ABSTRACT
The functions of mammalian Atg8 proteins (mATG8s) expand beyond canonical autophagy and include processes collectively referred to as Atg8ylation. Global modulation of protein synthesis under stress conditions is governed by MTOR and liquid-liquid phase separated condensates containing ribonucleoprotein particles known as stress granules (SGs). We report that lysosomal damage induces SGs acting as a hitherto unappreciated inhibitor of protein translation via EIF2A/eIF2α phosphorylation while favoring an ATF4-dependent integrated stress response. SGs are induced by lysosome-damaging agents, SARS-CoV-2 open reading frame 3a protein (ORF3a) expression, Mycobacterium tuberculosis infection, and exposure to proteopathic MAPT/tau. Proteomic studies revealed recruitment to damaged lysosomes of the core SG proteins NUFIP2 and G3BP1 along with the GABARAPs of the mATG8 family. The recruitment of these proteins is independent of SG condensates or canonical autophagy. GABARAPs interact directly with NUFIP2 and G3BP1 whereas Atg8ylation is needed for their recruitment to damaged lysosomes. At the lysosome, NUFIP2 contributes to MTOR inactivation together with LGALS8 (galectin 8) via the Ragulator-RRAGA-RRAGB complex. The separable functions of NUFIP2 and G3BP1 in SG formation vis-a-vis their role in MTOR inactivation are governed by GABARAP and Atg8ylation. Thus, cells employ membrane Atg8ylation to control and coordinate SG and MTOR responses to lysosomal damage.Abbreviations: Atg8: autophagy related 8; ATG: autophagy related; ATF4: activating transcription factor 4; EIF2A/eIF2α: eukaryotic translation initiation factor 2A; GABARAP: GABA type A receptor-associated protein; G3BP1: G3BP stress granule assembly factor 1; LLOMe: L-leucyl-L-leucine methyl ester; LysoIP: lysosome immunopurification; mRNA: messenger ribonucleic acid; MTOR: mechanistic target of rapamycin kinase; NUFIP2: nuclear FMR1 interacting protein 2; ORF3a: open reading frame 3a protein; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SG: stress granule; TIA1: TIA1 cytotoxic granule associated RNA binding protein
Lysosomes, membrane-bound degradative organelles, provide a spectrum of housekeeping activities and serve as critical signaling hubs. Therefore, the integrity of lysosomes is critical for cellular homeostasis. Due to the nature of their function, lysosomes are exposed to many extrinsic and intrinsic damaging agents. These include microbial pathogens, environmental agents, toxic protein aggregates, endogenous crystals composed of cholesterol or uric acid, and a variety of lysosomotropic drugs, all inducing lysosomal membrane damage. Lysosomal damage has been implicated in many human diseases such as infections, neurodegeneration, autoimmunity, metabolic disorders, and cancer, as well as in normal aging. Thus, it is important to delineate homeostatic activities countering lysosomal damage.
Cells elicit a complex set of responses to lysosomal damage such as inactivation of MTOR. MTOR is the cardinal metabolic regulator controlling a number of cellular processes including protein synthesis. SGs provide an additional level of control of protein synthesis as a component of an integrated stress response. SGs contribute to generalized translational arrest upon stress transduced by several kinases that converge upon and phosphorylate EIF2A. Phosphorylation of EIF2A results in reduction of general protein synthesis while favoring expression of integrated stress response proteins including transcription factor ATF4. The mRNA sequestered within SGs can reenter translation upon removal of environmental stressors and cell recovery.
We found that lysosomal damage induces SG formation using the conventional markers of SGs such as G3BP1, TIA1 and mRNA by high content microscopy in a panel of cell lines and primary cells in response to the treatment with lysosomal damaging agents including Leu-Leu-OMe (LLOMe), glycyl-L-phenylalanine 2-naphthylamide and silica crystals [Citation1] (). The hallmark of a conventional SG response, EIF2A phosphorylation, is detected in response to lysosomal damage. Among the known kinases phosphorylating EIF2A, only EIF2AK2/PKR knockdown abrogates EIF2A phosphorylation and SG formation during lysosomal damage. The SG response subsides during recovery from lysosomal damage. SG formation triggered by lysosomal damage is blocked by cycloheximide, a known inhibitor of SG condensate formation in response to standard SG-induced stressors such as arsenite. We found that LLOMe treatment causes general translational shutdown documented by a puromycin incorporation assay, whereas it selectively increases expression of ATF4. Thus, we conclude that lysosomal damage is a newly identified stimulus for induction of SGs causing generalized translational arrest while promoting an integrated stress response. These relationships are observed under physiological conditions including Mycobacterium tuberculosis infection, SARS-CoV-2 ORF3a expression, and exposure to a proteotoxic form of MAPT/tau.
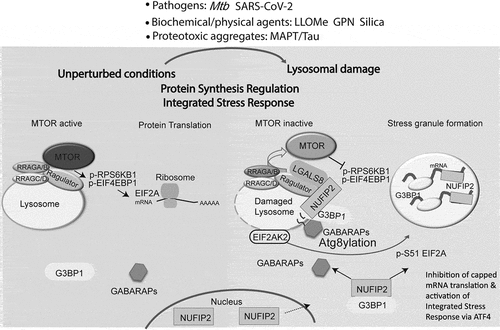
Figure 1. Lysosomal damage results in generalized protein synthesis inhibition by induction of stress granule (SG) formation and MTOR inactivation. The signal from damaged lysosomes is transduced to the SG machinery via a pool of EIF2AK2/PKR that resides on lysosomes. Upon damage, PKR phosphorylates EIF2A/eIF2α, a key regulator of translational arrest and SG formation. Inhibition of capped mRNA translation due to EIF2A phosphorylation and SG formation elicits an ATF4-driven integrated stress response in response to lysosomal damage. Complementary to SGs, lysosomal damage also inactivates MTOR. Under normal conditions, MTOR phosphorylates its substrates RPS6KB1/S6K1 and EIF4EBP1 to promote protein translation. Damaged lysosomes are Atg8ylated by GABARAPs which directly bind and recruit SG proteins NUFIP2 and G3BP1 to the lysosomal surface where they act to inactivate MTOR. The pools of NUFIP2 and G3BP1 are split between SG formation and the Ragulator-RRAG complex to control MTOR activity together with LGALS8 on the lysosomes. This results in both a competition (limiting SG formation) and a synergy (inhibiting translation by both sequestering mRNAs in SGs and inhibiting MTOR) thus coordinating these two roles of NUFIP2 and G3BP1 for the optimal contribution of both aspects of protein translation.
We preformed quantitative proteomic analyses of damaged vs. undamaged lysosomes using a well-established lysosome immunopurification (LysoIP) protocol. The LysoIP proteomics and immunoblotting analyses revealed increased association of individual SG proteins including G3BP1, TIA1 and NUFIP2 with damaged lysosomes. We observed by imaging analysis only a low-level association between SGs and lysosomes, indicating that individual SG proteins on damaged lysosomes may have additional functions independent of the roles they play within SGs. The enhanced levels of NUFIP2, G3BP1 and TIA1 in LysoIP preparations from cells treated with LLOMe are not inhibited by cycloheximide, confirming that the recruitment of individual SG proteins to damaged lysosomes is independent of SG formation.
What might be the functions of individual SG proteins on the surface of lysosomes? We found that NUFIP2, the essential component of SGs, translocates from the nucleus to lysosomes upon damage, and that it is responsible for MTOR inactivation. We have previously shown that MTOR is inactivated by LGALS8 during lysosomal damage via the Ragulator-RRAGA-RRAGB system, and this is reflected in our LysoIP proteomic analysis indicating that MTOR dissociates from damaged lysosomes. Using an established assay for measuring the activation state of the Ragulator guanine nucleotide exchange factor/GEF complex with RRAGs, we found that NUFIP2 together with LGALS8 causes RRAGA-RRAGB inactivation thus inhibiting MTOR during lysosomal damage.
Our LysoIP proteomic analysis furthermore revealed enrichment of mATG8s (MAP1LC3/LC3s and GABARAPs) on damaged lysosomes. We found by GST affinity isolation that NUFIP2 interacts directly with GABARAPs but not with LC3s. Further LysoIP proteomic and immunoblotting analyses show that GABARAPs are responsible for the recruitment of NUFIP2 to damaged lysosomes, and that this is key to MTOR inactivation. The conjugation of GABARAPs to damaged lysosomal membranes as a manifestation of Atg8ylation responses during stress have a balancing effect on MTOR inactivation vs. SG formation. Thus, we found that Atg8ylation controls both SG formation and MTOR inactivation during lysosomal damage, balancing via a shared factor, NUFIP2, the two key aspects of translational arrest.
In conclusion, this study shows that lysosomal damage is a hitherto unknown inducer of SG formation and integrated stress response and that membrane Atg8ylation coordinates SG formation with MTOR inactivation in response to lysosomal damage affecting protein translation regulation, which is of relevance for multiple physiological conditions and disease states.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Jia J, Wang F, Bhujabal Z, et al. Stress granules and mTOR are regulated by membrane atg8ylation during lysosomal damage. J Cell Biol. 2022 Sep 30;221(11):e202207091.
