ABSTRACT
In neuronal synapses, autophagosome biogenesis is coupled with the activity-dependent synaptic vesicle cycle via ATG-9. How vesicles containing ATG-9 are sorted at the presynapse is unknown. We performed forward genetic screens at single synapses of C. elegans neurons for mutants that disrupt ATG-9 presynaptic localization, and identified the long isoform of the active zone protein CLA-1 (Clarinet; CLA-1 L). We find that disrupting CLA-1 L results in abnormal accumulation of ATG-9-containing vesicles enriched with clathrin. The adaptor protein complexes and proteins at the periactive zone genetically interact with CLA-1 L in ATG-9 sorting. Moreover, the phenotype of the ATG-9 protein in cla-1(L) mutants was not observed for integral synaptic vesicle proteins, suggesting distinct mechanisms that regulate sorting of ATG-9-containing vesicles and synaptic vesicles. Our findings reveal novel roles for active zone proteins in the sorting of ATG-9 and in presynaptic macroautophagy/autophagy.
Article
ATG-9, a transmembrane protein necessary for autophagy, is transported from the trans-Golgi network of the neuronal cell body to synapses in C. elegans via the AP-3 adaptor protein complex and the synaptic vesicle kinesin, UNC-104/KIF1A. At the presynaptic sites, ATG-9 undergoes exo-endocytosis sorting using the synaptic vesicle cycling machinery such as UNC-13/MUNC-13 and UNC-26/Synaptojanin 1. Yet, the mechanisms that sort ATG-9 at presynaptic sites and the relationship of these mechanisms to those that sort integral synaptic vesicle proteins are not well understood.
To identify molecules required for presynaptic sorting of ATG-9, we performed forward genetic screens and identified the long isoform of Clarinet (CLA-1 L), which bears a functional similarity to the large vertebrate active zone proteins Piccolo and Bassoon. In cla-1(L) mutants, ATG-9 abnormally accumulates to subsynaptic regions enriched for clathrin. The phenotype is suppressed by mutants for synaptic vesicle exocytosis, such as unc-13/Munc-13 [Citation1], suggesting that the abnormal subsynaptic enrichment of ATG-9 observed in cla-1(L) mutants is dependent on synaptic activity. Our ultrastructural analyses revealed that the observed phenotype for ATG-9 in cla-1(L) mutants is not due to a general problem in synaptic morphology. Interestingly, the subsynaptic enrichment phenotype was not observed for integral synaptic vesicle proteins, suggesting that despite using the same exo-endocytosis machinery, distinct mechanisms are involved in regulating sorting of ATG-9-containing vesicles and synaptic vesicles.
Because the ATG-9 phenotype in cla-1(L) mutants was also observed in the canonical endocytic mutants, such as unc-26/synaptojanin 1, and because the abnormal ATG-9 foci are enriched for clathrin, we next examined the genetic relationship between clathrin adaptor protein complexes and CLA-1 L in ATG-9 localization. Through genetic analyses, we found that mutants of the adaptor complexes AP-2 and AP180 phenocopy and enhance the ATG-9 phenotypes in cla-1(L) mutant, whereas mutants for the AP-1 adaptor complex suppress the phenotype. The subsynaptic ATG-9-rich foci might represent endocytic intermediates, from which the AP-2 (and the associated AP180) adaptor complexes bind to and sort out cargoes, whereas AP-1 mediates the sorting of ATG-9 to the endocytic intermediates. A similar process with different adaptor complexes involved at distinct stages is found in the activity-dependent bulk endocytosis (ADBE) of synaptic vesicles. During ABDE, the F-BAR protein SDPN-1/syndapin 1 plays important roles in early stages of membrane invagination. We hypothesized that if similar sorting mechanisms were involved for ATG-9, disrupting SDPN-1 should prevent ATG-9 accumulation at the endocytic intermediates. Indeed, sdpn-1 mutants suppressed the abnormal ATG-9 foci for cla-1(L) and AP180 mutants. We therefore favor a model in which AP-1 and SDPN-1 mediate trafficking of ATG-9 to a transient sorting station from which AP2-AP180 complexes facilitate clathrin-mediated ATG-9 vesicle budding. We hypothesize that the active zone protein, CLA-1 L, facilitates the AP2-AP180-mediated ATG-9 vesicle budding ().
Figure 1. Schematic diagrams of presynaptic sorting of ATG-9 and genetic regulations. (A) Schematic of the presynaptic compartment. The active zone and the adjacent periactive zone cooperate in regulating the sorting of synaptic vesicles and ATG-9 containing vesicles. The large active zone protein CLA-1 (long isoform; CLA-1 L) is anchored, via its C terminus, to the active zone, whereas its N terminus interacts with periactive zone proteins EHS-1/EPS15 and ITSN-1/intersectin 1 and regulates presynaptic events such as ATG-9 sorting. (B) Genetic model of presynaptic sorting of ATG-9. We hypothesize based on our data that the adaptor complex AP-1 and SDPN-1/syndapin 1 mediate trafficking of ATG-9 to a transient sorting station from which AP2-AP180 complexes facilitate clathrin-mediated ATG-9 vesicle budding. CLA-1 L, together with presynaptic endocytic proteins that reside in the periactive zone, facilitates the AP2-AP180-mediated ATG-9 vesicle budding. Mutations in the active zone gene cla-1 L result in abnormal accumulation of ATG-9 into endocytic intermediates, and defects in activity-dependent autophagosome formation. The italicized words adjacent to arrows refer to the genes encoding proteins that are necessary for the processes indicated by the arrows.
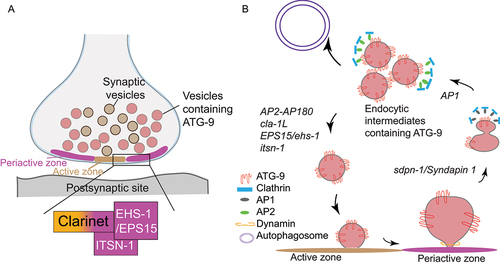
How does CLA-1 L facilitate the AP2-AP180-mediated ATG-9 vesicle budding? CLA-1 L-like proteins Piccolo and Bassoon extend from the active zone to the periactive zone, a property hypothesized to be important for sorting synaptic components during exo-endocytosis. Using CRISPR strategies, we labeled the endogenous CLA-1 L (8922 amino acids) in vivo with GFP either at the N terminus or C terminus and compared their localizations to a periactive zone marker. While the C-terminally tagged CLA-1 L localizes to small puncta corresponding to the active zone, the N-terminally tagged CLA-1 L displays a more distributed presynaptic distribution and colocalizes with a periactive zone marker. Disrupting the periactive zone proteins EHS-1/EPS15 and ITSN-1/intersectin 1 phenocopies and enhances the ATG-9 phenotypes in cla-1(L) mutant. These results suggest that CLA-1 L is anchored, via its C terminus (common to all CLA-1 isoforms), to the active zone, but extends to the periactive zone, where its unique N terminus interacts with periactive zone proteins to regulate presynaptic sorting of ATG-9.
To determine a potential cross-talk between CLA-1 L-mediated presynaptic sorting of ATG-9 and autophagy, we generated epg-9/Atg101;cla-1(L) double mutant animals. The ATG-9 phenotype is enhanced in the double mutants, compared to single mutants Furthermore, we found that CLA-1 L-mediated presynaptic sorting of ATG-9 likely plays a role in activity-dependent autophagosome biogenesis. Unlike wild-type animals, the average number of LGG-1/Atg8/GABARAP (autophagosome marker) puncta did not increase in cla-1(L) mutants upon neuronal stimulation. It is interesting that in cla-1(L) mutants, only activity-induced autophagy, but not baseline autophagy, is affected in the neuron we examined. We speculate that other molecules play a redundant role in supporting baseline autophagy.
Protein turnover (e.g., via autophagy) is correlated with activity states of synapses and is necessary for optimal synaptic function. Large active zone proteins, like CLA-1 L, could monitor synaptic transmission at the active zone and regulate presynaptic sorting of ATG-9 at the periactive zone to boost synaptic autophagy for the degradation of damaged synaptic components under high activity states.
Disclosure statement
No potential conflict of interest was reported by the authors.
