ABSTRACT
The autophagic-lysosomal pathway of microglia plays a key role in myelin debris removal in white matter damage. As the lipid-rich myelin debris are engulfed by microglia, the cellular autophagic level increases, accompanied by lysosomal dysfunction. However, several issues such as how to regulate this pathway to ensure the effective degradation of myelin debris, and maintain the balance of lipid metabolism are still to be elucidated. Recently, we have demonstrated that the excessive activation of macroautophagy/autophagy leads to lipid overload in lysosomes and lipid droplets accumulation, which could be the initiator of microglial dysfunction and secondary inflammatory white matter damage. Interestingly, staged suppression of autophagic activation in the acute phase of demyelination could benefit microglia allowing them to regain the lipid metabolism balance, and reduce the excessive accumulation of lipids, thus promoting the removal of myelin debris. The neuroprotective effects of microglial autophagy regulation may be related to intracellular linoleic acid (LA) production and PPARG pathway activation.
Macroautophagy/autophagy impairment is one of the common features shared by several brain disorders, including the central nervous system (CNS) inflammatory demyelinating diseases such as multiple sclerosis, and most neurodegenerative diseases including Alzheimer disease, in which autophagy dysfunction and substrate accumulation in neurons have been thoroughly investigated. However, autophagic dysfunction in other neural cells, particularly microglia, still requires further investigation.
Demyelination is a key pathological characteristic of white matter damage caused by various etiologies (including but not limited to ischemia, inflammation, degeneration, etc.). Effective clearance of myelin debris is one of the core elements during the process of the repair and regeneration of white matter damage. The vast majority of myelin debris engulfed by microglia enters lysosomes for degradation through autophagy. Based on the key role of microglia in the removal of myelin debris, several studies suggest that activation of microglial phagocytosis and autophagy is beneficial for remyelination. However, other studies indicate that inhibition of autophagy can be neuroprotective in certain circumstances of white matter injury. Such conflicting findings suggest that the role of autophagy activation in demyelinated lesions is not a simple black-and-white matter. In our study, we established a mouse model of double-point injection of lysophosphatidylcholine-induced demyelination to observe the dynamic process from white matter damage to myelin repair under a severe demyelinating attack. We found that the microglial autophagy is overactivated in the acute phase of demyelination, and a large number of engulfed lipid-rich myelin debris are transported to lysosomes. Lipid overload can directly inhibit the lysosomal acidification and acid hydrolase activity, trigger permeabilization of the lysosomal membrane, resulting in a large amount of content being released into the cytoplasm, disturbing cell homeostasis. Meanwhile, lipids that cannot be effectively degraded will accumulate in cells as lipid droplets, further affecting various cellular functions [Citation1].
There are also arguments regarding the possible consequences of lipid droplets accumulation in phagocytes. Lipid-droplet-containing microglia are dysfunctional and exhibit a pro-inflammatory phenotype during demyelination in the aging brain, while intracellular lipid droplets are neuroprotective against lipotoxicity in another model of demyelination. In our study, we found that the accumulation of lipid droplets in microglia impairs microglial functions, particularly in phagocytosis and the autophagic-lysosomal pathway, which further leads to lipid metabolism dysregulation and lipid droplet aggregation, forming a vicious circle. In addition, the lipid-droplet-accumulating microglia exhibit an enhanced-inflammatory phenotype that aggravates demyelinating injury. Disturbance of intracellular lipid metabolism could be the intrinsic mechanism of microglia-mediated propagation of demyelination.
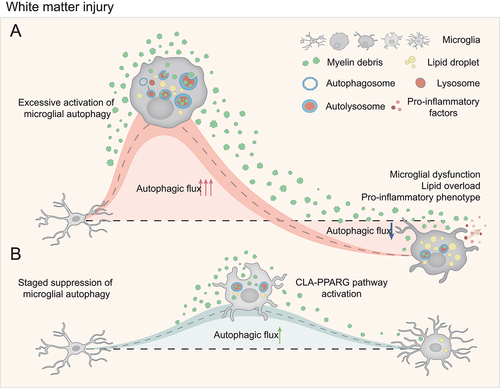
Our findings suggest that the autophagy overactivation in microglia might be the initial stage of intracellular lipid overload during demyelination. However, can the simple inhibition of the activation of microglial autophagy play a protective role in demyelination? To this end, we adopted two different strategies of autophagy inhibition: 1) Genetically, microglia-specific atg5-deficient mice (Cx3cr1-CreER+ atg5fl/fl) which present sustained lower autophagy levels in microglia; 2) pharmacologically, BAF A1, a specific and potent V-ATPase inhibitor, was intracerebroventricularly administered to inhibit the classical autophagy-lysosomal pathway. The protective effects of autophagy inhibition can be clearly observed in the early stage of acute demyelination. Surprisingly, continuous inhibition of autophagy inversely results in more severe demyelination area and neurological deficits at a relatively late stage (28 days post injury). More lipid droplets accumulation is also observed in microglia at a late stage when the autophagy pathway is continuously inhibited, suggesting that several compensatory pathways such as those involving endosomes might be activated to transport myelin debris into lysosomes, albeit at a lower efficiency. As the most effective degradation pathway via the autophagy-lysosome is inhibited, lipid metabolism disorder aggravates, resulting in more severe demyelination.
One of the most intriguing findings of our research is that the white matter damage is mostly alleviated when BAF A1 is administered in the first 5 days post lysophosphatidylcholine injection to suppress the transient autophagy activation at the early stage of demyelination. We innovatively proposed that transiently inhibiting the activation of autophagy only at the acute phase of demyelination can order intracellular lipid metabolism and avoid the acquired lysosomal dysfunction due to acute lipid overload, as well as the microglial dysfunction caused by the lipid droplets accumulation. Then the autophagic-lysosomal pathway in microglia at the late stages can continue to degrade myelin debris, reducing the secondary white matter damage caused by the debris. The staged regulation of the autophagic-lysosomal pathway we proposed in the CNS demyelinating diseases, might be extended to other neurological diseases in which excessive activation of autophagy is involved in their pathological processes.
Furthermore, our research also explored the specific changes of lipid metabolism in microglia under autophagy inhibition in vitro. Combined analysis of transcriptomic and metabolomic data suggest that the cellular abundance of LA and the activation of related metabolic pathways can be the key metabolic characteristics after inhibiting autophagy in microglia. Our findings highlight the intracellular LA accumulation as a good metabolic sign. Exogenous supplementation of conjugated LA (CLA) in microglia in vitro could rebuild the balance of cellular lipid metabolism and suppress the microglia-mediated inflammation. Administration of CLA can also regulate the microglial autophagic-lysosomal pathway, assisting its lipid metabolism, thereby reducing white matter damage. Our results indicate a clinical translational value of CLA in the CNS demyelinated diseases. Mechanistically, CLA is likely to achieve the neuroprotective effects by activating the PPARG pathway. Although this pathway is a well-studied in several different diseases, there is still a lack of evidence for its translational application in diseases other than diabetes. Our results also suggest that other agonists of the PPARG pathway, such as rosiglitazone, might also exhibit similar protective effects, and thus warrant more investigation in treating CNS demyelination and other diseases.
In summary, our research demonstrated a novel mechanism of myelin debris clearance dysregulation in microglia during white matter injury, which has important implications for all CNS demyelinating diseases and even some lysosomal disorders. We innovatively proposed that staged regulation of the autophagic-lysosomal pathway in microglia could assist the orderly removal of myelin debris, which will be a key therapeutic direction for CNS demyelinating injury ().
Figure 1. A schematic model for the staged regulation of the microglial autophagic-lysosomal pathway during white matter injury. (A) in severe demyelination, myelin debris are engulfed by microglia, and the large amount of lipid floods into lysosomes and interrupts the degradation system, leading to a dysfunctional and pro-inflammatory phenotype. (B) Staged suppression of over-activated autophagy orderly balances intracellular lipid metabolism and avoids the acquired lysosomal dysfunction as well as the microglial dysfunction caused by the lipid droplets accumulation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Zhou LQ, Dong MH, Hu ZW, et al. Staged suppression of microglial autophagy facilitates regeneration in CNS demyelination by enhancing the production of linoleic acid. Proc Natl Acad Sci, USA. 2023;120:e2209990120.
