ABSTRACT
Phosphatidylinositol-3-phosphate (PtdIns3P) is essential for generating autophagosomes and regulating endocytic trafficking. Recently, we have shown that the activities of human PIK3C3/VPS34-containing complexes I and II, which synthesize PtdIns3P, are greatly affected by three membrane physicochemical parameters: lipid unsaturation, membrane curvature, and negative charge. Both complexes are more active on membranes composed of unsaturated lipids than saturated lipids, and high membrane curvature can compensate for the negative effect of high lipid saturation. Negatively charged phosphatidylserine (PS) activates the complexes, as well as PIK3C3/VPS34 alone. The kinase activity of complex I depends critically on the ATG14 BATS domain, whereas complex II relies on the BECN1 BARA domain. Our findings highlight the importance of the membrane character as sensed by the unique membrane binding motifs/domain of the complexes for regulating PIK3C3/VPS34 activity.
Phosphatidylinositol-3-phosphate (PtdIns3P) is a critical component of vesicular membranes, and important for endocytic sorting at early endosomes and macroautophagy/autophagy. In mammalian cells, most PtdIns3P is produced from phosphatidylinositol (PtdIns) by the phosphatidylinositol 3-kinase (PtdIns3K) PIK3C3/VPS34, which is found mainly in two heteromeric complexes, complexes I and II. Both complexes have PIK3C3/VPS34, PIK3R4/VPS15, and BECN1/Beclin 1, whereas complex I has ATG14 and NRBF2, and complex II has UVRAG as unique subunits. The four subunits excluding NRBF2 are organized in a Y-shaped structure, with a catalytic arm and an adaptor arm. The catalytic arm has the PIK3C3/VPS34 lipid kinase domain bound to the pseudokinase domain of PIK3R4/VPS15, while the adaptor arm bears BECN1 and a complex-specific subunit, either ATG14 for complex I or UVRAG for complex II. Complex I localizes to phagophores (autophagosome precursors), whereas complex II localizes to early endosomes. How each complex becomes activated on specific membranes has not been clear. Importantly, many studies of PIK3C3/VPS34 activity used pure PtdIns (either water soluble or as liposomes) or simple mixtures of PtdIns and phosphatidylserine (PS) as substrates. Because biological membranes contain various glycerophospholipids, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), PS, and PtdIns, how the complexes are activated on physiologically relevant membranes has remained unexplored.
Membrane-protein association is greatly affected by three physicochemical parameters, lipid unsaturation, membrane curvature, and electrostatics (). Saturated lipids cause tight membrane packing. Highly curved membranes such as ER membranes enable proteins carrying motifs such as amphipathic helices to associate with them. Negatively charged PS and phosphoinositides (PIs) can attract proteins carrying positively charged patches. To examine the effects of these parameters on PtdIns3K complexes, we developed an in vitro method to quantitatively measure PIK3C3/VPS34 activity on giant unilamellar vesicles (GUVs), whose properties can be tuned by altering their lipid composition [Citation1]. This analysis revealed that unsaturation of either the non-substrate lipids or the substrate lipid PtdIns greatly increases activities of the complexes. Both complexes have maximal activity on membranes with dioleoyl lipids (a double bond in each acyl chain), but neither complex is active on lipids with only one unsaturated bond (1-stearoyl-2-oleoyl). Nevertheless, increased saturation could be partially compensated by high membrane curvature. Increasing PS significantly increases the activity of both complexes, as well as PIK3C3/VPS34 alone. Several PIs such as PtdIns4P and PtdIns(4,5)P2 have been reported to affect autophagic activities. Although we found modest activation of complex I by PtdIns4P and PtdIns(4,5)P2, and complex II by PtdIns4P, these PIs are much less influential than the other parameters.
Figure 1. Membrane properties have critical impact on the intrinsic activities of PIK3C3/VPS34 complexes. (A) Lipid packing, membrane curvature and electrostatic charge have important functional consequences. Acyl chain unsaturation (top) and higher membrane curvature (middle) cause lipid packing defects. The electrostatic charge of the membrane depends on anionic lipids such as phosphatidylserine (PS) or phosphoinositides, which can be recognized by basic amino acids in proteins (bottom). (B) PIK3C3/VPS34 complex I produces PtdIns3P on omegasomes at the ER, where It is recruited by the ALPS motif in the ATG14 BATS domain, and partly by the aromatic finger 1 (AF1) in the BECN1 BARA domain. PtdIns3K complex II produces PtdIns3P on early endosomes (EE), which are enriched in saturated lipids, cholesterol and anionic lipids. In contrast to complex I, complex II uses aromatic fingers 1 and 2 (AF1/2) and a hydrophobic loop (HL) in the BECN1 BARA domain for membrane recruitment
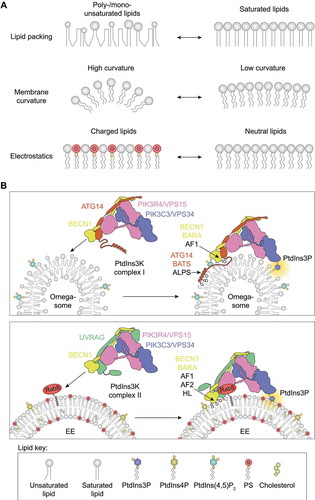
Next, we examined whether complexes I and II use the same motifs/domains for membrane binding. Human complex I is typically more active and has a higher affinity for membranes than complex II. However, the difference in activity is diminished by increasing amounts of PS, indicating that complex II is more dependent on electrostatics than complex I. Hydrogen-deuterium exchange mass-spectrometry/HDX-MS with complexes I and II in the presence and absence of liposomes revealed that the adaptor arm has a decisive role in membrane binding. Complex I associates with membranes primarily via the BATS domain in ATG14. In contrast, we found two new motifs in complex II, aromatic finger 2 (AF2) and a hydrophobic loop (HL), in addition to the previously noted aromatic finger 1 motif (AF1), all of which are in the BARA domain of BECN1. Mutants showed that the BATS domain is necessary for kinase activity of complex I. Whereas complex I activity is only mildly affected by the mutations in the three motifs (AF1, AF2, and HL) of the BECN1 BARA domain, complex II activity is significantly decreased by these mutations. Our results highlighted intrinsically different activation mechanisms between complexes I and II, which involve different membrane-associating motifs.
Complex I is recruited by ULK1 to phagophores at the ER, where membranes are flexible and highly curved. An amphipathic-lipid-packing-sensor (ALPS) motif in the ATG14 BATS domain is responsible for membrane association, enabling complex I activity. High unsaturation of ER lipids might make the ER ideally suited for complex I activity. In contrast, complex II is recruited by RAB5 to early endosomes, whose composition is more similar to plasma membranes, with a relatively high PS content. Because complex II activity is more dependent on PS than complex I, high PS, together with recruitment by RAB5, might contribute to complex II activity on early endosomes. The vast complexity of lipid species, variation in lipid organization among various cell compartments, and interaction with regulatory proteins will cooperate in tuning the activities of PtdIns3K complexes in distinct locations.
Disclosure statement
No potential conflict of interest was reported by the authors. The work was supported by the MRC (File reference number MC_U105184308 to RLW) and Cancer Research UK (Programme grantC14801/A21211 to RLW).
Additional information
Funding
Reference
- Ohashi Y, Tremel S, GR M, et al. Membrane characteristics tune activities of endosomal and autophagic human VPS34 complexes. Elife. 9 2020. Epub 2020/07/01. PubMed PMID: 32602837. doi: 10.7554/eLife.58281.
