ABSTRACT
Ferroptosis is a form of regulated cell death caused by iron accumulation and oxidative injury. BECN1 is a key regulator of macroautophagy/autophagy, a catabolic process of degradation induced by starvation or other stressors. Our recent findings reveal a novel alternative mechanism by which BECN1 can promote ferroptosis through the regulation of activity of the cysteine and glutamate antiporter system xc− in cancer cells. BECN1-dependent autophagy requires the formation of the BECN1-containing class III phosphatidylinositol 3-kinase (PtdIns3K) complex, whereas BECN1-dependent ferroptosis requires the formation of a BECN1-SLC7A11 complex. Furthermore, AMP-activated protein kinase (AMPK) is required for BECN1 phosphorylation to trigger formation of the BECN1-SLC7A11 complex in the process of inhibiting system xc− activity and inducing lipid peroxidation. These findings suggest that the autophagy-dependent and -independent functions of BECN1 play distinct roles in regulated cell death.
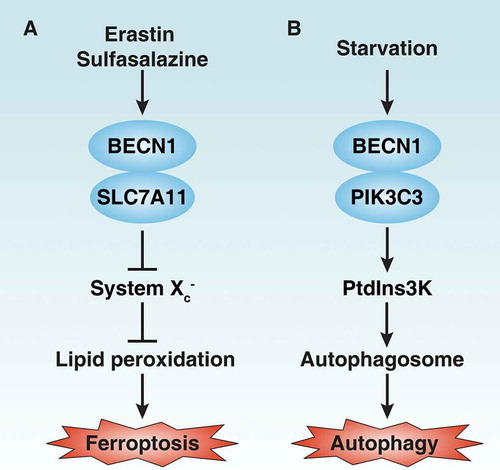
Autophagy is an evolutionarily conserved, self-degradative process that is used to either promote survival or induce death in response to various stresses. BECN1 (Vps30/Atg6 in yeast) is a well-known regulator of autophagy that is involved in the generation of the PtdIns3K complex involved in activating autophagy. Recently, we demonstrated that BECN1 is a critical regulator of ferroptosis, which is independent of the formation of the PtdIns3K complex.
BECN1 promotes system xc− inhibitor-induced ferroptosis
Ferroptosis is a non-apoptotic form of cell death that can be triggered by small molecules targeting the antioxidant system or enzymes such as system xc− and GPX4 (glutathione peroxidase 4). To determine the role of BECN1 in ferroptosis, we treated cancer cells such as HCT116, CX-1, and HT1080 with system xc− inhibitors (e.g., erastin, sulfasalazine, and sorafenib), GPX4 inhibitors (e.g., RSL3 and FIN56) or a GSS (glutathione synthetase) inhibitor (e.g., buthionine sulfoximine) [Citation1]. Among them, BECN1 expression only affects system xc− inhibitor-induced ferroptosis. Knockdown of Becn1 by RNA interference (RNAi) blocks ferroptosis, whereas knockin of Becn1 by gene transfection promotes ferroptosis in cancer cells in response to erastin, sulfasalazine, and sorafenib. In contrast, suppression of Becn2 (a paralog of BECN1) expression by RNAi does not affect erastin-, sulfasalazine-, or sorafenib-induced ferroptosis, suggesting BECN1, but not BECN2, is required for system xc− inhibitor-induced ferroptosis.
Major biochemical events leading to ferroptosis include iron accumulation and lipid peroxidation. Our data demonstrate that BECN1 does not affect intracellular iron accumulation or iron metabolism-associated expression. In contrast, BECN1 promotes lipid peroxidation, assessed by means of the fluorescent biosensor C11-BODIPY or by quantifying the oxidative stress marker malondialdehyde. These findings suggest that BECN1 promotes ferroptosis through regulation of lipid peroxidation.
Promotion of ferroptosis by BECN1 is ptdins3k-independent
We previously demonstrated that ferroptosis is a process of autophagic cell death, which requires ATG5 (autophagy related 5; part of an E3-like ligase essential for the lipidation of members of the MAP1LC3 [microtubule associated protein 1 light chain 3], or GABARAP [GABA type A receptor-associated protein] families) and NCOA4 (nuclear receptor coactivator 4; a cargo receptor-mediating FT/ferritin degradation via selective ferritinophagy). Knockdown of Atg5 inhibits erastin-induced conversion of MAP1LC3B-I to -II, as determined by immunoblot analysis. Furthermore, knockdown of NCOA4 blocks FT/ferritin degradation and suppresses ferroptosis. In contrast, knockdown or knockin of Becn1 does not affect the formation of lipidated MAP1LC3B and MAP1LC3B-positive puncta in ferroptosis. As a positive control, knockdown of Becn1 significantly blocks starvation-induced conversion of MAP1LC3B-I to -II. Importantly, the formation of a BECN1-PtdIns3K complex is only observed in cancer cells in response to starvation, but not ferroptotic stimulus. These findings indicate that BECN1 may play a different role in the regulation of ferroptosis compared to starvation-induced autophagy.
BECN1 promotes ferroptosis through inhibition of system xc- activity
System xc−, an antiporter expressed in cell membranes, imports cystine and exports glutamate to sustain anti-oxidant defenses in vitro or in vivo. SLC7A11 (solute carrier family 7 member 11) is the major component of system xc−. An increase in SLC7A11 expression often reflects an enhancement of cystine transport in ferroptosis. However, alteration of BECN1 does not affect the expression of SLC7A11. In contrast, a glutamate release assay revealed that the activity of system Xc− is significantly blocked in BECN1-overexpressing cells, whereas it is increased in BECN1-knockdown cells resulting in the induction of ferroptosis. Remarkably, immunoprecipitation analysis shows that BECN1 binds to SLC7A11 during ferroptosis, but not as part of starvation-induced autophagy. Indeed, the levels of the BECN1-SLC7A11 complex determine ferroptosis sensitivity and resistance in cancer cells, suggesting that BECN1 is a new regulator of system xc−.
AMPK-mediated BECN1 phosphorylation promotes ferroptosis
The function of the ATG proteins including BECN1 is modulated by posttranslational modifications, especially phosphorylation. Our protein domain mutation analysis indicated that amino acids 1–150 of BECN1 are required for BECN1-SLC7A11 complex formation and lipid peroxidation during ferroptosis. Furthermore, immunoprecipitation analysis shows that only the S90,93,96A mutant (but not any other Ser/Thr mutants in amino acids 1–150 of BECN1) limits the binding of BECN1 to SLC7A11 and malondialdehyde production, suggesting that the phosphorylation of BECN1 at S90, S93 and S96 contributes to BECN1-SLC7A11 complex formation and subsequent lipid peroxidation that occurs as part of ferroptosis.
AMPK is a central regulator of energy metabolism. AMPK-mediated BECN1 phosphorylation at S90 and S93 not only promotes autophagy and cell trafficking, but also induces ferroptosis. Genetic or pharmacological blocking of PRKAA/AMPKα by siRNA or by compound C suppresses BECN1 phosphorylation, BECN1-SLC7A11 complex formation, and subsequent lipid peroxidation during ferroptosis. However, the exact signal transduction mechanism by which BECN1 phosphorylation promotes ferroptosis in cancer cells has not been clearly defined, although the ferroptotic and autophagic response machineries share common AMPK pathways.
In summary, we provide the first evidence showing that BECN1 is involved in the regulation of ferroptosis via directly blocking system xc− activity. This pathway is different from the previously identified function of BECN1 in the regulation of autophagy via directly promoting PtdIns3K activity (). Our study further suggests that manipulating the BECN1 pathway may improve anticancer therapy via the induction of ferroptosis.
Acknowledgments
We thank Christine Burr (Department of Surgery, University of Pittsburgh) for her critical reading of the manuscript. This work was supported by grants from the US National Institutes of Health (R01GM115366, R01CA160417, R01CA211070, R01GM127791, and R01GM053396), the Natural Science Foundation of Guangdong Province (2016A030308011), the American Cancer Society (Research Scholar Grant RSG-16-014-01-CDD), the National Natural Science Foundation of China (31671435, 81400132, and 81772508), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2017).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Song X, Zhu S, Chen P, et al. AMPK-Mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system X(c)(-) activity. Curr Biol. 2018 Aug 6;28(15):2388–2399.e5. Epub 2018 Jul 26. PubMed PMID: 30057310.