ABSTRACT
More than 55 million people are suffering from Alzheimer's disease (AD), but there is still no effective treatment for it. Therefore, novel therapeutic approaches and regulatory mechanisms of protein quality control need to be further evaluated and dissected. The lysosome is one of the major degradative organelles that maintain cellular homeostasis and protein quality control. In our recent study, we have identified a group of LYsosome-Enhancing Compounds (LYECs), which significantly promote the activation of TFEB (transcription factor EB) and lysosome biogenesis via inhibiting dopamine transporters (DAT). Injection of LH2-051, a member of the LYECs identified in this study, significantly improves learning, memory, and cognitive function of APP-PSEN1 mice, in which the enhanced capability of lysosomal degradation promotes the clearance of amyloid protein aggregates. In summary, this study reports novel mechanisms of neurotransporter-mediated lysosome biogenesis and shows that DAT inhibition can alleviate the pathogenesis of Alzheimer's disease.
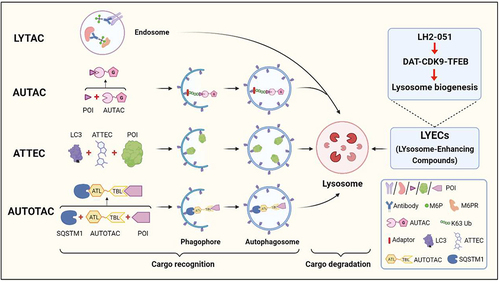
The pathogenesis of Alzheimer's disease (AD) is characterized by the accumulation of protein aggregates, including β amyloids (Aβ) and MAPT/Tau, which subsequently results in neuron loss and dysfunction of human brains. In recent years, new technologies have been applied for the degradation of pathogenic protein aggregates in proteasome- or autolysosome-dependent pathways. The proteolysis-targeting chimera (PROTAC) technology provides an efficient strategy to degrade the target protein of interest (POI) by specific E3 ligase-mediated ubiquitination and subsequent proteasome-mediated degradation. In fact, there are several novel approaches focusing on the intracellular autolysosomal system, including lysosome targeting chimera (LYTAC), autophagy-targeting chimera (AUTAC), autophagosome-tethering compound (ATTEC), and autophagy-targeting chimera (AUTOTAC) technologies. All these approaches facilitate the recognition and delivery of the POI into lysosomes, where the POI is degraded by the lysosomal hydrolases ().
Figure 1. A schematic illustration of LYTAC, AUTAC, ATTEC, AUTOTAC and LYECs. LYTACs mark the extracellular POI with mannose-6-phosphate (M6P)-conjugated antibodies for lysosomal degradation. AUTACs contain a degradation tag (guanine derivative), which triggers K63 polyubiquitination of the POI, and the POI is recognized by the selective macroautophagy/autophagy pathway for degradation. ATTECs tether the POI to the phagophore for autophagic degradation by direct binding to the POI and LC3. AUTOTACs bring the POI to the phagophore for autophagic degradation by binding to the POI and SQSTM1/p62. LYECs promote activation of TFEB and lysosome biogenesis, which enhances the degradation efficiency of autolysosomes (created with BioRender.Com).
However, lysosomes become dysfunctional and their degradative capacity declines during the pathogenesis of neurodegenerative diseases. These changes of lysosomes further aggravate the toxic protein-induced burden within neuronal cells. Therefore, strategies to promote lysosome function and its degradative capacity could act as potential therapeutic approaches for neurodegenerative diseases. By establishing the screening system on the basis of a TFEB-EGFP stable cell line and LysoTracker staining, we recently identified a group of LYECs that could significantly promote TFEB activation and lysosome biogenesis [Citation1]. Further profiling indicated that LH2-051 exerts its biological roles by the inhibition against DAT and may act as a potential drug candidate for AD therapy.
We here provide novel insights into DAT inhibition and dissect the DAT–CDK9–TFEB signaling axis, which is a novel regulatory mechanism of lysosome biogenesis (). In this cellular process, lysosomes function as the hubs for this signal transduction. LH2-051 treatment induces translocation of DAT from plasma membranes onto lysosomal membranes, where DAT promotes CDK9 inactivation and release from lysosomes into the cytosol. Notably, the inhibition of DAT has beneficial effects on lysosome biogenesis and AD progression independently of intracellular dopamine concentration because neither administration of additional dopamine nor knockdown of intracellular SLC18A2/VMAT2 (solute carrier family 18 member A2) can mimic/reverse LH2-051-induced TFEB activation and lysosome biogenesis. Our results provide the first link between intracellular protein quality control and functions of neurotransmitter transporters and explain how DAT translocation from plasma membranes participates in signal transduction on lysosome membranes. This work helps us to understand new biological functions of neurotransporters.
Taking all the results together, our study offers a new perspective to help us understand protein quality control under pathophysiological conditions. We think that there are potential translational values of DAT inhibition for the treatment of neurodegenerative diseases. In the future, combining LYECs with AUTAC, ATTEC, LYTAC or AUTOTAC maybe have synergetic effects and further enhance the degradation efficiency of autolysosomes ().
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Yin L, Zhou J, Li T, et al. Inhibition of the dopamine transporter promotes lysosome biogenesis and ameliorates Alzheimer’s disease–like symptoms in mice. Alzheimer’s Dement. 2022:1–15. DOI:10.1002/alz.12776
