Abstract
Context: Clinical toxicologists may be called upon to determine the appropriateness of medical monitoring following documented or purported exposures to toxicants in the occupational, environmental, and medical settings.
Methods: We searched the MEDLINE database using the Ovid® search engine for the following terms cross-referenced to the MeSH database: (“occupational exposures” OR “environmental exposures”) AND (“physiologic monitoring” OR “population surveillance”). The titles and abstracts of the resulted articles were reviewed for relevance. We expanded our search to include non-peer-reviewed publications and gray literature and resources using the same terms as utilized in the MEDLINE search. There were a total of 48 relevant peer-reviewed and non-peer-reviewed publications. Publications excluded contained no information relevant to medical monitoring following potentially harmful toxicologic exposures, discussed only worker screening/surveillance and/or population biomonitoring, contained redundant information, or were superseded by more recent information.
Approaches to medical monitoring: A consensus exists in the peer-reviewed medical literature, legal literature, and government publications that for medical monitoring to be a beneficial public health activity, careful consideration must be given to potential benefits and harms of the program. Characteristics of the exposure, the adverse human health effect, the screening test, and the natural history of the disease are important in determining whether an exposed population will reap a net benefit or harm from a proposed monitoring program.
Broader interpretations of medical monitoring: Some have argued that medical monitoring programs should not be limited to exposure-related outcomes but should duplicate general preventive medicine efforts to improve public health outcomes although an overall reduction of morbidity, mortality and disability by modifying correctable risk factors and disease conditions. This broader approach is inconsistent with the targeted approach advocated by the Agency for Toxic Substances and Disease Registry and the United States Preventive Services Task Force and the bulk of the peer-reviewed medical literature.
Medical monitoring in legal contexts: Numerous medical monitoring actions have been litigated. Legal rationales for allowing medical monitoring claims often incorporate some of the scientific criteria for the appropriateness of monitoring programs. In the majority of cases in which plaintiffs were awarded medical monitoring relief, plaintiffs were required to demonstrate both that the condition for which medical monitoring was sought could be detected early, and that early detection and treatment will improve morbidity and mortality. However, the treatment of medical monitoring claims varies significantly depending upon jurisdiction.
Examples of large-scale, comprehensive medical monitoring programs: Large-scale, comprehensive medical monitoring programs have been implemented, such as the Fernald Medical Monitoring Program and the World Trade Center Health Program, both of which exceeded the scope of medical monitoring typically recommended in the peer-reviewed medical literature and the courts. The Fernald program sought to prevent death and disability due to non-exposure-related conditions in a manner similar to general preventive medicine. The World Trade Center Health Program provides comprehensive medical care for World Trade Center responders and may be viewed as a large-scale, federally--funded research effort, which distinguishes it from medical monitoring in a medico-legal context.
Synthesis of public health approaches to medical monitoring: Medical monitoring may be indicated following a hazardous exposure in limited circumstances. General causation for a specific adverse health effect must be either established by scientific consensus through a formal causal analysis using a framework such as the Bradford–Hill criteria. The exposure must be characterized and must be of sufficient severity that the exposed population has a significantly elevated risk of an adverse health effect. Monitoring must result in earlier detection of the condition than would otherwise occur and must confer a benefit in the form of primary, secondary or tertiary prevention. Outcome tables may be of use in describing the potential benefits and harms of a proposed monitoring program.
Conclusions: In the context of litigation, plaintiffs may seek medical monitoring programs after documented or putative exposures. The role of the clinical toxicologist, in this setting, is to evaluate the scientific justifications and medical risks and assist the courts in determining whether monitoring would be expected to result in a net public health benefit.
Introduction
Medical monitoring, as a clinical activity, aimed at early detection of disease, is commonly undertaken by clinicians of all specialties. Most medical monitoring takes the form of clinical testing for the presence, or indicia (distinguishing marks), of specific diseases or disease processes in patient populations at risk for an adverse health outcome. By way of example, dermatologists often monitor patients for clinical signs of dermatologic cancer, gynecologists monitor patients for breast and gynecologic cancers, and ophthalmologists often monitor patients for the development of elevated intraocular pressure.
In the field of clinical toxicology, judicial oversight via the court system may be involved in determining which individuals should be monitored for the development of disease related to various exposures, and for what duration. Clinical toxicologists are often called upon to determine the necessity of establishing medical monitoring programs. Issues surrounding medical monitoring, and its appropriateness, arise in many settings including those associated with occupational exposures, environmental exposures, and medically related exposures to drugs and pharmaceuticals. Although medical monitoring, as a concept, may be appropriate to a variety of human exposure settings, little scientific research has been conducted regarding its application, or regarding the terms and conditions under which medical monitoring programs are effective.
Defining medical monitoring in clinical toxicology
In the context of populations potentially exposed to hazardous substances, medical monitoring may be defined as “periodic medical testing to screen people at significant risk for disease” [Citation1]. Other types of medical monitoring exist (e.g., the monitoring of patients with chronic disease), but are intrinsically distinct from the medical monitoring of a population due to concerns related to potentially hazardous exposures. Careful consideration must be given to the establishment of a medical monitoring program, including the selection of participants, the scope of monitoring, and the monitoring frequency and modalities. Consideration of such factors maximizes the likelihood that the net benefits of the program will outweigh potential harms and that monetary costs remain reasonable.
Medical monitoring should be distinguished from the related activities of medical screening and medical surveillance. While these terms are sometimes used interchangeably in the peer-reviewed medical literature, they are distinct but complementary entities [Citation2].
Medical screening refers to the cross-sectional testing, often non-recurrent, of a population or group of workers for early indicia of a disease. Medical screening is distinct from pre-employment screening, drug screening, or genetic screening.
Medical surveillance is the recurrent, longitudinal tracking of a population or group of workers for early indicia of disease. While both screening and surveillance are preventative in nature, only surveillance occurs at regular follow-up intervals. Medical screening may be repeated as necessary, but is not scheduled at regular intervals as is the case with medical surveillance.
Intent of medical screening
Medical monitoring is distinguished from medical screening and medical surveillance in its intent. Medical monitoring aims to identify indicia of disease in a population at significantly increased risk of disease due to a past exposure so as to benefit the individual being screened. No additional benefit accrues to the population as a result of monitoring because there are no modifiable risk factors present (e.g., ongoing exposure) [Citation3]. Conversely, both medical screening and medical surveillance test for indicia of disease in a population with the intent of protecting both the individual and the population. Interventions to protect the population as a whole, e.g. modifications to exposure control processes including engineering and administrative controls and protective equipment are not considered medical monitoring.
Methods
We searched the MEDLINE database using the Ovid® search engine for the following terms cross-referenced to the MeSH database: (“occupational exposures” OR “environmental exposures”) AND (“physiologic monitoring” OR “population surveillance”). This search yielded 1750 results. Limiting those results to the English language and records that pertain to human subjects yielded 1431 results. The titles and abstracts of the resulted articles were reviewed for relevance. We expanded our search to include non-peer-reviewed publications and gray literature, literature not formally published or outside traditional academic publishing, and resources using the same terms as utilized in the MEDLINE search. There were a total of 48 relevant peer-reviewed and non-peer-reviewed publications. Publications that were not cited contained no information relevant to medical monitoring following potentially harmful toxicologic exposures, discussed only worker screening/surveillance and/or population biomonitoring, contained redundant information, or were superseded by more recent information.
Approaches to medical monitoring
Various approaches to determine the appropriateness of medical monitoring have been proposed in the peer-reviewed medical literature, by governmental agencies, and by the courts. The criteria used to establish the appropriateness of medical monitoring, the desired outcomes and the importance of cost vary between these approaches. In assessing the appropriateness of medical monitoring in a specific context, clinical toxicologists may apply principles and processes from these approaches.
Agency for Toxic Substances and Disease Registry criteria for medical monitoring programs
The Agency for Toxic Substances and Disease Registry (ATSDR) has promulgated seven criteria () that a medical monitoring program must satisfy to be deemed an appropriate public health activity under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). The Agency for Toxic Substances and Disease Registry approach is of particular interest for clinical toxicologists because it is tailored specifically to environmental exposures. The Agency for Toxic Substances and Disease Registry approach considers medical monitoring to be a “service to individuals in communities where there is believed to be an increased risk of disease from exposure to hazardous substances released into the environment” [Citation1].
Table 1. Agency for toxic substances and disease registry (ATSDR) criteria for appropriateness of medical monitoring programs as a public health activity.
The Agency for Toxic Substances and Disease Registry criteria fall into two phases: Phase 1 consists of an evaluation of the exposure and outcome criteria, while Phase 2 consists of aspects of the medical monitoring plan itself.
Phase 1 criteria
The first two criteria (exposure criteria) require that a target population is identifiable in whom an exposure has occurred to a hazardous substance at a sufficient level to increase the risk of an adverse human health effect. These criteria, therefore, exclude monitoring populations with medically- or toxicologically-insignificant exposures or in those cases where an exposure is only putative or of unclear severity.
The next two criteria, (outcome criteria) specify that medical monitoring should only be undertaken when a known adverse human health effect is causally related to exposure to a substance, is easily detectable, and is amenable to prevention or intervention. In scenarios where the association between an exposure and an adverse human health effect is not well established, the Bradford Hill criteria may be utilized to support or refute a causal relationship. Of note, the Agency for Toxic Substances and Disease Registry does not consider animal model studies sufficient to determine whether an exposure results in an adverse human health effect. The adverse health effect must be easily detectable, such as by clinical examination or by the use of simple, outpatient diagnostic testing. Lastly, “early detection and treatment or intervention interrupts the progress to symptomatic disease, improves the prognosis of the disease, improves the quality of life of the individual, or is amenable to primary prevention”.
Phase 2 criteria
The phase 2 criteria (systems criteria) delineate characteristics of appropriate medical monitoring programs. Due to the similarities between medical monitoring and medical screening, discussed above, medical monitoring programs should meet the requirements of medical screening programs, which include the availability of a screening test with sufficient sensitivity and specificity so as to yield clinically useful positive and negative predictive values in the population in whom monitoring is being considered. Any screening test utilized in a medical monitoring program should be recommended for medical screening in other settings. The screening test must also be of acceptable cost, feasible, and acceptable to the population in question. Lastly, the proposed monitoring program should be well defined, including who is being monitored, how often, and for how long. Considerations of disease latency and progression should inform monitoring frequency and duration. A referral system should be delineated prior to initiation of monitoring for persons with positive screening test results.
United States Preventive Services Task Force criteria for clinical preventive services
The US Preventive Services Task Force has developed a structured framework for the evaluation of clinical preventive services. Although the process was designed to evaluate the appropriateness of medical screening, it may be applied to the evaluation of the appropriateness of medical monitoring in a toxicology setting.
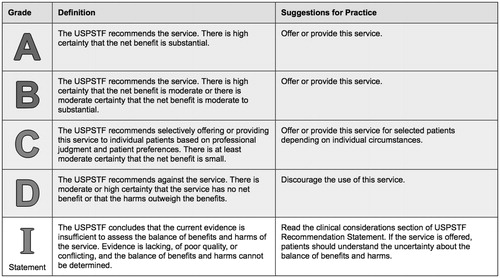
The US Preventive Services Task Force issues letter recommendation grades () based on the likelihood that screening the population in question will result in net benefit to that population. In order for screening to be recommended, there must either be high certainty that the net benefit to the screened population is at least moderate or moderate certainty that the net benefit is moderate to substantial. Screening that only yields a small net benefit for the population may be selectively offered, while screening that yields no net benefit should be discouraged. This consideration somewhat aligns with the Agency for Toxic Substances and Disease Registry mandate that the adverse health effect must be amenable to prevention or intervention, but goes farther in weighing the risks of screening against the potential benefits in formulating a recommendation.
Figure 1. U.S. Preventive Services Task Force Grades for clinical preventive services. Adapted from http://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions.
The US Preventive Services Task Force has defined eight questions to be used in the determination of the degree of net benefit that will accrue to a population by institution of medical screening. The key questions with numerals corresponding to their designation in the analytic framework () are
Figure 2. U.S. Preventive Services Task Force analytic framework for evaluation of a clinical preventive service. Adapted from: United States Preventive Services Task Force, Procedure Manual, Agency for Healthcare Research and Quality, 2008.
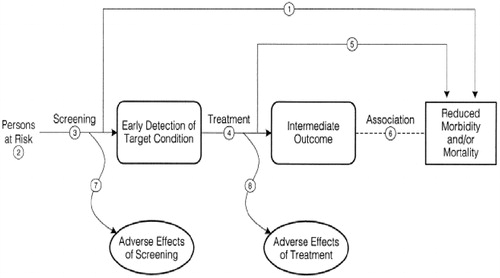
Does screening for “X” reduce morbidity and/or mortality?
Can a group at high risk for “X” be identified on clinical grounds?
Are there accurate (i.e., sensitive and specific) screening tests available?
Are treatments available that make a difference in intermediate outcomes when the disease is caught early, or detected by screening?
Are treatments available that make a difference in morbidity or mortality when the disease is caught early, or detected by screening?
How strong is the association between the intermediate outcomes and patient outcomes?
What are the harms of the screening test?
What are the harms of the treatment? [Citation4]
Parameters that must be defined prior to the initiation of screening should include sensitivity and specificity of the screening test, prevalence of the condition of concern, adherence to screening, effectiveness of screening, and costs [Citation4]. Outcome measures include the number of cases of disease or death expected to be averted, gain in quality-adjusted life years, and potential harms associated with screening. The “number needed to screen” refers to the number of screening interventions in the target population to prevent one case of disease or one death.
In order to realize a net benefit from a medical screening program, potential benefits must be weighed against potential harms. Harms associated with medical screening programs include adverse effects from screening e.g., psychological harm associated with patient “labeling”, or adverse effects of the screening test itself [Citation4]. The harms of early treatment include the potential for adverse effects from invasive diagnostic testing to confirm or refute the presence of the condition; from invasive treatments; and from “over-treatment,” i.e., treatment of healthy patients mistakenly diagnosed due to false positive diagnostic testing results. Medical screening may subject individuals to harm from early treatment, because their conditions would not have received medical attention in the absence of the screening program. Other harms may include the time, effort, and expense expended by individuals in the target population and the healthcare system.
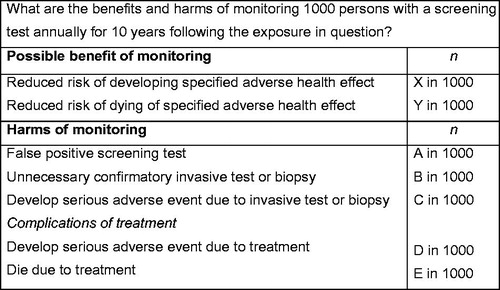
Application of the US Preventive Services Task Force framework to proposed medical monitoring programs in order to determine their appropriateness as a public health activity is appropriate. The same procedural and outcome criteria important to the evaluation of medical screening apply to medical monitoring. In presenting their findings on the appropriateness of a medical monitoring program to the medical community, the courts or the lay public, clinical toxicologists may use the outcome table recommended by the US Preventive Services Task Force. These tables present, in a standardized fashion, the benefits and harms that will accrue to a population by implementation of a screening program over a set time horizon (e.g., 5- or 10-years). Benefits and harms are typically expressed as number of people benefited or harmed per 1000 people screened [Citation4]. These same outcomes tables may be used to express the benefits and harms expected from a proposed medical monitoring program. An example of an outcome table is shown in . The frequency of monitoring, duration of monitoring, and benefits and harm of monitoring were chosen arbitrarily in this example table; in an actual table, they will be determined by the exposure scenario.
Peer-reviewed medical literature
Discussions of medical monitoring in the peer-reviewed medical literature similarly emphasize that for monitoring to be appropriately indicated, the person or community must be at sufficiently increased risk so that the potential benefits of monitoring outweigh costs and potential risks [Citation2,Citation5]. The utility of a medical monitoring program depends on the magnitude of risk and prevalence of the disease condition that is to be monitored. Obtaining information on those two parameters may then inform the decision as to whether medical monitoring would be efficacious [Citation2].
Monitoring must result in earlier detection of the adverse health effect than would otherwise occur and the early detection must confer a benefit [Citation5]. Tests used for medical monitoring should be sensitive, specific, precise, rapid, easy, inexpensive, and involve, at most, minimal risk. The positive predictive value of the test should be calculated based on the anticipated prevalence of disease in the monitored population to determine whether the costs and risks of monitoring are reasonable and whether a net benefit is anticipated to accrue to the monitored population [Citation6]. In developing a medical monitoring program for former workers at the US Department of Energy weapons sites and national laboratories, monitoring for a “particular exposure-induced health outcome” was only considered if two criteria were met:
“Screening tests with acceptable sensitivity and specificity (US Preventive Service Task Force, 1996) are available for the health outcome associated with the specific exposure under consideration.
An intervention that decreases disease severity, morbidity, and/or mortality is available” [Citation7].
Conversely, in a medical monitoring program of former workers at the US Department of Energy Rocky Flats site, participants received a comprehensive medical examination that included tests not designed to identify exposure-related adverse health effects (e.g., cholesterol panel, electrocardiogram, etc.) [Citation8]. In that program, a follow-up or referral system for abnormal results was also not delineated although participants were recommended to share their testing results with their personal physicians. These two approaches, both designed for former US Department of Energy workers, demonstrate two diametrically opposite approaches to medical monitoring.
The use of tests and test panels (e.g., liver function panel) without a scientifically sound and evidence-based rationale increases the risk that results will be abnormal due to chance alone [Citation6]. Further investigation (e.g., invasive confirmatory testing) of these abnormal results may then lead to greater risk and increased costs. In the occupational setting, abnormal results due to chance may lead to inappropriate medical removal protection of the worker, which engenders further risks to the worker and costs to the worker’s employer. Inappropriate use or overuse of radiographic studies may increase the risk of cancer [Citation9]. Therefore, a risk–benefit analysis should be undertaken for each test proposed for a medical monitoring program. Monitoring programs should be regularly reassessed to determine whether the monitoring is consistent with interval changes in medical and scientific knowledge [Citation6].
The intervals at which monitoring should occur should be chosen to provide sufficient probability of a change in the outcome being monitored to justify risks and costs of monitoring and early intervention [Citation5]. In conditions with long latency periods, monitoring should not commence before reasonable latency has accrued [Citation6]. Monitoring undertaken too early carries both the risks inherent to the test being monitored and the additional risk of false reassurance of those being monitored. If monitoring is undertaken, it should be discontinued when the harms and costs of monitoring outweigh the potential benefits [Citation5].
Prior to implementation of a medical monitoring program, protocols should be developed as to what specific test results will trigger an action, such as referral for further diagnostic evaluation, and the recommended level of action for each degree of abnormality [Citation6]. A referral system to higher-level care and/or specialty care should be in place prior to the initiation of monitoring. Notification of primary care providers about monitoring programs and appropriate follow-up care prior to implementation and release of monitoring results is reasonable.
Proposed medical monitoring programs should be acceptable to the community being monitored and issues of convenience, attendant risk, and perceived value should be considered [Citation6]. Onerous monitoring programs may result in low community participation thereby failing to provide a public health benefit and resulting in an unfavorable cost–benefit ratio. Even in occupational settings, where participation may be compulsory, onerous monitoring programs may alienate workers from the medical community, resulting in the potential for increased health risks to those workers.
Medical monitoring programs may be demanded by asymptomatic communities and/or workers who have been notified of a potentially harmful exposure or an increased risk of an adverse health effect [Citation6]. In cases where medical monitoring would be inappropriate, such as where the anticipated prevalence of the adverse health effect is low, no suitable screening test exists, or early detection is unlikely to offer a benefit – education and counseling provide more appropriate public health services.
Broader interpretations of medical monitoring
Some authors have argued for a much broader application of the principles of medical monitoring. It has been argued that medical monitoring programs should not be limited to “specific exposure-related outcomes” but rather should “be aimed at an overall reduction of premature morbidity, mortality, and disability by identifying correctable risk factors and disease conditions” [Citation10]. This approach holds that general preventive medicine is beneficial and should be incorporated into medical monitoring programs as a way to offset the increased risk due to the adverse health outcome associated with the putative hazardous exposure.
However, others have argued this comprehensive approach duplicates “preventive strategies… advocated for use in the general population” and that preventive medicine strategies should not be tied to environmental contamination [Citation11]. It is also unclear whether a comprehensive medical monitoring program with preventive medicine components actually offsets adverse health risks associated with occupational or environmental exposures. This broader approach is inconsistent with the targeted approach advocated by the Agency for Toxic Substances and Disease Registry, US Preventive Services Task Force and the bulk of the peer-reviewed medical literature.
Medical monitoring in legal contexts
Numerous medical monitoring actions have been litigated in the Federal and state courts of the US.
Friends for All Children Inc. v Lockheed Aircraft Corp
In 1984, medical monitoring was first established as a legal remedy in the case of Friends for All Children Inc. v Lockheed Aircraft Corp [Citation12]. In that case, a group of Vietnamese orphans were onboard a Lockheed aircraft that lost cabin pressure and crash-landed. Friends For All Children, an organization serving as legal guardian of the surviving children, filed a complaint alleging that Lockheed’s negligent manufacture of the aircraft caused the disaster. The court issued an injunction requiring that defendant Lockheed create a fund to provide for the surviving children’s reasonable expenses for diagnostic examinations for Minimal Brain Dysfunction.
Ayers v Township of Jackson
Another significant development in medical monitoring jurisprudence is reflected in the 1987 New Jersey case of Ayers v Township of Jackson. In this case, residents of Jackson Township, NJ, who were exposed to contaminated drinking water from leaching of hazardous waste from a landfill into an aquifer, filed suit seeking a medical monitoring program [Citation13]. The New Jersey Supreme Court decided the Ayers case in favor of the Plaintiffs, based upon the rationale that they should have access to medical testing to facilitate rapid diagnosis and treatment of exposure-related adverse health effects.
Initial successful medical monitoring claims argued that the injury sustained was economic: the tortious conduct of the defendant rendered it medically necessary for the Plaintiffs to undergo costly periodic preventive screening that would otherwise have been unnecessary.
Decisions favorable to plaintiffs in medical monitoring cases are often based upon the rationale that the relief granted to the plaintiff serves the public interest by preventing morbidity and mortality and in deterring tortious conduct by industry [Citation14]. For conditions with long latency periods, medical monitoring claims would result in accountability to industry for activities that might otherwise result in no adverse judgments due to statutes of limitation.
Legal claims seeking relief in the form of a medical monitoring program are distinct from claims seeking damages for enhanced risk of future disease or non-intentional infliction of emotional distress: medical monitoring claims do not necessarily seek damages for future disease or present emotional distress. While medical monitoring claims have had mixed success in the US courts, the majority of claims based upon enhanced risk and non-intentional infliction of emotional distress have been rejected [Citation14–16]. Medical monitoring claims generally seek to recover “ongoing medical diagnostic costs that a reasonable physician would suggest to the plaintiff in order to ensure prompt diagnosis of any illness that a defendant’s activity has made more likely” [Citation12]. The legal rationale for allowing a medical monitoring claim has been articulated as follows:
When a person is threatened with the substantial likelihood of contracting a serious disease in the future that could be effectively treated if detected early through readily available medical monitoring procedures, in most cases, a physician would recommend a monitoring regime… It seems elementary that the defendant, having wrongfully put the plaintiff in the position she is in, should alleviate it by paying for medical monitoring when such monitoring would, under current professional medical judgments, be efficacious in improving plaintiff's mortality and long-term health prospects [Citation12].
This legal rationale embodies some of the scientific criteria for the appropriateness of a medical monitoring program, including the stipulation that a person should have a “substantial likelihood of contracting a serious disease” and that the medical monitoring should “under current professional medical judgments, be efficacious in improving… mortality and long-term health prospects” [Citation12]. Proponents of medical monitoring have stated that “medical monitoring claims should be allowed, but only when re-characterized as equitable claims to cover the cost of medical surveillance where the screening has substantial medical efficacy in providing early detection and possible cure and is likely to mitigate severe emotional distress caused by the threat of disease caused by defendant” [Citation12].
In the majority of cases in which plaintiffs were awarded medical monitoring relief, plaintiffs were required to demonstrate both that the condition for which medical monitoring was sought could be detected early, and that early detection and treatment will improve morbidity and mortality [Citation12]. This legal interpretation aligns with the scientific criterion that medical monitoring should only be undertaken when it will result in significant primary, secondary, or tertiary disease prevention. However, in a minority of cases, plaintiffs were not required to show that medical monitoring would improve morbidity or mortality, which contradicts the scientific approach to appropriate medical monitoring programs. In those cases, the decision to grant monitoring relief is “to give the victim advanced warning that she was in for a long-term illness, thus enabling her to make plans for the inevitable outcome” [Citation12]. This view allows medical monitoring relief to be granted more as a remedy for emotional distress, with the possibility of very limited economic benefit, than as a mechanism to prevent morbidity and mortality.
The treatment of medical monitoring claims still varies significantly depending upon jurisdiction: some jurisdictions recognize medical monitoring recovery, others do not, and still other jurisdictions have divided law [Citation17,Citation18].
Bower v Westinghouse Electric Corp
In a 1999 West Virginia case, Bower v Westinghouse Electric Corp., the court ruled that a medical monitoring claim can be approved even when effective treatment for the disease is unavailable [Citation16,Citation18]. It further allowed for payments to plaintiffs in the form of lump-sum damages that may be used by the plaintiffs for any purpose, as distinguished from court-administered funds used solely to pay for medical monitoring. In Bower v. Westinghouse Electric Corp, the West Virginia State Medical Association appeared as amicus curiae (“friend of the court”), arguing that the serial CT scans outlined in the plaintiff’s medical monitoring plan were potentially harmful because they subjected plaintiffs to ionizing radiation and increased the risk of cancer. However, the West Virginia State Supreme Court of Appeals rejected that argument, stating, “this Court has previously disapproved of the argument that medical monitoring should be rejected due to its risk of harm” [Citation18]. This approach is outside the mainstream treatment of medical monitoring claims in the courts.
Legal claim for medical monitoring
It has been proposed that a legal claim for medical monitoring should consist of the following seven elements:
The exposure to a substance at greater than normal background levels.
The substance is hazardous, dangerous or toxic.
The exposure results from the wrongful conduct of a defendant.
There is a significant increased risk of contracting a serious latent disease.
There is a monitoring procedure in existence for the early detection of the disease.
The monitoring procedure is different from that normally recommended in the absence of exposure to or ingestion of the hazardous or dangerous substance.
The monitoring regime is reasonably necessary according to modern scientific principles [Citation19].
These elements align with the scientific criteria for the appropriateness of medical monitoring programs, to an extent. This list of seven elements does not expressly include the criteria requiring that the adverse health outcome be amenable to treatment, and that early detection measures result in reduced morbidity and mortality. However, the seventh element stating that the “monitoring regime is reasonably necessary” could be construed to imply those criteria. Of note, the proponents of this set of criteria served as plaintiff’s counsel in a class action suit against the manufacturers and sellers of the weight loss drugs fenfluramine and phentermine (fen-phen), seeking damages for medical monitoring, among other relief.
Other authors have proposed that a medical monitoring claim should consist of the following six elements:
A significant exposure.
To a hazardous substance with a probable (or proven) link to a human disease.
That creates a (a) quantifiable; and (b) significant increase in the exposed individual’s risk.
Of developing one or more “serious” diseases.
Such that there is a demonstrable clinical value of medical monitoring (that is, as a result of early detection and diagnosis, treatment exists that improves either morbidity or mortality statistics).
The monitoring is in excess of or in addition to a diagnostic regime that is either: (a) recommended for a part or all of the population at large by contemporary medical consensus; or (b) necessary as a result of individual risk factors distinct from the defendant’s tortious exposure (such as hereditary and lifestyle factors, as well as alternative sources of exposure) [Citation3].
Opponents of medical monitoring relief have advanced several arguments, asserting that medical monitoring is rarely efficacious, that scientific uncertainty exists as to the degree of increased risk of disease prior to symptom manifestation, and that allowing medical monitoring claims would result in expensive and wasteful litigation that would threaten the financial viability of industry [Citation16]. Opponents also express doubt about the ability of courts and juries to adequately assess what constitutes “a significant increase of risk of serious disease which a reasonable physician might think could be substantially mitigated by currently available methods of early detection” [Citation12]. The modifiers “significant”, “serious”, and “substantially” have not been set to objective measurable standards by the courts, creating the risk of non-uniformity in the award of damages. It has been argued that due to the complexity of the issues involved, the legislature is the appropriate forum for standards allowing recovery for medical monitoring claims [Citation16].
Further opposition to medical monitoring claims includes arguments that the costs of such monitoring would likely outweigh any medical benefits; that diagnostic testing itself may be unpredictable or result in harms; that early detection of disease may not result in measurable improvement in morbidity and mortality; and that medical monitoring claims will result in over-deterrence, bankrupt industry, and leave other victims who suffer serious injury without recourse for the recovery of damages [Citation12]. Proponents of medical monitoring relief argue that such claims are substantially exaggerated or without merit.
Metro-North Commuter Railroad Co. v Buckley
In 1997, the US Supreme Court issued an important ruling on the legal approach to medical monitoring claims in its Metro-North Commuter Railroad Co. v Buckley decision. In this case, a railroad employee with a history of occupational asbestos exposure for 1 h per working day over a 3-year period filed a negligence action against the defendant railroad corporation, seeking a lump sum damage award for costs of medical monitoring that would be prescribed by a reasonable physician and that would not have been necessary absent the occupational asbestos exposure. The Court ruled in favor of the defendants, based on the difficulty in assessing the necessity and cost of the proposed monitoring activities, and concerns that awarding medical monitoring damages would open the floodgates of litigation due to the breadth of potentially toxic exposures experienced in everyday life. The Court also noted that awarding medical monitoring relief ignored the availability of medical monitoring that might otherwise be available to plaintiff, e.g., through existing occupational surveillance.
In the Metro-North case, the Court expressed particular concern regarding the plaintiff’s demand for lump sum damages, noting that previous medical monitoring decisions had utilized mechanisms such as insurance coverage and court-supervised funds. Such mechanisms ensure that the damage award is indeed used for medical monitoring, and allows for adjustments due to changes in screening costs [Citation12]. Lump-sum awards have been reported to be unlikely spent on medical monitoring, and do not seem to further the goals of medical monitoring programs [Citation16]. The Court was also “troubled” by the concern that successful medical monitoring claims would affect the “interests of other potential plaintiffs who are not before the court and who depend on a tort system that can distinguish between reliable and serious claims on the one hand, and unreliable and relatively trivial claims on the other hand” [Citation16].
C-8 Medical Monitoring Program
The C-8 Medical Monitoring Program was implemented as part of the settlement of the class action lawsuit Jack Leach et al. v E.I. du Pont de Nemours and Co in February 2005 [Citation20]. This suit was filed by residents living near the DuPont Washington Works Plant in the Parkersburg, WV metropolitan area in response to releases of perfluorooctanoic acid (PFOA, C-8) from the plant. Releases of C-8 into air and the Ohio River occurred at that plant from the 1950s through the early 2000s, resulting in contamination of several drinking water supplies in water districts in the vicinity of the plant.
The settlement called for a medical monitoring program to be established only if a Science Panel created by the settlement found a probable link between C-8 exposure and human disease [Citation20]. A probable link between C-8 and human disease was defined as “more likely than not” based on the weight of the available scientific evidence. The Science Panel consisted of three epidemiologists and was tasked with investigating whether C-8 was associated with human disease based on a DuPont study of workers at the Washington Works plant and studies of the potentially exposed community. From December 2011 to October 2012, the Science Panel submitted 17 probable link evaluation reports to the court addressing a comprehensive list of human diseases. Those 17 evaluations reported a probable link between exposure to C-8 and pregnancy-induced hypertension (including preeclampsia), kidney cancer, testicular cancer, thyroid disease, ulcerative colitis, and hypercholesterolemia but not to any other human disease.
The finding of a probable link between C-8 exposure and the above-listed six human diseases therefore triggered the implementation of a medical monitoring program for the settlement class members. In November 2013, a Medical Panel established by the settlement composed of three physicians developed medical monitoring protocols for each of the “probable link conditions” based on medical practice guidelines and the medical literature. Participation in the medical monitoring program was available to all settlement class members regardless of their past C-8 exposure. Recommended monitoring protocols were developed for class members of various ages and are to be performed initially and at a 3-year follow-up appointment (). The Medical Panel also recommended that all class members have a blood C-8 concentration performed to evaluate for the need for future monitoring. The class members were given a list of symptoms to self-monitor related to each of the probable link conditions and instructed to schedule a follow-up appointment should they develop those symptoms.
Table 2. Initial monitoring protocols for the C-8 medical monitoring program.
Pharmaceutical-related litigation and medical monitoring
Diet drugs (phentermine/fenfluramine/dexfenfluramine) products liability litigation
Medical monitoring claims have been litigated in the setting of pharmaceutical exposures. During the 1990s, numerous lawsuits were filed against manufacturers and distributors of the weight loss drugs fenfluramine and phentermine (fen-phen), as well as against the physicians, pharmacies, and weight-loss clinics providing these drugs, after a causal association with valvular heart disease was established [Citation21]. Over 130 class action suits and the claims of more than 35,000 individual plaintiffs were transferred to a single venue in the federal district court for the Eastern District of Pennsylvania [Citation22].
A settlement was reached in that matter with regard to medical monitoring. Some class members were awarded the costs of an echocardiogram and one visit to a cardiologist as well as reimbursement or cash for medical monitoring of valvular heart disease if the echocardiogram demonstrated valvular regurgitation. The settlement agreement also provided for the establishment of a medical research and education fund as well as for awards for class members with “serious” valvular heart disease. In this case, the court ruled the following elements as necessary for a medical monitoring recovery:
Plaintiff was significantly exposed to a proven hazardous substance through the negligent actions of the defendant.
As a proximate result of exposure, plaintiff suffers a significantly increased risk of contracting a serious asymptomatic disease.
That increased risk makes periodic diagnostic medical examinations reasonably necessary.
Monitoring and testing procedures exist that make the early detection and treatment of the disease possible and beneficial.
A reasonable physician would prescribe a monitoring regime different than the one that would have been prescribed in the absence of that particular exposure [Citation22].
The court also relied upon the following public health criteria, set forth in hearing testimony, as prerequisites for implementing a medical monitoring program:
Asymptomatic progression of disease following toxic exposure.
The existence of a test with high sensitivity.
An exposed population with relatively high prevalence.
The test has a high predictive value.
The test is relatively low cost.
Monitoring is capable of integration into standard clinical follow-up of those with disease.
Monitoring should allow early preventive care.
Monitoring should allow appropriate timing of definitive care [Citation22].
In approving the medical monitoring portion of the settlement, the court decided that both the legal and medical prerequisites for a medical monitoring program were met and that the incorporation of medical monitoring into the settlement furthered important public policy and public health objectives [Citation22].
In Re West Virginia Rezulin Litigation
In other pharmaceutical cases, the courts have deemed medical monitoring inappropriate. In the 2001 West Virginia case In Re West Virginia Rezulin Litigation, the plaintiff class sought medical monitoring for hepatic injury against manufacturers of troglitazone [Citation13]. The court applied the same criteria as in the above-mentioned fen-phen case, and found in favor of the defense. In the West Virginia Rezulin case, the court held that there was insufficient evidence that troglitazone caused latent hepatic injury; that no high-sensitivity screening tests or procedures were available to assess for that specific adverse health outcome; that it was unknown if the target population had a sufficiently high prevalence of hepatic injury; and that a liver biopsy test was inappropriate for medical monitoring because it is expensive, invasive, and risky.
Examples of large-scale, comprehensive medical monitoring programs
Compared with the scientific and legal approaches, discussed above, that limit medical monitoring to populations with documented exposures above an established reference level and limit screening to adverse health effects with established associations to the known exposure; some medical monitoring programs have been much broader in their ambitions. Such programs include the Fernald Medical Monitoring Program and the World Trade Center Health Program discussed as follows.
The Fernald Medical Monitoring Program
The Fernald Medical Monitoring Program was established based upon a class action lawsuit against National Lead of Ohio, Inc., and the US Department of Energy [Citation23]. Residents in the vicinity of the Feed Material Production Center (a Superfund site located in Hamilton and Butler Counties, Ohio), a uranium processing plant, may have been exposed to ionizing radiation and uranium particulates due to plant activities from 1952 to 1989. Medical monitoring was included as a legal remedy in that case as a method to mitigate emotional distress suffered by class members as a result of their alleged exposures. The use of medical monitoring for such purposes was more expansive than previously accepted public health goals of primary, secondary, and tertiary disease prevention. With respect to the aims of Fernald Medical Monitoring Program, the program leadership has stated:
“The key design feature of the program was that the examination was comprehensive—it was focused on conditions that had the most potential to improve subsequent health without regard to whether those conditions were potentially related to exposures to hazards from the plant. The rationale for this was that the known health effects of exposures such as radiation or the metal toxicity of uranium did not have very effective treatments (e.g., lung cancer, renal disease, interstitial pulmonary fibrosis). In contrast, regardless of what exposures may have occurred in this population, we were certain that the leading causes of death and disability in the participating population would be the same as the general population—coronary heart disease, common cancers, and stroke. The risk of death and disability from some of these common conditions is modifiable through the application of known screening practices and risk factor reduction. In short, the program was focused on conditions with the most potential to affect future health rather than conditions potentially related to environmental exposures” [Citation23].
The Fernald Medical Monitoring Program was, in part, a duplication of the responsibilities of primary care providers unrelated to the exposure of concern, such as monitoring of serum lipid profiles. Other Fernald Medical Monitoring Program interventions, such as serial chest radiographs, are not considered valid screening modalities for the outcome of interest, i.e., lung cancer. The Fernald Dosimetry Reconstruction Project and Fernald Risk Assessment Project, both conducted by the US Centers for Disease Control and Prevention, reported that among the 46,000 potentially exposed persons there would be an estimated 85 additional cases of lung cancer deaths, five additional cases of leukemia, and one additional case each of breast, bone, and kidney cancer [Citation24]. The attributable risk for each type of cancer due to Fernald-related exposures as compared with the expected number of background cases is summarized in .
Table 3. Estimated total cancer cases attributable to Fernald-related radiation exposure compared with background cancer cases.
World trade center health program
The terrorist attacks on the World Trade Center on 11 September 2001 resulted in inhalational exposure of numerous workers, volunteers, and survivors to a mixture of chemicals, combustion products, and aerosolized building materials [Citation25]. The World Trade Center Worker and Volunteer Medical Screening Program received federal funding and began medical monitoring of workers and volunteers in 2002. The stated goals of the program were:
Identification of individuals who sustained exposures at the World Trade Center site during rescue and recovery operations.
Provision of clinical assessments for exposed individuals to identify persistent World Trade Center-related medical conditions.
Provision of coordinated referral for follow-up clinical care for affected individuals.
Education of individuals about their exposures and the associated health risks, as well as advice on available benefit and entitlement programs.
Establishment of a “baseline” clinical status for individuals exposed at or near “Ground Zero” for the purpose of comparison with future clinical assessments [Citation25].
The program included self-administered physical and mental health questionnaires, interview-administered medical and exposure assessment questionnaires, physical examination, spirometry, blood count, serum chemistries, urinalysis, and chest radiographs. Further funding was received in 2004 and federal funding for treatment services was appropriated in 2006, resulting in the renaming of the program to World Trade Center Medical Monitoring and Treatment Program.
The World Trade Center Medical Monitoring and Treatment Program transitioned to the current program, the World Trade Center Health Program, with the passage of the James Zadroga 9/11 Health and Compensation Act of 2010. Medical monitoring was continued for both worker and volunteer World Trade Center responders, while World Trade Center survivors who worked, lived or attended school/daycare in the vicinity of the site were offered an initial health evaluation. Medical monitoring of World Trade Center responders includes annual medical history and mental health questionnaires, 9/11 exposure assessment, vital signs, physical examination, spirometry/pulmonary function testing, blood count, chemistries, and urinalysis and biannual chest radiographs. Annual electrocardiograms, biannual mammograms, and colonoscopies are offered to selected program members based on age and health situation.
In addition to medical monitoring, the World Trade Center Health Program also pays for treatment of a wide variety of health conditions provided that a program physician determines that 9/11-related exposures were a significant factor in causing or exacerbating the condition. Numerous studies have been published in the peer-reviewed medical literature using data collected by the World Trade Center Health Program and related World Trade Center Health Registry [Citation26–38]. As with the FMMP, the World Trade Center Health Program is an extensive program, which may be more reflective of American sentiments towards World Trade Center responders than strict scientific principles, with funding in excess of $1 billion. In addition to its intent to provide comprehensive medical care for World Trade Center responders, the World Trade Center Health Program may also be viewed as a large-scale federally-funded research effort, which distinguishes it from medical monitoring in a medico-legal context [Citation39].
Synthesis of public health approaches to medical monitoring following occupational, environmental, or pharmaceutical exposures
Causation
Medical monitoring may be indicated following a potentially hazardous exposure in limited circumstances. The substance or mixture to which the population was exposed must be associated with one or more specific adverse health effects. When general causation between the substance in question and specific adverse health effect(s) has not been firmly established, formal causal analysis using a framework such as the Bradford Hill criteria may be considered [Citation40]. Although not extolled by Hill, evaluation of relative risks (RRs) and confidence intervals are informative in a general causation analysis provided that they are interpreted with concern for bias, confounding, and validity in the underlying studies [Citation41]. It is particularly difficult to assert causality with small-to-moderate RRs, which are unlikely to fulfill Hill’s “strength” and “biological gradient” criteria, and competing theories to explain the possible association between exposure and disease should be explored.
Greater weight should be given to human epidemiological studies over animal model and in vivo studies, which may be hampered by inter-species differences in toxicodynamics/toxicokinetics or unrealistic exposure conditions, respectively. It is of some note that the Agency for Toxic Substances and Disease Registry does not consider animal model studies to be sufficient for determining that an exposure results in an adverse human health effect. Within human epidemiological studies themselves, different study designs (i.e., case–control, cohort, ecological, cross-sectional) each have their strengths and weaknesses in contributing to a causation analysis [Citation41]. Characteristics of the adverse human health effect, such as induction and latency periods, should be considered when critically analyzing study designs. In some cases (e.g., cigarette smoke and lung cancer), general causation may already have been sufficiently established such that causal analysis is unnecessary.
The exposure must be characterized and must be documented to be of sufficient severity that the exposed population has a significantly elevated risk of an adverse health effect. An exposure assessment or exposure modeling must be performed in the characterization of the exposure. Dose, route, duration, and chronicity of exposure are all important in this evaluation. When conducted retrospectively, recall bias and litigation bias are potential confounders in exposure assessment, particularly in community environmental exposures [Citation41–43].
A number of considerations may determine what degree of additional risk, or attributable risk, is deemed “significantly elevated risk”, including the morbidity/mortality associated with the adverse health outcome, whether the adverse health outcome is a cancer or non-cancer endpoint, the population who was exposed, and public health constructs of acceptable risk. A frequently referenced value for de minimus risk that derives from an FDA rule issued in 1977 is a maximum lifetime risk of 10−6, although there is no scientific underpinning for this arbitrary value [Citation44]. The US EPA designates maximum concentrations for carcinogens in drinking water based on an excess risk of cancer of 10−4–10−6, while the World Health Organization bases their guideline values for genotoxic carcinogens in drinking water on an upper bound estimate of excess risk of cancer of 10−5 [Citation45]. Higher levels of risk may be tolerated in the occupational setting over environmental exposures. US OSHA considers a working lifetime risk of death of over 10−4 as significant [Citation46]. The UK Health and Safety Executive designates two tiers of acceptable risk for two populations: the upper limit of “tolerable” risk of death for workers is 10−4, while for the public, it is 10−5; the upper limit of “broadly acceptable” risk of death is 10−6 for both workers and the general public [Citation47].
Quantifying the degree of additional risk is more difficult following low-level exposures when a low-concentration dose–response curve must be extrapolated from a known dose–response relationship at higher exposure concentrations [Citation48]. Such an extrapolation involves assumptions as to whether risk increases monotonically with exposure (i.e., linear-no threshold model) or whether low-level protective effects, threshold effects, or plateau effects may be relevant [Citation49,Citation50]. In some cases where an exposure results in a small effect size with respect to a health outcome, the degree of additional risk may be relevant at the population risk level but not clinically significant at the individual risk level [Citation48]. In such cases, medical monitoring of individuals due to medico-legal action following the cessation of exposure would not be anticipated to contribute to public health.
Disease clusters may lead concerned workers, community members, or scientists to seek out an occupational or environmental exposure to explain the apparent excess incidence of disease [Citation51]. The tendency to search for an occupational/environmental pollutant as a contributing factor may be particularly strong with cancer clusters, due to overestimation of environmental factors in the causation of cancer [Citation52]. Such clusters have occasionally led to advances in clinical toxicology (e.g., vinyl chloride and hepatic angiosarcoma) [Citation53]. Depending on the scenario, a cluster investigation may be undertaken by the responsible public health agency.
Requirements for beneficial medical monitoring programs
Monitoring for the adverse health effect in question must result in earlier detection of the condition than would otherwise occur and that earlier detection must confer a benefit to the person being monitored. The health condition must be amenable to primary, secondary or tertiary prevention.
The proposed monitoring test or procedure must be simple, inexpensive, and relatively non-invasive. Cost-effectiveness analyses may be indicated to determine the cost per quality-adjusted life-year (QALY) of a proposed medical monitoring program. Historically, ratios of $50,000 and $100,000 per QALY have been cited in the peer-reviewed medical literature as cutoffs to determine that a healthcare intervention is cost-effective. However, there is no solid scientific basis for these arbitrary cutoffs and authors have suggested other, typically higher, ratios as cutoffs to determine cost-effectiveness [Citation54]. Unfortunately, no consensus exists, which leaves the upper bound ratio of cost per QALY that should be determined cost-effective to interpretation. In addition, these healthcare intervention cost-effectiveness cutoffs have been used in the setting of general screening and intervention as opposed to the medico-legal setting where issues of product liability or tortious conduct may affect the interpretation of what threshold of cost effectiveness monitoring must meet to be considered indicated.
Similarly, a risk–benefit analysis may be of use in determining whether the risks of the monitoring test outweigh the benefits for the exposure and population in question or susceptible sub-populations. Non-invasive monitoring such as serial physical examinations or spirometry is more likely to have favorable risk–benefit profiles than minimally invasive monitoring tests (e.g., phlebotomy, plain radiographs). Diagnostic testing that is significantly invasive, experimental, time-consuming, or requires inpatient hospitalization is inappropriate as a medical monitoring activity. Application of the USPSTF analytic framework and use of Outcome Tables may be of benefit in the evaluation of risk–benefit.
It is important to recognize that medical monitoring, may at times, be considered for so-called vulnerable populations such as pregnant females, infants, children, elderly, and immunocompromised individuals. When such recommendations are being considered, it is important to apply the basic principles of exposure and dose. If the basic principles of exposure and dose are not fulfilled, the so-called vulnerable populations may not require special consideration.
The proposed monitoring test or procedure must be both sensitive and specific for the adverse health effect being monitored. The attributable risk for the adverse health effect due to the exposure should be estimated so that the positive and negative predictive values for the monitoring test may be calculated and evaluated for favorability. Monitoring tests with low positive predictive values will result in unfavorable risk–benefit ratios due to over-diagnosis and unnecessary further diagnostic testing and treatment.
Monitoring should be performed for only the time period that the exposed population is at substantially increased risk of the adverse health effect(s). In establishing the monitoring period, onset and resolution of exposure and minimum and maximum latencies for the adverse health effect should be considered.
Conclusions
Unless stringent criteria are met, the establishment of a medical monitoring program should be considered imprudent. In the context of litigation, plaintiffs may seek medical monitoring programs after documented or putative exposures. The role of the clinical toxicologist, in this setting, is to evaluate the scientific justifications and medical risks and assist the courts in determining whether monitoring would be expected to result in a net public health benefit.
Disclosure statement
The authors report no declarations of interest.
References
- Agency for Toxic Substances and Disease Registry. ATSDR's Final Criteria for Determining the Appropriateness of a Medical Monitoring Program Under CERCLA. Federal Register. 1995;60:388–404.
- Gochfeld M. Medical surveillance and screening in the workplace: complementary preventive strategies. Environ Res. 1992;59:67–80.
- Guzelian CP, Hillner BE, Guzelian PS. Quantitative methodology for determining the need for exposure-prompted medical monitoring. Indiana Law J. 2004;79:57.
- United States Preventive Services Task Force. Procedure Manual. In: Agency for Healthcare Research and Quality, editor; 2008.
- Beeler MF, Sappenfield R. Medical monitoring. What is it, how can it be improved? Am J Clin Pathol. 1987;87:285–288.
- Halperin WE, Ratcliffe J, Frazier TM, et al. Medical screening in the workplace: proposed principles. J Occup Environ Med. 1986;28:547–552.
- Breysse PN, Weaver V, Cadorette M, et al. Development of a medical examination program for former workers at a Department of Energy national laboratory. Am J Ind Med. 2002;42:443–454.
- Daugherty NM, Falk RB, Furman FJ, et al. Former radiation worker Medical Surveillance Program at Rocky Flats. Health Phys. 2001;80:544–551.
- Brenner DJ, Hall EJ. Cancer risks from CT scans: now we have data, What Next? Radiology. 2012;265:330–331.
- Gochfeld M, Bogden JD, Louria DB. Ethics and principles in medical monitoring of populations exposed to environmental hazards. J Occup Environ Med. 2009;51:1363–1366.
- Mandel JH, Maldonado G. Comprehensive medical monitoring after environmental contamination: not ready for prime time. J Occup Environ Med. 2010;52:465. Author reply -6.
- Bourne R. Medical monitoring without physical injury: the least justice can do for those industry has terrorized with poisonous products. SMU Law Rev. 2005;58:251–302.
- Studdert DM, Mello MM, Brennan TA. Medical monitoring for pharmaceutical injuries: tort law for the public's health? JAMA. 2003;289:889–894.
- LaRatta AR, Paszamant BS. Diagnosing medical monitoring costs under CERCLA: checking for a pulse. Villanova Environ Law J. 1996;7:81–107.
- Saxon AE. Tobacco litigation: medical monitoring of healthy smokers. J Law Med Ethics. 2002;30:755–758.
- Schwartz VE, Lorber L, Laird EJ. Medical monitoring: the right way and the wrong way. Missouri Law Rev. 2005;70:349.
- McDavid GE, Laccabue MG. 2006: a difficult year for medical monitoring proponents. Reed Smith LP 2007 January 8; 2007.
- Williams ME. The scientific case against medical monitoring. In-House Defense Q. 2011;40–51.
- Gonzalez EA, Valori RW. Medical monitoring claims are viable in Florida. Florida Bar J. 2001;75:66.
- Jack W. Leach, et al. v E.I. du Pont de Nemours and Co Class Action Settlement Agreement.
- Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9.
- United States District Court for the Eastern District of Pennsylvania. In Re: Diet Drugs (Phentermine/Fenfluramine/Dexfenfluramine) Products Liability Litigation. MDL Docket No 1203; 28 August 2000.
- Wones R, Pinney SM, Buckholz JM, et al. Medical monitoring: a beneficial remedy for residents living near an environmental hazard site. J Occup Environ Med. 2009;51:1374–1383.
- US Centers for Disease Control and Prevention. Fernald Risk Assessment Project 2010. Available from: http://www.cdc.gov/nceh/radiation/fernald/default.htm
- Moline JM, Herbert R, Levin S, et al. WTC medical monitoring and treatment program: comprehensive health care response in aftermath of disaster. Mt Sinai J Med. 2008;75:67–75.
- Berger KI, Reibman J, Oppenheimer BW, et al. Lessons from the World Trade Center disaster: airway disease presenting as restrictive dysfunction. Chest. 2013;144:249–257.
- de la Hoz RE, Mallea JM, Kramer SJ, et al. Polysomnographic diagnoses among former world trade center rescue workers and volunteers. Arch Environ Occup Health. 2012;67:239–242.
- Ekenga CC, Friedman-Jimenez G. Epidemiology of respiratory health outcomes among World Trade Center disaster workers: review of the literature 10 years after the September 11, 2001 terrorist attacks. Disaster Med Public Health Prep. 2011;5 Suppl 2:S189–S196.
- Feldman DM, Baron SL, Bernard BP, et al. Symptoms, respirator use, and pulmonary function changes among New York City firefighters responding to the World Trade Center disaster. Chest. 2004;125:1256–1264.
- Guidotti TL, Prezant D, de la Hoz RE, et al. The evolving spectrum of pulmonary disease in responders to the World Trade Center tragedy. Am J Ind Med. 2011;54:649–660.
- Jordan HT, Brackbill RM, Cone JE, et al. Mortality among survivors of the Sept 11, 2001, World Trade Center disaster: results from the World Trade Center Health Registry cohort. Lancet. 2011;378:879–887.
- Nolan A, Naveed B, Comfort AL, et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest. 2012;142:412–418.
- Schenck EJ, Echevarria GC, Girvin FG, et al. Enlarged pulmonary artery is predicted by vascular injury biomarkers and is associated with WTC-Lung Injury in exposed fire fighters: a case–control study. BMJ Open. 2014;4:e005575.
- Soo J, Webber MP, Hall CB, et al. Pulmonary function predicting confirmed recovery from lower-respiratory symptoms in World Trade Center-exposed firefighters, 2001 to 2010. Chest 2012;142:1244–1250.
- Udasin I, Schechter C, Crowley L, et al. Respiratory symptoms were associated with lower spirometry results during the first examination of WTC responders. J Occup Environ Med. 2011;53:49–54.
- Webber MP, Moir W, Crowson CS, et al. Post-September 11, 2001, incidence of systemic autoimmune diseases in world trade center-exposed firefighters and emergency medical service workers. Mayo Clin Proc. 2016;91:23–32.
- Wisnivesky JP, Teitelbaum SL, Todd AC, et al. Persistence of multiple illnesses in World Trade Center rescue and recovery workers: a cohort study. Lancet. 2011;378:888–897.
- Zvolensky MJ, Kotov R, Schechter CB, et al. Post-disaster stressful life events and WTC-related posttraumatic stress, depressive symptoms, and overall functioning among responders to the World Trade Center disaster. J Psychiatr Res. 2015;61:97–105.
- Savitz DA, Oxman RT, Metzger KB, et al. Epidemiologic research on man-made disasters: strategies and implications of cohort definition for World Trade Center worker and volunteer surveillance program. Mt Sinai J Med. 2008;75:77–87.
- Hill AB, The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300.
- Acquavella JF, Friedlander BR, Ireland BK. Interpretation of low to moderate relative risks in environmental epidemiologic studies. Annu Rev Public Health. 1994;15:179–201.
- Lees-Haley PR, Williams CW. Neurotoxicity of chronic low-dose exposure to organic solvents: a skeptical review. J Clin Psychol. 1997;53:699–712.
- Woodside FC III, Lydon DR. Litigation bias. N Eng J Med. 1983;308:1604.
- Kelly K. The Myth of 10−6 As a Definition of Acceptable Risk. 84th Annual Meeting of the Air and Waste Management Association. Vancouver, BC, Canada: Environmental Toxicology International, Inc.; 1991.
- Fewtrell L, Bartram J, editors. Water Quality – Guidelines, Standards and Health: Assessment of risk and risk management for water-related infectious disease. London, UK: World Health Organization; 2001.
- US Occupational Safety and Health Administration. Occupational Exposure to Asbestos; 1994.
- UK Health and Safety Executive. Reducing risks, protecting people – HSE's decision-making process. Norwich, UK: Her Majesty's Stationery Office; 2001.
- Bellinger DC. Interpretation of small effect sizes in occupational and environmental neurotoxicology: individual versus population risk. Neurotoxicology. 2007;28:245–251.
- Hoel D, Kaplan N, Anderson M. Implication of nonlinear kinetics on risk estimation in carcinogenesis. Science. 1983;219:1032–1037.
- Juni RL. Hormesis and toxic torts: traditional torts and claims for subclinical harm. Hum Exp Toxicol. 2008;27:109–112.
- Alexander FE. Clusters and clustering of childhood cancer: a review. Eur J Epidemiol. 1999;15:847–852.
- Wold KS, Byers T, Crane LA, et al. What do cancer survivors believe causes cancer? (United States). Cancer Causes Control. 2005;16:115–123.
- Collins JJ, Jammer B, Sladeczek FM, et al. Surveillance for angiosarcoma of the liver among vinyl chloride workers. J Occup Environ Med. 2014;56:1207–1209.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness – the curious resilience of the $50,000-per-QALY threshold. N Eng J Med. 2014;371:796–797.