ABSTRACT
Lysosomal damage activates AMPK, a regulator of macroautophagy/autophagy and metabolism, and elicits a strong ubiquitination response. Here we show that the cytosolic lectin LGALS9 detects lysosomal membrane breach by binding to lumenal glycoepitopes, and directs both the ubiquitination response and AMPK activation. Proteomic analyses have revealed increased LGALS9 association with lysosomes, and concomitant changes in LGALS9 interactions with its newly identified partners that control ubiquitination-deubiquitination processes. An LGALS9-inetractor, deubiquitinase USP9X, dissociates from damaged lysosomes upon recognition of lumenal glycans by LGALS9. USP9X’s departure from lysosomes promotes K63 ubiquitination and stimulation of MAP3K7/TAK1, an upstream kinase and activator of AMPK hitherto orphaned for a precise physiological function. Ubiquitin-activated MAP3K7/TAK1 controls AMPK specifically during lysosomal injury, caused by a spectrum of membrane-damaging or -permeabilizing agents, including silica crystals, the intracellular pathogen Mycobacterium tuberculosis, TNFSF10/TRAIL signaling, and the anti-diabetes drugs metformin. The LGALS9-ubiquitin system activating AMPK represents a novel signal transduction system contributing to various physiological outputs that are under the control of AMPK, including autophagy, MTOR, lysosomal maintenance and biogenesis, immunity, defense against microbes, and metabolic reprograming.
Abbreviations
AMPK: AMP-activated protein kinase; APEX2: engineered ascorbate peroxidase 2; ATG13: autophagy related 13; ATG16L1: autophagy related 16 like 1; BMMs: bone marrow-derived macrophages; CAMKK2: calcium/calmodulin dependent protein kinase kinase 2; DUB: deubiquitinase; GPN: glycyl-L-phenylalanine 2-naphthylamide; LLOMe: L-leucyl-L-leucine methyl ester; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MAP3K7/TAK1: mitogen-activated protein kinase kinase kinase 7; MERIT: membrane repair, removal and replacement; MTOR: mechanistic target of rapamycin kinase; STK11/LKB1: serine/threonine kinase 11; TNFSF10/TRAIL: TNF superfamily member 10; USP9X: ubiquitin specific peptidase 9 X-linked
Endomembrane damage, especially lysosomal damage, is a threat to cellular function and survival. Lysosomal damage profoundly affects MTOR, which normally promotes anabolic processes, and AMPK, which marshals catabolic activities. Coordination between MTOR and AMPK balances nutrient and energy status, and equilibrates various cellular pathways, including autophagy. AMPK and MTOR act as gas and brake pedals for autophagy, respectively. Galectins are sensors that set off upon lysosomal injury the multi-tiered cellular response termed MERIT, orchestrating an ESCRT-dependent lysosomal repair, autophagy-mediated lysosomal removal, and TFEB-driven biogenesis and replacement of injured lysosomes. MTOR is negatively controlled by LGALS8 (galectin 8) whereas AMPK is positively controlled by LGALS9 (galectin 9). In addition to galectins, ubiquitin influences autophagy to clear the injured lysosomes, a process known as lysophagy. Whether the ubiquitin response and the galectin system are interlinked during lysosomal damage is unknown.
Here, we tested whether galectins affect the ubiquitin response during lysosomal damage. The ubiquitin response is reduced in the absence of LGALS9 during lysosomal damage with LLOMe, GPN or silica. To understand how LGALS9 regulates ubiquitination, we have employed the proteomic proximity biotinylation strategy using chromosomally integrated APEX2-LGALS9. In cells with damaged lysosomes, APEX2-LGALS9 is found in proximity to the lysosomal proteins LAMP1 and LAMP2, reflecting accumulation of LGALS9 on damaged lysosomes confirmed by microscopy, immunoblotting of LysoIP preparations, and BioWeb assay. This depends on R65 and R239 residues that endow LGALS9 with the capacity to bind β-galactoside glycans. WT LGALS9 can rescue the loss of ubiquitination response in LGALS9 KO cells during lysosomal damage, whereas mutant LGALS9R65A,R239A cannot.
The proteomic analyses with APEX2-LGALS9 have singled out among a number of ubiquitin transaction enzymes (ubiquitin ligases and deubiquitinases/DUBs) the DUB USP9X as showing a reduction in association with LGALS9 during lysosomal injury. The dissociation between LGALS9 and USP9X depends on the glycan-recognition ability of LGALS9, thus representing a signal transduction of the recognition of the lumenal glycans by LGALS9 transmitted to USP9X, with the latter acting upon ubiquitin. LGALS9 KO cells knocked down for USP9X display restored ubiquitination response to lysosomal damage. Thus, USP9X negatively regulates the ubiquitin response, whereas LGALS9 promotes the ubiquitination response on damaged lysosomes by preventing USP9X-mediated removal of ubiquitin.
How do ubiquitination, LGALS9 and USP9X contribute to the activation of AMPK in response to lysosomal damage? AMPK is positively controlled by three upstream kinases, STK11/LKB1, CAMKK2 and MAP3K7/TAK1. STK11/LKB1 and CAMKK2 kinases have clear physiological contexts for activation of AMPK, but MAP3K7/TAK1 has remained enigmatic. Our BioWeb data confirm that STK11/LKB1 associates with PRKAA1/AMPKα1 under mitochondrial stress or glucose starvation, and that CAMKK2 is in the proximity of PRKAA1/AMPKα1 in response to Ca2+ increases. However, during lysosomal damage MAP3K7/TAK1 is the specific upstream kinase that associates with PRKAA1/AMPKα1. It is known that activation of MAP3K7/TAK1 is mediated by ubiquitination. The aforementioned K63 ubiquitination response during lysosomal damage affects the activation of AMPK by MAP3K7/TAK1. This is positively regulated by LGALS9 and negatively controlled by USP9X.
What are the physiological contexts that activate AMPK during lysosomal damage? We have found that AMPK activation is important for control of Mycobacterium tuberculosis, a membrane-damaging microbe. Furthermore, signaling such as by TNFSF10/TRAIL can induce lysosomal permeabilization. In response to TNFSF10, LGALS9 enhances ubiquitination, thus activating AMPK. We have also found the AMPK agonist metformin, a widely prescribed anti-diabetes drug, affects lysosomal integrity – in cells that possess metformin transporters such as SLC22A1/OCT1 – as assessed by multiple measures of lysosomal quality and detected by galectin and ESCRT activation. Thus, TNFSF10 and metformin cause lysosomal permeabilization and induce AMPK activation. AMPK is a well-known positive regulator of autophagy. Given that LGALS9 regulates AMPK activation during lysosomal damage, we tested whether LGALS9 affects autophagy responses during lysosomal injury. Response of autophagic markers, such as ATG13, ATG16L1 and LC3B, is reduced in LGALS9 KO cells, whereas autophagic response can be restored by knocking down USP9X in the LGALS9 KO cells. This indicates that LGALS9 and USP9X regulate autophagic responses during lysosomal damage. Finally, beyond autophagy, global metabolomics changes are noticeable in cells exposed to lysosomal damage by LLOMe.
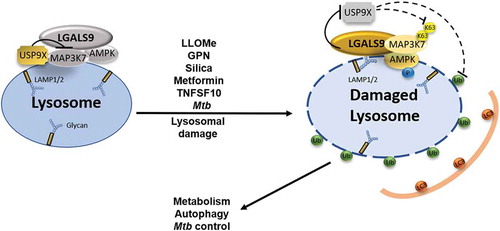
In conclusion, this study [Citation1] establishes a galectin-ubiquitin-governed signal transduction system for AMPK activation in response to lysosomal damage (), which is of relevance for multiple physiological processes.
Figure 1. Model depicting the galectin-ubiquitin-governed signal transduction system for AMPK activation in response to lysosomal damage. At resting state, USP9X’s tonic presence and activity inhibits activation of MAP3K7/TAK1, which is associated with LGALS9 and AMPK on or in the vicinity of the lysosomal surface. Following lysosomal membrane damage, LGALS9 recognizes and contacts the exposed glycosylation groups decorating lumenal domains of lysosomal proteins such as LAMP1/2. This triggers USP9X departure from damaged lysosomes, promoting MAP3K7/TAK1 K63-ubiquitination followed by AMPK activation and its various outputs such as autophagy induction, metabolic reprograming, and control of intracellular Mycobacterium tuberculosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Jia J, Bissa B, Brecht L, et al. AMPK, a regulator of metabolism and autophagy, is activated by lysosomal damage via a novel galectin-directed ubiquitin signal transduction system. Mol Cell. 2020;77:951–969.e9. PMID: 31995728.
