal motor neurons
Although LAMP1 and LAMP2 target to lysosomes, they are not static components of degradative lysosomal membranes. Rather, newly synthesized LAMP1/2 exit the trans-Golgi network and enter the plasma membrane and endo-lysosomal pathway, where they are in dynamic equilibrium between endosomes, lysosomes, amphisomes, and autolysosomes. Neurons are highly-polarized cells with complex dendritic arbors and an extended long axon. To achieve effective degradation, endocytic and autophagic organelles undergo long-distance retrograde transport from distal regions toward the soma, where mature lysosomes are enriched. These unique structural features and transport dynamics add another layer of complexity to maturation of neuronal lysosomes. Impaired autophagic-lysosomal or endolysosomal trafficking causes an aberrant accumulation of immature organelles in axons, leading to reduced proteolytic capability associated with major neurodegenerative diseases. Similar phenotypes also manifest in hereditary lysosomal storage diseases that affect the nervous system. With these clinical implications, there is growing interest in understanding the causal link between impaired autophagic and lysosomal systems and disease progression. The current practice of using LAMP1/2 as lysosome markers leads to misinterpretation of the actual roles of degradative lysosomes or autolysosomes in neuronal growth, function, and survival in the healthy brain, and their pathological impact on neurodegenerative diseases. Thus, there is an urgent need to comprehensively and quantitatively characterize LAMP1-labeled degradative and nondegradative organelles in in vitro and in vivo nervous systems. In our recent study [Citation1], we aimed to address 2 fundamental questions: (1) whether neuronal LAMP1-labeled organelles contain major lysosomal hydrolases and function as degradative lysosomes; and (2) whether altered LAMP1 intensity in diseased neurons represents a sensitive indicator for lysosomal deficits or lysosomal response to pathological conditions.
A mature degradative lysosome is defined as: (A) a storage organelle for active forms of degradative enzymes with acidic pH optimum, allowing substrate degradation; (B) limiting membranes with specific glycosylated membrane-associated proteins such as LAMP1/2; and (C) lacking non-lysosomal proteins such as IGF2R/CI-M6PR (insulin like growth factor 2 receptor). Because one prerequisite for a degradative lysosome is the presence of hydrolases, we first chose to label lysosomes for CTSD (cathepsin D), an aspartyl protease that represents a major category of lysosomal hydrolases. We investigated the distribution and colocalization of LAMP1 and CTSD in mouse dorsal root ganglions (DRGs), because its axon bundle structures make it an ideal model to trace axonal organelles in vivo. Surprisingly, we found differential distribution patterns of LAMP1 and CTSD in DRG axons, where a large portion of LAMP1-labeled organelles lack CTSD staining.
We confirmed the light imaging results at the ultrastructural level by iTEM. In mouse spinal motor neurons (sMNs) at age postnatal day 80 (P80), LAMP1 resides on the surface of various organelles, including lysosomes, autolysosomes, endosomes, multivesicular bodies, and multilamellar bodies. In contrast, CTSD was only detected in the lumenal side of lysosomes or autolysosomes, but rarely found in autophagosomes and endocytic organelles. Next, we performed a BSA pulse-chase assay combined with LAMP1-iTEM to examine the degradative capacity of LAMP1-labeled organelles. BSA conjugated with colloidal gold (BSA-gold) is internalized after fluid phase endocytosis, and gold particles would flocculate in the lumen of mature lysosomes, where proteolytic degradation of BSA occurs, but should show a discrete pattern in the lumen of nondegradative lysosomes. This study revealed that a sizable portion of LAMP1-labeled organelles with discrete BSA-gold, indicating a lack of degradative capacity.
To provide a comprehensive and quantitative analysis of LAMP1-positive degradative and nondegradative organelles in neurons, we (1) examined 2 types of cultured neurons: mouse embryonic cortical neurons and adult DRG neurons; (2) characterized LAMP1 in 3 neuronal compartments: cell body, dendrite and axon; (3) applied 2 types of colocalization analysis: Mander’s colocalization coefficient-based and particle-based analysis; and (4) used a modified fixation method (50% Bouin’s) to detect lysosomal lumenal hydrolases; this modified fixation allows for optimal co-detection of both lumenal hydrolases and membrane proteins and significantly enhances CTSD signals by ~ 3 fold compared to 4% paraformaldehyde fixation in neurons. We found a significant portion of LAMP1-positive organelles that are devoid of CTSD. In the soma, 44.8 ± 1.87% of LAMP1-labeled organelles contain CTSD. This number further drops to 28.6 ± 2.07% in dendrites and 30.31 ± 2.39% in axons. The Airyscan super-resolution microscopy showed LAMP1 decorating the limiting membrane of the organelle where a large portion are without CTSD staining. By triple-staining with RAB7 or IGF2R, markers for late endosomes, we showed that some LAMP1-positive, CTSD-negative organelles are late endosomes in nature. By co-staining cortical neurons with BODIPY-pepstatin A, that specifically binds active CTSD in acidic environments, we showed that the majority of LAMP1 organelles co-labeled with IGF2R lack degradative capacity.
We further revealed that a significant portion of LAMP1-labeled organelles do not contain 2 other major lysosomal hydrolases, CTSB and GBA. LAMP2 also shared a similar distribution pattern as LAMP1. In addition, we provided quantitative evidence indicating a heterogeneous population of LAMP1-labeled organelles across the endolysosomal pathway (), including early/late endosomes, and immature lysosomes. Percoll gradient fractionation displays differential distribution patterns of LAMP1/2 and CTSD/B in cortical neurons; while LAMP1/2 are widely present in most fractions, both CTSD and CTSB are mainly restricted to the heavier gradient fractions. Thus, our studies with 3 major lysosomal hydrolases consistently support the notion that a significant portion of LAMP1/2-labeled organelles do not contain detectable lysosomal hydrolases.
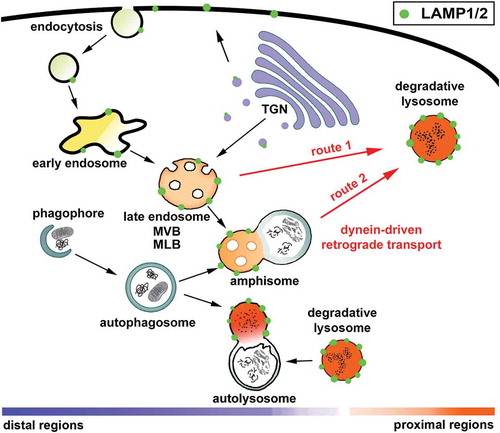
Figure 1. LAMP1/2 are distributed among a heterogenous population of autophagic and endolysosomal organelles. Lysosomes serve as the degradation hubs for autophagic and endocytic components. Newly synthesized LAMP1/2 exit the trans-Golgi network and enter the plasma membrane and endolysosomal pathway, where they are in dynamic equilibrium between endosomes, lysosomes, amphisomes, and autolysosomes. Endolysosomal trafficking, from early endosomes to late endosomes and finally into mature lysosomes (route 1), is essential for delivering endocytosed materials for degradation. Autophagosomes deliver bulk cytoplasmic components and damaged organelles to lysosomes through a stepwise maturation by fusing with endosomes into intermediate amphisomes or with lysosomes into degradative autolysosomes (route 2). To achieve effective degradation, both endocytic and autophagic organelles undergo dynein-driven retrograde transport from distal regions toward the soma, where degradative lysosomes are relatively enriched. MLB, multilamellar body; MVB, multivesicular body.
We next asked whether LAMP1 is a sensitive marker for assessing lysosomal defects in neurodegenerative diseases. Our previous study revealed progressive lysosomal deficits in sMNs of the familial amyotrophic lateral sclerosis-linked HsSOD1G93A mouse starting at an early, asymptomatic stage (P40). We co-immunostained ventral root sMNs of both WT and HsSOD1G93A mice at P80 with antibodies against LAMP1, CTSD, and RBFOX3/NeuN. Compared to WT sMNs, CTSD mean intensity is dramatically reduced (54.86 ± 7.59%, p < 0.001) in HsSOD1G93A sMNs. In contrast, there is no detectable change in LAMP1 mean intensity in the same HsSOD1G93A sMNs.
Our study indicates that LAMP1/2 are neither specific markers to assess lysosome distribution and trafficking, nor sensitive indicators to reveal the pathological response of the autophagy-lysosome system in some neurodegenerative diseases. We suggest that labeling a set of lysosomal hydrolases combined with various autophagic and endolysosomal markers would be more accurate than simply relying on LAMP1/2 staining to assess neuronal lysosome distribution and trafficking under physiological and pathological conditions. Therefore, our study provides a practical guideline for correctly labeling degradative lysosomes and autolysosomes in nervous systems in vitro and in vivo, thus advancing our understanding of how autophagy-lysosome functionality contributes to neuronal health and disease progression.
Acknowledgments
This work is supported by the Intramural Research Program of NINDS, NIH ZIA NS003029 (Z-H. Sheng) and ZIA NS002946 (Z-H. Sheng).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cheng X-T, Xie Y, Zhou B, et al. Characterization of LAMP1-labeled non-degradative lysosomal and endocytic compartments in nervous systems. J Cell Biol. 2018 April 25. (Epub ahead of print).
