ABSTRACT
Macroautophagy/autophagy is a resistance mechanism to targeted therapy in BRAF mutant cancers. Preclinical evidence and clinical trial data demonstrate that hydroxychloroquine (HCQ) is an effective autophagy inhibitor at clinically achievable concentrations. Here we highlight the results of a recently published single-arm phase I/II multi-institution trial of dabrafenib, trametinib, and the autophagy inhibitor HCQ (the BAMM trial) that established the safety and activity of this regimen in BRAF V600-mutant melanoma patients. Compared to the pivotal trials that led to FDA approval of dabrafenib and trametinib, the BAMM trial enrolled a high percentage of patients with elevated LDH and prior immunotherapy, reflecting the trend that poorer-prognosis patients are treated with targeted therapy in the modern era where multiple immunotherapy regimens are available for melanoma. Dabrafenib, trametinib, and hydroxychloroquine are safe and produce a high response rate (85%). Progression-free survival does not meet the pre-specified threshold for the entire cohort but looks especially promising in patients with elevated LDH and prior treatment. A national randomized study has been launched to study this regimen further in poor-prognosis BRAF V600-mutant melanoma patients.
Multiple groups have demonstrated autophagy as a key resistance mechanism to targeted therapy in BRAF mutant cancers. MAPK targeted therapy elicits an ER stress response, activates AMPK, and engages a transcriptional program, all contributing to increased autophagic flux (). In melanoma, combined BRAF and MAP2K/MEK inhibition is the standard of care for advanced BRAF mutant melanoma patients. Resistance to targeted therapy in BRAF mutant melanoma is common, and preclinical studies suggest that targeting autophagy could significantly improve outcomes for these patients. The results of the BAMM trial (NCT02257424), a four-center, investigator-initiated phase I/II clinical trial of dabrafenib, trametinib, and hydroxychloroquine (HCQ) in BRAF inhibitor-naïve patients with advanced BRAF mutant melanoma were recently published [Citation1].
Figure 1. Combined BRAF, MAP2K/MEK and autophagy inhibition in BRAF mutant melanoma. (A) Schematic of concurrent targeting of the MAPK pathway and autophagy. Mu: Mutant. (B) Chest CT images of a stage IV BRAF mutant melanoma patient treated on the BAMM trial with dabrafenib, trametinib and hydroxychloroquine (HCQ). Mo.: Months. Arrow: melanoma metastases to the lung.
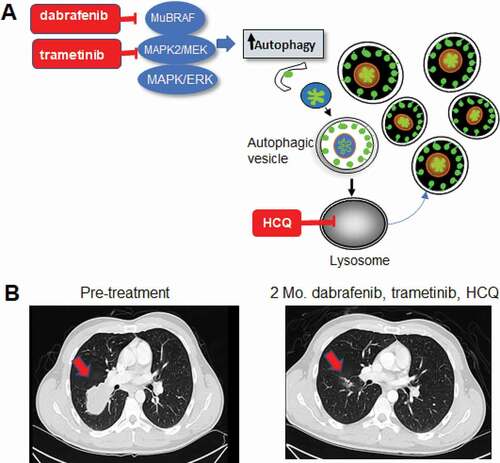
In this single-arm, open-label trial, 34 patients with advanced BRAF mutant melanoma were treated with the BRAF inhibitor dabrafenib, the MAP2K/MEK inhibitor trametinib, and the autophagy inhibitor HCQ. There was no dose-limiting toxicity in the phase I component, and the recommended phase II dose of HCQ was 600 mg twice daily, the highest dose allowed by the FDA. The response rate for the three-drug regimen was 85%, with a completion response rate of 41%. Responses were usually rapid, often providing symptomatic relief for patients (). In some cases, the responses lasted for years whereas in other cases, the duration of response was transient. The median progression-free survival (PFS) was 11.2 months, and the one-year PFS rate was 48%. This regimen was well tolerated with no significant excess toxicity compared to previously published reports of dabrafenib and trametinib. An extensive serial ocular exam was included in the study and did not find any clinically significant ocular toxicity. Finally, RNA-seq in pre-treatment tumors identified several autophagy-lysosome genes (ATG12, BNIP3, and those encoding V-ATPase subunits), ALDH1A1, and the TGFB pathway, which are differentially regulated in patients with short versus prolonged PFS.
When the trial was initially designed, BRAF and MAP2K/MEK inhibitors were tested and used in the frontline setting for BRAF mutant melanoma. Based on these earlier dabrafenib and trametinib studies, the criteria for success stated in the original design of the BAMM trial was a one-year PFS rate of 53%. Therefore, the BAMM trial did not meet its primary endpoint. However, this does not rule out the activity of this regimen. As immunotherapy regimens became available for melanoma patients, including BRAF mutant melanoma, targeted therapy became more commonly used in the second- or third-line setting after tumors had already become therapy-resistant and large. These poor-prognosis patients often have elevated serum LDH, and the BAMM study was enriched for such patients. A more contemporary study, the DREAMseq study presented in 2021 at the ASCO plenary session, found that dabrafenib and trametinib produce a 43% response rate and a 36% response rate 1-year PFS rate in a similar population of BRAF mutant melanoma patients that BAMM had enrolled. In this context, while officially a negative study, the BAMM trial demonstrated excellent tolerability and clinical activity that warrants further study.
Tumors with elevated LDH have increased autophagic flux and lysosomal acidification. Therefore, there may be a mechanistic reason why targeting autophagy may be especially effective in patients with elevated LDH, a group of patients who rarely respond to any treatment. The BAMM regimen produced an 88% response rate in patients with elevated serum LDH. Based on this finding, a double-blind placebo-controlled randomized trial (BAMM2/EA6191) of dabrafenib, trametinib, and HCQ versus placebo in advanced BRAF mutant melanoma patients with elevated LDH who were previously treated with immunotherapy is being conducted through the National Clinical Trial Network. This trial will answer whether or not the addition of HCQ to targeted therapy is significantly better than targeted therapy alone. The findings of the BAMM trial suggest novel autophagy inhibitors may also have activity in combination with BRAF and MAP2K/MEK inhibitors in BRAF mutant melanoma. The limitations of this trial are: 1) it had a small number of patients; 2) despite the high response rates, there was still slow accrual as most patients are being preferentially treated with immunotherapy; 3) and the study was stopped early due to slow accrual and COVID-19 lockdown. Future studies will be focused on elucidating possible resistance mechanisms to autophagy inhibition when combined with targeted therapy. This could include TGFB signaling, transcriptional modulation of autophagy and lysosome genes, or other reported resistance mechanisms such as NFE2L2/NRF2 signaling. Nevertheless, the 85–88% response rate with this well-tolerated regimen is noteworthy and demonstrates the potential of autophagy inhibition for BRAF mutant cancers.
Disclosure statement
RKA is the inventor of patents related to novel chloroquine derivatives for cancer licensed to Pinpoint Therapeutics. RKA is the Founder of Pinpoint Therapeutics. He is a consultant for Deciphera, Sprint Biosciences, and Immunacell, Merck.
Additional information
Funding
Reference
- Mehnert JM, Mitchell T, Huang AC, et al. BAMM (BRAF autophagy and MEK inhibition in melanoma): a phase I/II trial of dabrafenib, trametinib and hydroxychloroquine in advanced BRAFV600-mutant melanoma. Clin Cancer Res. 2022 Jan 12:clincanres.3382.2021. Online ahead of print. PMID: 35022320. DOI:https://doi.org/10.1158/1078-0432.CCR-21-3382.
