ABSTRACT
Several cytotoxic agents used in cancer therapy cause DNA damage and replication stress. Understanding the metabolic determinants of the cell response to replication stress-inducing agents could have relevant implications for cancer treatment. In a recent study, we showed that cell survival during replication stress is influenced by the availability of amino acids, as well as by TORC1 and Gcn2-mediated amino acid sensing pathways. Amino acid starvation, or TORC1 inhibition, sensitizes cells to replication stress conditions, whereas Gcn2 ablation promotes cell survival by stimulating protein synthesis. The Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG phosphatidylinositol-3-phosphate (PtdIns3P) complex at the endosomes sets the balance between survival and death signals during replication stress and amino acid starvation. The Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG axis promotes the degradation of amino acid transporters, thus sensitizing cells to amino acid starvation, while Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG inactivation promotes cell survival by enabling synthesis of stress response proteins mediating survival under replication stress conditions. Our study unravels an autophagy-independent mechanism through which Vps34-Vps30/Atg6/BECN1 promotes lethal events during replication stress.
Cytotoxic chemotherapy remains the most commonly used and the most effective anticancer strategy for the treatment of human malignancies. Several chemotherapeutical agents, including platinum salts, topoisomerase inhibitors and alkylating agents, exert their antineoplastic activity by causing DNA damage or by interfering with DNA structure or with the dynamics of DNA replication, finally inducing replication stress. Eukaryotic cells respond to replication stress by activating the DNA-damage response (DDR), which promotes several survival mechanisms, including the synthesis of DNA repair proteins and the biosynthesis of nucleotides required for DNA replication. However, the metabolic circuits and mechanisms that influence cell survival during replication stress conditions remain elusive.
Vps30/Atg6/BECN1 is a tumor suppressor protein that can associate with two different phosphatidylinositol 3-kinase complexes, namely Vps34-Vps15-Vps30/Atg6/BECN1-Atg14, which activates autophagy, and Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG, which regulates endosomal trafficking of proteins that are delivered to the vacuole (lysosome).
In a recent study, that employed the budding yeast S. cerevisiae as a model system, we described a novel function of Vps30/Atg6/BECN1 in influencing cell survival during replication stress [Citation1]. Different replication stress-inducing agents were used, such as hydroxyurea (HU), which inhibits dNTP production, nitrogen mustard (NM), which induces DNA intra-strand adducts, and methyl methanesulfonate (MMS), a DNA alkylating agent. We showed that an impaired activity of the Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG complex, which results in lower PtdIns3P levels at endosomes, prevents the degradation of plasma membrane amino acid transporters in the vacuole (lysosome); this in turn, causes an increased exposure of amino acid transporters in the plasma membrane and a consequent boost in cellular uptake of essential amino acids (EAAs), such as aromatic amino acids or branched chain amino acids (). Notably, an increased uptake of EAAs in cells with inactive Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG complexes generates resistance to replication stress-inducing agents. By contrast, cells with higher PtdIns3P levels at the endosomes exhibit impaired EAA uptake as a result of an increased degradation of EAA plasma membrane transporters, and, consequently, they become more sensitive to replication stress conditions.
Figure 1. Vps30/Atg6 at the endosomes regulates the response to replication stress. Once ubiquitinated, plasma membrane proteins, including amino acid transporters, are targeted for degradation in the vacuole (lysosome). PtdIns3P produced by the Vps34-Vps15-Vps30/Atg6-Vps38 complex at endosomes recruits ESCRT 0 (Vps27), which recognizes ubiquitinated cargos that are then internalized through the ESCRT III complex (Vps60); finally, these endosomes fuse with the vacuole, where they are degraded. Deletion of the VPS30/ATG6 gene results in an increase of plasma membrane amino acid transporters compared to WT cells, and in consequence increased uptake of EAAs. This reduces Gcn2 activation during replication stress and increases protein synthesis capacity. Finally, an enhanced synthesis of proteins involved in cellular response to replication stress boosts the DDR and makes cells resistant to replication stress. AA: amino acids; EAA: essential amino acids; DDR: DNA damage response; ub: ubiquitin.
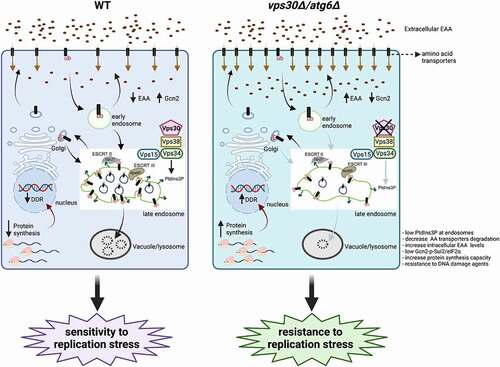
Intriguingly, the function of Vps30/Atg6/BECN1 in affecting the response to replication stress is independent of its role in autophagy. Indeed, the deletion of the autophagy ATG1 gene, or the inactivation of the autophagic Vps34-Vps15-Vps30/Atg6/BECN1-Atg14 complex, does not affect cell survival to HU, NM, or MMS. Conversely, the mechanism linking the Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG activation status and EAA uptake to cell sensitivity to replication stress is mediated by EAA-dependent synthesis of stress response proteins relevant for replication stress, including enzymes involved in DNA repair or in nucleotide biosynthesis, such as Rnr3.
These conclusions are supported by the following major findings: 1) cell exposure to cycloheximide, an inhibitor of protein synthesis, prevents cell cycle progression through the S phase under replication stress conditions; consistently, the inhibition of TORC1, which promotes ribosome biogenesis and protein synthesis, sensitizes cells to replication stress; 2) changes in PtdIns3P levels at endosomes affect the phosphorylation status of Maf1, a target of TORC1 involved in ribosome biogenesis; 3) the activation of the Gcn2-Sui2/eiF2α-Gcn4 axis during EAA starvation aggravates the sensitivity of cells with high PtdIns3P to replication stress conditions by preventing the synthesis of specific proteins; 4) the supplementation of EAA rescues cell sensitivity to replication stress-inducing agents.
The finding that the Gcn2-Sui2/eiF2α-Gcn4 axis contributes to lethal events during replication stress conditions was surprising. Indeed, the Gcn2-Sui2/eiF2α-Gcn4 axis is acknowledged as a survival pathway that is activated in response to amino acid starvation, and which promotes pro-survival functions, including the inhibition of global protein synthesis – to spare energy and essential amino acids in amino acid-depleted conditions – and the synthesis of plasma membrane amino acid transporters – to promote amino acid uptake and to restore a physiological intracellular concentration of amino acids. The recent observations indicate that Gcn2-Sui2/eiF2α-Gcn4-induced inhibition of global protein synthesis can become toxic in specific stress response conditions, which require a boost in the synthesis of proteins that allow cell survive.
Some details about the role of the endosomal Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG complex in affecting cell sensitivity to replication stress remain to be elucidated. First, it is unclear if Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG promotes the internalization and consequent degradation of plasma membrane amino acid transporters, if it prevents amino acid transporter recycling to the plasma membrane, or if it affects the trafficking of vesicles containing amino acid transporters that are directed from the Golgi to the vacuole for degradation (). Second, it remains to be elucidated whether PtdIns3P levels at the endosome can directly affect TORC1 signaling during amino acid imbalance and replication stress.
These recent findings have potentially relevant consequences for cancer therapy. BECN1 is a known tumor suppressor protein whose functions have been historically attributed to its role in modulating autophagy. BECN1 loss in human cancers could promote tumor cell growth by boosting amino acid uptake and protein synthesis. Pharmacological activators of the Vps34-Vps15-Vps30/Atg6/BECN1-Vps38/UVRAG axis capable of promoting the degradation of plasma membrane amino acid transporters may result in synergistic antitumor effects in combination with cytotoxic chemotherapy and/or EAA starvation, as well as with an imbalanced concentration of extracellular EAAs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Ajazi A, Bruhn C, Shubassi G, et al. Endosomal trafficking and DNA damage checkpoint kinases dictate survival to replication stress by regulating amino acid uptake and protein synthesis. Dev Cell. 2021 Sep 27;56(18):2607–2622 e6.
