Abstract
Background: Insect consumption is a common practice in the Asian culture and all over the world. We are reporting an outbreak investigation of histamine poisoning from ingestion of fried insects.
Methods: On 24 July 2014, a group of students at a seminar presented to Angthong Provincial Hospital, Thailand, with pruritic rash after ingesting snacks consisting of fried insects from a vendor. We initiated an outbreak investigation with retrospective cohort design and collected samples of remaining foods for analyses. Attack rates, relative risks and their confidence intervals (CI) were calculated.
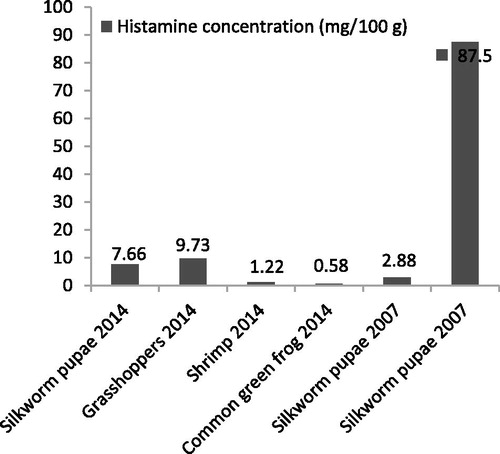
Results: Out of 227 students, 28 developed illnesses that were consistent with our case definition which included, flushing, pruritus, urticarial rashes, headache, nausea, vomiting, diarrhea, dyspnea and bronchospasm. Two children were hospitalized for progressive bronchospasm overnight without serious complications. The types of food ingested included a lunch that was provided at the seminar for all students and snacks that 41 students bought from the only vendor in the vicinity. The snacks included fried grasshoppers, silkworm pupae, common green frogs, bamboo borers, crickets and meat balls. The attack rates were highest (82.6 and 85.0%) among students who ingested fried grasshoppers and silkworm pupae and lowest (4.4 and 5.3%) among those who did not ingest them, with relative risk of 18.7 (95% CI 9.6–36.4) for grasshoppers and 16.0 (95% CI 8.8–29.3) for silkworm pupae. Histamine concentrations in the fried grasshoppers and silkworm pupae were 9.73 and 7.66 mg/100g, respectively.
Discussion and conclusion: Through epidemiological analysis and laboratory confirmation, we have illustrated that histamine poisoning can occur from ingestion of fried insects. We postulate that histidine, which is present in high concentration in grasshoppers and silkworm pupae, is decarboxylated by bacteria to histamine, a heat stable toxin. The ingestion of histamine is responsible for the clinical pictures being reported.
Introduction
Insects as a group has been a part of the human diet since ancient times and continues to be a staple food in many cultures to this day due to its abundant proteins, vitamins and low fat contents [Citation1]. Traditionally, insects are processed on small scales for household consumption and the method of processing can vary, depending upon the geographic location, availability of insect species in the area and existing regional or cultural practices. Such small scale consumptions are uncomplicated and the batch of insects being produced is often consumed at once. Currently, insects as food is gaining popularity, both as sustenance in underdeveloped countries where protein sources are limited, as delicacies in tourist destinations, and are sold over the internet and by street vendors for those seeking a new food experience [Citation1–3]. Consequently, the consumption and production of insect foods are now done on an industrial scale all across the world such as in China, the United States, Africa and Southeast Asia [Citation1–5]. In 2013, the FAO issued a document “Edible insects: future prospects for food and feed security” which emphasizes the prospects of insects as worldwide human food in the future [Citation1]. Not surprisingly, as the food becomes more popular, reports of illnesses associated with consumption of insects begin to emerge in the medical literatures and in the news, thus reiterating the importance of a standardized production method that will yield contaminant-free insects for human consumption [Citation1]. Despite the frequency with which such unwanted events occur, however, the information regarding the symptoms of toxicity from insect foods are still sparse, due in part to the case-by-base nature of these reports [Citation6]. A large outbreak, on the other hand, can surreptitiously provide key analytical epidemiologic information that is currently lacking in case reports.
In Thailand during December 2007 to January 2008, Mungaomklang et al. reported a large outbreak of scombroid poisoning-like illnesses with 118 patients. Consumption history and food sample analysis suggested that fried silkworm pupae were the cause of the outbreak. In that case series, the first of its kind on this topic, the investigators hypothesized that poor refrigeration of raw silkworm pupae allowed the bacteria to grow and cause the decarboxylation of histidine to histamine, leading to the appearance of a hypersensitivity-like reaction reported in the literatures. Nevertheless, as with all previous publications, epidemiological analysis was lacking, as well as the necessary laboratory tests which would exclude an endogenous release of histamine were missing. Moreover, the paper was published only in the Thai language, thus barring its distribution in the international scientific community [Citation7]. On the other hand, the incident did reiterate the need for a carefully conducted epidemiologic investigation of future outbreaks which would confirm the association between the consumption of such food and histamine poisoning and fill in the knowledge gap regarding the toxicologic and pathophysiologic features of this poisoning.
Such opportunity arose on 24 July 2014, when Matichon Online™, a Thai online newspaper, reported that 18 teenagers presented to Angthong Provincial Hospital with nausea, vomiting, diarrhea, headache, dizziness, difficulty in breathing and pruritic skin rash while attending a school seminar being held at a temple nearby. Initially, they were assumed to have acute insecticide poisoning, based on the history that all the teenagers involved had consumed fried snacks which included insects 1-6 hours before the onset of illness. Upon hearing this news, two clinical toxicologists from the Siriraj Poison Control Center recognized the symptoms to be that of histamine poisoning, due to their clinical similarities to previously reported cases. The Angthong Provincial Health Office and the Angthong Provincial Hospital were contacted and gave the investigators permission to participate in an on-going outbreak investigation on this cluster of patients. The assumption was that the illness was an acute poisoning with type 1 hypersensitivity-like syndrome from ingestion of insects.
Methods and materials
Recorded data were obtained with permission from Angthong Provincial Health Office. All personal patient information including names and birthdates were deleted prior to analysis by the investigators. The use of all secondary and analytical data obtained from this outbreak investigation was approved by the Siriraj Institutional Review Board.
Clinical interview
On 25 July 2014, the investigators started by meeting the attending physicians and the local outbreak investigation team who were in direct contact with the affected students during the outbreak the day before. A retrospective cohort design was selected because all the students who attended the seminar were available for interview [Citation8]. Standard food poisoning investigation form issued by the Bureau of Epidemiology, Ministry of Public Health of Thailand (MOPH) was used with some modifications to cover the issues of histamine poisoning. For the case definition of histamine poisoning, the investigators suggested that the team utilize a modified version of the 2011 criteria for scombroid toxin (histamine) published in the United States Center of Disease Control and Prevention’s Guide to Confirming an Etiology in Foodborne Disease Outbreak () [Citation9]. Interviews of affected persons were conducted by local health officers who were familiar with the standard food poisoning form and were educated regarding histamine poisoning. The data collected included age, gender, year group, symptoms and their onsets, food and beverages that were consumed before the symptoms developed, medical history and allergy and medication history. Data on clinical symptoms and signs, treatment and clinical course of each patient were extracted from the medical records of patients who presented at the hospital. Any available clotted blood samples were sent for serum butyrylcholinesterase and tryptase analysis. An epidemic curve was generated from the collected data. Patients who experienced at least one of the symptoms listed in the case definition were considered symptomatic and plotted in the epidemic curve. The vender of the fried insects was interviewed by the local health officers for information regarding the source and method of preparation of the insects.
Table 1. Case definition of histamine poisoning in the cluster investigation.
Analysis of left-over snack samples
The local health officials also obtained fried insects that were left-over from the previous day. These samples were preserved in an icebox during transportation and placed in freezers at the Angthong Health Office. They were analyzed for the presence of histamine and insecticide residues. The samples were separated by ion exchange chromatography and were analyzed for histamine by fluorometric method [Citation10]. Analyses for residues of organophosphorus, carbamate and pyrethroid insecticides were performed using standard Gas Chromatography-Mass Spectrometry at the Department of Medical Sciences, MOPH.
Statistical analyses
Descriptive data were reported as frequency, percentage, mean and standard deviation (SD), where appropriate. Attack rates, relative risks (RR) and the 95% confidence intervals (CI) for each food and beverage item were calculated. Chi square test was used to evaluate the association between food or beverage consumption and the symptoms. Alpha of 0.05 was used to determine statistical significance.
Results
Two hundred and twenty-seven school students aged 13–19 years who were studying in grade 7–12 attended the school seminar on the day of the incident. All of them agreed to be interviewed by the health officers.
Clinical symptoms and course of the illness
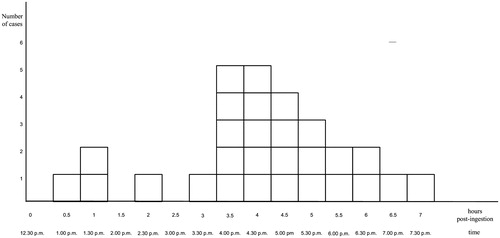
Of all the students who attended the seminar, twenty eight students developed at least one of the symptoms listed in the case definition. This included skin rashes, pruritus, nausea, vomiting, dizziness, headache, dyspnea, diarrhea, abdominal pain, numbness in the limbs and face and chest tightness during the period between 1.00 and 7.30 p.m. on 24 July 2014 (). Three cases (10.7%) had symptom onset that was within the 1.5 hours after the ingestion, as specified for scombroid case definition. The remaining 25 cases (89.3%) had onsets that ranged from 2–7 hours. Overall, 18 patients presented to the Angthong Provincial Hospital with two 14-year-old previously healthy males (patients A and B) whose symptoms started at 3.00 p.m. and had progressive dyspnea and bronchospasm. No hypotension was observed. They were hospitalized and treated with intravenous chlorpheniramine, domperidone and nebulized salbutamol. Symptoms completely resolved within 8 hours for patient A and 10 hours for patient B. The other sixteen patients were treated with oral chlorpheniramine and domperidone and sent home from the Acute Care Department. None of the patients were treated with corticosteroid. Patients A and B returned to the hospital for follow up on day 3 and were totally normal.
Table 2. Frequency of clinical manifestations among the symptomatic students (n = 28).
Food consumption interview
All 227 students who attended the seminar had breakfast from home prior to the start of the seminar at 8.30 a.m. Lunch was provided by the school and served between 12.00 and 1.00 p.m. It consisted of steamed rice, fried chicken and stirred fried pork with basil, fried vegetable and water. All 227 students ate lunch that was provided by the school and 41 students (18.1%) ate snacks from the mobile vender after lunch (12.30–1.00 p.m.). Twenty-two students reported eating more than one type of the snack. Incidentally, this vendor was the only vendor in the temple ground on that particular day. The deep-fried snacks included meatballs, shrimps, common green frogs, crickets, bamboo borers, grasshoppers and silkworm pupae. Commonly, these were popular snacks being peddled in the countryside of Thailand. Patient A ingested approximately 10 silkworm pupae and patient B ingested 10 silkworm pupae and 4–5 fried grasshoppers. Both had eaten these snacks before from other vendors but never experienced any illness.
Epidemic curve
Hour zero on the epidemic curve corresponded to 12.30 p.m. There was an observable rapid rise in the case number with a single peak at hours 3.5 to 4 and a steady decline afterward. The first case was at hour 0.5 and the last case was at hour 7. The median time of onset was approximately 4 hours. The epidemic plot had the appearance of a point source pattern, suggesting that a single source of exposure had caused the symptoms (). The fried grasshoppers and silkworms were associated with the highest attack rates (82.6 and 85.0%) while non-consumption of these items led to the lowest attack rates (4.4 and 5.3%). Grasshoppers and silkworms also accounted for the highest proportion of sick cases (67.9 and 60.7%). Relative risks and chi square tests revealed statistical significance for consumption of fried grasshoppers (RR 18.7, 95% CI 9.6–36.4, p value <.001) and fried silkworm pupae (RR 16.0, 95% CI 8.8–29.3, p value <.001) and the development of symptoms. In addition, when the consumption categories of fried grasshopper and fried silkworm pupae were combined, the attack rate among the insect consumers became even higher (84.4%) while that of non-consumers became even lower (0.5%); the proportion of sick cases increased (96.4%), yielding an extremely high relative risk of 164.5 (95% confidence interval 23.2–1168.8). None of those who consumed fried common green frogs experienced any symptoms in this cluster. Consumers of other food and snack items had attack rates ranging from 10.2 to 25.0 with no statistical significance found for the relative risks and chi square tests ().
Table 3. Ingested food and snacks, item-specific attack rates and relative risk (n = 227 cases).
Clotted blood analysis
Tryptase concentrations in the serum of patients A and B, which were drawn within 2.5 hours after the onset of symptoms, were less than 1.0 mg/L (reference range 1.0–15.0 mg/L). Serum butyrylcholinesterase concentrations for patients A and B were 2350 and 2600 U/L, respectively (reference range 1900–4100 U/L).
Insect histamine and insecticide analysis
Histamine contents in fried grasshopper, fried silkworm pupa, common green frog and fried shrimp samples were 9.73, 7.66, 0.58 and 1.22 milligram/100 grams, respectively (). No cricket and bamboo borer were available for the analysis. Analyses for residues of organophosphorus, carbamate and pyrethroid insecticides were negative.
Vendor interview
The mobile vendor bought his supplies of meatballs, insects and other cooking ingredients from the local market in the town of Angthong. It was his habit to purchase his supplies and store them in the freezer at home to be used every 2–3 days. The vendor prepped the meats by marinating them in fish sauce and refrigerated them for 24 hours before they were ready to be cooked. The vehicle the vendor used to peddle his goods was a motorcycle with a sidecar containing an icebox where he stored the uncooked meats. He often fried the insects and meatballs on-site. He had been in the same fried snack business for more than 5 years and was not aware that any of his customers were ever taken ill before this incident.
Discussion
The clinical manifestations of patients in this study resemble an immediate-type hypersensitivity reaction and should these findings be isolated to a single patient, hypersensitivity to food would be the most likely diagnosis [Citation11–14]. However, as a cluster, these symptoms fit best with the syndrome of histamine (scombroid) poisoning from food and was further confirmed by normal tryptase in the patients for whom the disease is the most severe [Citation11,Citation15,Citation16]. Our study reveals that, despite similarities in clinical presentations, scombroid originating from insects can have some distinguishing features compared to that from fish. Typically, the onset of symptoms in histamine or scombroid poisoning from fish is rapid, occurring within 0.5 and can be delayed for up to 2.5 hours after the ingestion [Citation15–18]. For histamine poisoning due to insects, a longer period before onset of symptoms is observed, both in this outbreak and in the outbreak of 2007–2008 which range from 0.25 to 22 hours with a median 4 hours before the onset of symptoms [Citation7]. In a case report and review by Ji et.al., 14 patients with anaphylaxis associated with silkworm pupae ingestion show a delay in symptoms onsets for up to 4 hours [Citation6].
Histamine exists as a neurotransmitter in insects. Yet, given its ubiquitous presence and despite widespread consumption of insect foods, human poisonings from histamine found in insects remain quite rare. This is because the amount present is minute and is unlikely to be the source of significant toxicity. On the other hand, we postulate that the histamine which causes the scombroid-like syndrome seen in this outbreak is a de novo product of bacterial decarboxylation of histidine. This is a well-known phenomenon which is recognized by the tuna-canning industry where the tuna supplied by fishing vessels were caught in distant waters and processed inland during which the refrigeration process may not be optimum [Citation18,Citation20–23]. Silkworm pupae and grasshoppers are insects with high histidine contents. Moreover, the histamine contents in these foods are higher than in cases where symptomatic scombroid poisoning are being reported (≥ 5.0 mg/100 gram) [Citation19]. Once histamine is produced, the toxin is heat-stable and cooking or deep frying cannot eliminate histamine from the contaminated food, as evident by the various outbreaks of histamine poisoning in canned tuna [Citation18,Citation20–23]. The explanation for occurrence of histamine poisoning in specific foods, namely silkworm pupa and grasshoppers, but not the cricket and bamboo borer, in our cluster is primarily logistical. The presently unregulated supply chains of insects rely heavily on standardized and predictable refrigeration to keep the possibilities of spoilage and bacterial overgrowth to a minimum. While the raw bamboo borers and crickets are supplied to the wholesale markets from domestic farms in Thailand, the grasshoppers and silkworm pupae are partly produced domestically and partly imported from neighboring countries [Citation24,Citation25]. This longer and more protracted supply chain renders them at risk of bacterial overgrowth due to variations in quality of refrigeration during multistep transportation and storage. Average concentrations of histidine, the precursor for histamine are virtually comparable in silkworm pupae (22.5–35.4 mg/gram protein), grasshopper (27.9 mg/gram protein), bamboo borers (23.3 mg/gram protein) and crickets (22.7–23.4 mg/gram protein) [Citation26,Citation27].
Despite such strong data supporting the conclusion that fried grasshoppers and fried silkworms are the culprits, one important factor that must not be overlooked is the contamination of cooking ingredients with histamine. This is a real possibility since, in Thailand, the salting agents is often made from fish sauce which is a well-known source of histamine [Citation28]. In this study, we are unable to obtain the marinating ingredients for histamine analysis. On the other hand, some foods such as fried shrimps, bamboo borers or crickets which use the same marinade are found to have very low attack rates and insignificant relative risks. Most importantly, none of those who consumed fried common green frogs reported any symptoms. Therefore, it is reasonable to exclude the possibility of fish sauce as a cause of histamine poisoning in our cluster.
Public health interventions
On 25 July 2014, the snack vender was educated to refrigerate the uncooked insects to prevent spoilage. Results of the epidemiological analysis and histamine contents were reported to the Angthong Provincial Health Office within 2 weeks of the incidents. The students, parents and teachers were informed by the health officers about the results. Moreover, the MOPH issued a nationwide health message to raise awareness of potential untoward effects from insect consumption. No further cluster of histamine poisoning cases from insect ingestion had been reported since July 2014. Lastly, the investigators acting on behalf of the Siriraj Hospital Poison Control Center made recommendations that the same standard for regulating and monitoring the production and supply chain of uncooked and cooked insects be applied nationwide [Citation23].
Limitations
Limitations of our outbreak investigation are the inability to obtain leftover cricket and bamboo borer samples and the marinating sauce for histamine analyses. In addition, we are unable to trace the supply chain of the uncooked insects to clarify the quality of insect preservations. For serum tryptase concentrations, we do not have convalescent or follow-up concentrations at 24 hours post-onset of the sickness, which serve as baseline concentrations for the patients. In the future, we suggest that a study be conducted on the different methods of storage of fresh, raw insects to demonstrate the production of histamine from histidine.
Conclusion
Insects are promising source of nutrients for human and an up-and-coming solution for the problem of food security faced by the world’s population today. Histamine (scombroid) poisoning can occur not only from fish ingestion, but also from insect consumption. We reported an outbreak investigation that confirms insects as the cause of histamine poisoning in human and demonstrates its varying clinical presentation. In order to detect such outbreaks in the future, the diagnostic criteria for histamine poisoning from insect consumption should accommodate longer onsets of symptoms.
Acknowledgements
The authors thank Ms. Woranut Chantaworn, Angthong Provincial Health Office, Mr. Sayan Kongchareon, Ban-it Subdistrict Health Clinic, Dr. Darunee Ngamphoophan, Angthong Provincial Hospital and Ms. Pinsumon Chomchai, Shrewsbury International School, Bangkok for assistance in investigating the outbreak. Special thanks to Dr. Chanida Hansawasdi, Mahidol University International College for histamine analyses in food contents.
Disclosure statement
The authors report no declaration of interest.
References
- van Huis A, Itterbeeck JV, Klunder H, et al. Food and Agriculture Organization of the United Nations. 2013, Edible insects: future prospects for food and feed security. Rome: Food and Agriculture Organization of the United Nations; 2013. 191 p.
- Feng Y, Chen XM, Zhao M, et al. Edible insects in China: utilization and prospects. Insect Science. 2017[Feb 22];[1–15]. [Epub ahead of print]. doi: 10.1111/1744-7917.12449
- House J. Consumer acceptance of insect-based foods in the Netherlands: academic and commercial implications. Appetite. 2016;107:47–58.
- Payne CL, Scarborough P, Rayner M, et al. Are edible insects more or less ‘healthy’ than commonly consumed meats? A comparison using two nutrient profiling models developed to combat over- and undernutrition. Eur J Clin Nutr. 2016;70:285–291.
- van Huis A. Edible insects are the future? Proc Nutr Soc. 2016;75:294–305.
- Ji KM, Zhan ZK, Chen JJ, et al. Anaphylactic shock caused by silkworm pupa consumption in China. Allergy. 2008;63:1407–1408.
- Mungaomklang A, Teeyapant P, Sangsawang C, et al. Fried pupa of silkworms food poisoning outbreak due to histamine toxicity in seven provinces-Thailand, Dec 2007 – Jan 2008. J Health Sci. 2009;18:504–514.
- Dicker RC. Field Epidemiology. 2nd ed,. New York: Oxford University Press; 2002. Chapter 7, Designing studies in the field; p. 117–31.
- Guide to Confirming an Etiology in Foodborne Disease Outbreak Atlanta (GA): Centers for Disease Control and Prevention. 2011 Available from: https://www.cdc.gov/foodsafety/outbreaks/investigating-outbreaks/confirming_diagnosis.html
- AOAC International. Official method: histamine in seafood fluorometric method. Rockville (MD): AOAC; 2003. p. 20.
- Attaran RR, Probst F. Histamine fish poisoning: a common but frequently misdiagnosed condition. Emerg Med Jl. 2002;19:474–475.
- Guly HR, Grant IC. Case of the month: lesson of the week: don't forget scombroid. EmergM J. 2006;23:955–956.
- Jantschitsch C, Kinaciyan T, Manafi M, et al. Severe scombroid fish poisoning: an underrecognized dermatologic emergency. J Am Acad Dermatol. 2011;65:246–247.
- O'Connor MM, Forbes GM. Scombroid poisoning: not fish allergy. Aust N Z J Med. 2000;30:520.
- Ricci G, Zannoni M, Cigolini D, et al. Tryptase serum level as a possible indicator of scombroid syndrome. Clinical Toxicol. 2010;48:203–206.
- Ridolo E, Martignago I, Senna G, et al. Scombroid syndrome: it seems to be fish allergy but… it isn't. Curr Opin Allergy Clin Immunol. 2016;16:516–521.
- Davis J, Henry SA, Rowland J, et al. Scombroid fish poisoning associated with tuna steaks–Louisiana and Tennessee, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:817–819.
- Feng C, Teuber S, Gershwin ME. Histamine (Scombroid) Fish Poisoning: a Comprehensive Review. Clin Rev Allergy Immunol. 2016;50:64–69.
- Bartholomew BA, Berry PR, Rodhouse JC, et al. Scombrotoxic fish poisoning in Britain: features of over 250 suspected incidents from 1976 to 1986. Epidemiol Infect. 1987;99:775–782.
- Colombo FM, Cattaneo P, Confalonieri E, Bernardi C. Histamine food poisonings: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2016[Oct 28];[1?21]. [Epub ahead of print]. doi: 10.1080/10408398.2016.1242476
- Hungerford JM. Scombroid poisoning: a review. Toxicon. 2010;56:231–243.
- Prester L. Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:1547–1560.
- Visciano P, Schirone M, Tofalo R, et al. Histamine poisoning and control measures in fish and fishery products. Front Microbiol. 2014;5:500.
- Hanboonsong Y, Durst PB, Jamjanya T, Six-legged Livestock: edible Insect Farming, Collection and Marketing in Thailand. Bangkok: RAP Publication; 2013.
- Halloran A, Hanboonsong Y, Roos N, et al. Life cycle assessment of cricket farming in north-eastern Thailand. J Clean Prod. 2017;156:83–94.
- Rumpold BA, Schluter OK. Nutritional composition and safety aspects of edible insects. Mol Nutr Food Res. 2013;57:802–823.
- Wang D, Zhai SW, Zhang CX, et al. Nutrition value of the Chinese grasshopper Acrida cinerea (Thunberg) for broilers. Anim Feed Sci Tech. 2007;135:66–74.
- Brillantes S, Paknoi S, Totakien A. Histamine formation in fish sauce production. J Food Sci. 2002;67:2092–2094.