Abstract
Introduction: Treatment of acute organophosphorus or carbamate insecticide self-poisoning is often ineffective, with tens of thousands of deaths occurring every year. Researchers have recommended the addition of magnesium sulfate or calcium channel blocking drugs to standard care to reduce acetylcholine release at cholinergic synapses.
Objective: We aimed to review systematically the evidence from preclinical studies in animals exposed to organophosphorus or carbamate insecticides concerning the efficacy of magnesium sulfate and calcium channel blocking drugs as therapy compared with placebo in reducing mortality or clinical features of poisoning. We also systematically reviewed the evidence from clinical studies in patients self-poisoned with organophosphorus or carbamate insecticides concerning the efficacy of magnesium sulfate and calcium channel blocking drugs as therapy compared with placebo, in addition to standard therapy, in reducing mortality, atropine requirement, need for intubation and ventilation, and intensive care unit and hospital stay.
Methods: We performed a systematic review for articles on magnesium sulfate and calcium channel blocking drugs in organophosphorus or carbamate insecticide poisoning using PubMed and China Academic Journals Full-text (Medicine/Hygiene Series) databases and keywords: “organophosphorus or organophosphate poisoning”, “cholinesterase inhibitor poisoning” OR “carbamate poisoning” AND “magnesium”, “calcium channel blocker”, or generic names of different calcium channel blocking drugs. Review of titles and abstracts revealed 2262 papers of potential relevance. After review of the full papers, a total of 19 papers relevant to the question were identified: five preclinical studies, nine case reports or small case series, and five clinical studies and trials. We also obtained primary data from three unpublished clinical trials of magnesium sulfate, providing data from a total of eight clinical studies and trials for analysis. All studies were of organophosphorus insecticides; no studies of carbamates were found. No pre-clinical or clinical studies of calcium channel blocking drugs and magnesium sulfate in combination were found. We extracted data on study type, treatment regimens, outcome, and side effects.
Pre-clinical studies: Two rodent studies indicated a benefit of calcium channel blocking drugs treatment on mortality if given before or soon after organophosphorus exposure, in addition to atropine and/or oxime. In poisoned minipigs, treatment with magnesium sulfate after organophosphorus insecticide poisoning reduced cholinergic stimulation and hypertension. Of note, magnesium sulfate further suppressed serum butyrylcholinesterase activity in one rat study.
Observational clinical studies: Calcium channel blocking drugs and magnesium sulfate have been used to treat cardiac dysrhythmias and hypertonic uterine contractions in organophosphorus poisoned patients. A small neurophysiological study of magnesium sulfate reported reversion of neuromuscular junction effects of organophosphorus insecticide exposure.
Comparative clinical studies: Only four of eight studies were randomized controlled trials; all studies were of magnesium sulfate, of small to modest size, and at substantial risk of bias. They included 441 patients, with 239 patients receiving magnesium sulfate and 202 control patients. The pooled odds ratios for magnesium sulfate for mortality and need for intubation and ventilation for all eight studies were 0.55 (95% confidence interval [CI] 0.32–0.94) and 0.52 (95% CI 0.34–0.79), respectively. However, there was heterogeneity in the results of higher quality phase III randomized controlled trials providing more conservative estimates. Although a small dose-escalation study suggested benefit from higher doses of magnesium sulfate, there was no evidence of a dose effect across the studies. Adverse effects were reported rarely, with 11.1% of patients in the randomized controlled trials receiving the highest dose of magnesium sulfate requiring their infusion to be stopped due to hypotension.
Conclusions: Both preclinical and clinical data suggest that magnesium sulfate and calcium channel blocking drugs might be promising adjunct treatments for acute organophosphorus insecticide poisoning. However, evidence is currently insufficient to recommend their use. Mechanistic and large multi-center randomized controlled trials testing calcium channel blocking drugs and magnesium sulfate are required to provide the necessary evidence, with careful identification of the insecticides ingested and measurement of surrogate markers of toxicity, including butyrylcholinesterase activity.
Introduction
Acute organophosphorus (OP) and carbamate insecticide self-poisoning is a major public health problem [Citation1–3], likely accounting for over 100,000 suicides worldwide each year [Citation4] as well as thousands of deaths following unintentional poisoning [Citation5]. OP and carbamate compounds inhibit acetylcholinesterase (AChE) and other esterases, causing increased acetylcholine concentrations at, and over-stimulation of, cholinergic synapses in the autonomic nervous system, central nervous system, and neuromuscular junction (NMJ). The main cause of death is acute respiratory failure during an acute cholinergic crisis, due to reduced central respiratory drive, NMJ dysfunction, and bronchorrhea [Citation6–9]. Delayed NMJ dysfunction may also occur after resolution of the acute cholinergic syndrome (type II respiratory failure [Citation10] or intermediate syndrome [Citation11,Citation12]) in OP insecticide poisoning, prolonging the need for ventilation and leaving patients at risk of pneumonia, and other complications of ventilation. A small number of OP poisoned patients develop a delayed polyneuropathy, OP-induced peripheral neuropathy (OPIDN), several weeks after exposure.
Treatment of acute severe anticholinesterase insecticide poisoning is difficult with no new therapy introduced into routine clinical practice for 50 years [Citation13]. The anti-muscarinic drug atropine treats bronchorrhea and other muscarinic features of poisoning [Citation14,Citation15], but no therapy exists for acute NMJ dysfunction or failure of central respiratory drive. Respiratory failure must be treated with mechanical ventilation. Despite effective atropine therapy [Citation15], case fatality remains around 10% in patients who survive to hospital admission. Oximes, such as pralidoxime, are used to reactivate AChE after OP insecticide poisoning, potentially reducing acetylcholine concentrations at all cholinergic synapses, but evidence for effectiveness is currently unclear [Citation16,Citation17]. Other possible therapies have been tested in small clinical studies; including bicarbonate [Citation18], clonidine [Citation19], and particularly magnesium sulfate (MgSO4). Magnesium may work by blocking calcium channels, similar to calcium channel blocking drugs (CCB), such as nimodipine, thereby reducing acetylcholine release. If effective, magnesium or CCB could complement atropine treatment and improve therapy.
Objective
We aimed to review systematically the evidence from preclinical studies in animals exposed to OP or carbamate insecticides concerning the efficacy of MgSO4 and CCB as therapy, compared with placebo, in reducing mortality or clinical features of poisoning. We also systematically reviewed the evidence from clinical studies in patients self-poisoned with OP or carbamate insecticides concerning the efficacy of MgSO4 and CCB as therapy compared with placebo in reducing mortality, atropine requirement, need for intubation, and ventilation and ICU, and hospital stay.
Methods
PubMed and China Academic Journals Full-text Database (Medicine/Hygiene Series) searches were performed by MB and CMY using the terms “magnesium” or “calcium channel blocker”, and “organophosphorus” or “organophosphate” “poisoning” or “cholinesterase inhibitor poisoning”, or “carbamate poisoning” as either a subject term or keyword. Second searches used “magnesium”, or “nifedipine”, or “nimodipine”, or “nitrendipine”, or “verapamil”, and “organophosphate poisoning” or “cholinesterase inhibitor poisoning”, or “carbamate poisoning”. A full description of the search terms for each database can be found in Appendix A.
The Cochrane database of systematic reviews was searched, as was www.google.co.uk. There was no limitation on language. Articles retrieved from the searches were hand-searched for additional relevant studies and publications not found in our database search.
Three authors (MB, MYC, and ME) independently selected any article describing treatment of acute OP or carbamate insecticide poisoned animals or humans with magnesium or CCB, before all agreed on studies for inclusion. There were no exclusion criteria as we aimed to exhaustively identify all preclinical and clinical data, except the role of either therapy in preventing OPIDN, as has been shown for CCB in combination with calcium gluconate in poultry models of OP poisoning [Citation20] or when magnesium was administered as a cathartic in the gastric lavage [Citation21]. We did not include OP nerve agents in the search.
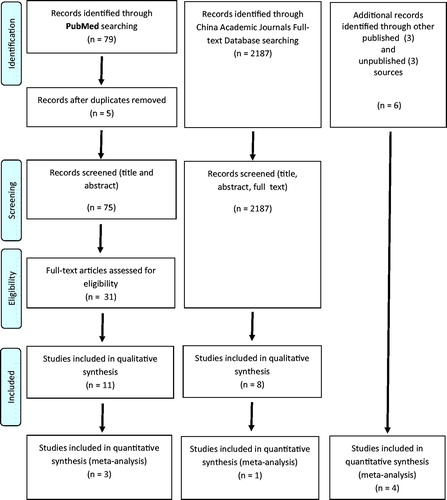
We identified relevant papers in three stages (). Review of titles and abstracts revealed 75 and 2187 papers of potential relevance in PubMed and China Academic Journals Full-text Database, respectively. The second stage, involving the screening of full-text articles for eligibility, revealed a total of 19 papers relevant to the question were identified: five preclinical studies [Citation22–26], nine case reports or small case series [Citation27–35], and five clinical studies, and trials [Citation36–40]. We also obtained primary data from three unpublished clinical trials of MgSO4, providing data from a total of eight clinical studies and trials for analysis. None of the studies was of carbamate insecticide poisoning; all were of OP insecticide poisoning. No pre-clinical or clinical studies of CCB and MgSO4 in combination were found.
Figure 1. Flow diagram illustrating stages of the review process. The very large number of papers identified in the Chinese database screen, compared to PubMed that were considered not relevant was due to the common practice of giving magnesium sulfate in the gastric lavage for OP insecticide poisoning in China. As the magnesium was not given as an antidote, but as a cathartic, these papers were excluded from the analysis.
Data on study type, treatments, outcomes, and complications were abstracted from papers. Outcomes of interest for clinical trials included mortality, need for intubation and ventilation, duration of ventilation, atropine dosing, and hospital, or ICU lengths of stay. Differences between authors were settled by consensus.
Some publications presented mean and standard deviations for non-normally distributed data. We, therefore, approached the authors for their primary data to allow it to be presented as medians with inter-quartile ranges (IQR). We also approached clinicians who had performed unpublished studies that we became aware of from the literature.
Clinical studies were assessed for risk of bias using the Cochrane Collaborations guidelines [Citation41]. Analysis was carried out using Statistical Package for Social Science for Windows (SPSS; SPSS Inc., Chicago, IL). Data are presented as median and interquartile range unless indicated otherwise. Chi-square tests were used for categorical data and the Mann–Whitney test for non-parametric variables. Odds ratios were calculated based on construction of two by two tables for each included study and presented by forest plots.
Results
Pre-clinical studies
Calcium channel blocking drugs
Two studies [Citation22,Citation23] reported the effect of CCB in rodent models of acute OP insecticide toxicity (). Nimodipine together with atropine and diazepam reduced lethality in paraoxon poisoned rats [Citation22]. Verapamil protected against muscle fasciculations and convulsions in dimethoate and omethoate poisoned rats and guinea pigs [Citation23].
Table 1. Pre-clinical studies of MgSO4 and CCB for acute organophosphorus insecticide toxicity.
Magnesium sulfate
Three studies [Citation24–26] reported the effect of MgSO4 in animal models of acute OP toxicity (). In a minipig parathion poisoning model, MgSO4 therapy prevented marked hypertension and tachycardia by reducing cholinergic stimulation [Citation24]. It improved skeletal muscle ATPase activity after OP poisoning in a rat model [Citation25]. In rats, prophylactic administration before dichlorvos increased nitric oxide concentrations in cardiac tissue and non-significantly decreased mortality [Citation26].
Of note, in one study, MgSO4 was associated with a further reduction in mean serum butyrylcholinesterase (BuChE) activity in rats receiving dichlorvos (control animals: 192.5 [standard deviation (SD) 51] mU/mL; dichlorvos: 93.6 [SD 18.2] mU/mL; MgSO4: 35.6 [SD 28.7] mU/mL) [Citation26]. The mechanism for this effect was unclear.
Observational clinical studies
Calcium channel blocking drugs
Three small studies reported the use of CCB in patients with cardiac dysrhythmias including cardiac arrests. One study reported the use of unnamed CCB and MgSO4 in individual patients but provided no details [Citation27]. A second study reported treatment of nine OP poisoned patients with diltiazem (1–3 mg/kg IV over 30–150 min) for a sinus tachycardia of around 165/min [Citation28]. The authors observed a decrease in heart rate in all but one patient; two patients died. A third study reported treatment with an unnamed CCB of seven patients with cardiac arrests 3–5 d post-OP poisoning; two patients died [Citation29].
We also found two Chinese observational studies of CCB in OP poisoned patients [Citation30,Citation31]. However, neither tested the effect of CCB as an OP antidote or provided data on mortality and intubation/ventilation according to treatment, focusing instead on their standard clinical use for cardiac dysrhythmias and injury. A study of 70 OP insecticide poisoned patients compared 34 patients treated with verapamil (5 mg intravenously three times daily) in addition to atropine, diazepam, and oxime for 3–5 d with 36 patients who had received atropine and oximes only [Citation30]. Verapamil treatment was associated with fewer pathological ECG changes (ST depression, QT prolongation, atrioventricular block), and lower serum creatine kinase (CK), aspartate transaminase, and lactate dehydrogenase (LDH) activity. A similar effect on CK, CK-MB and LDH activity was reported in 45 OP insecticide poisoned patients treated with verapamil (5 mg IV every 6 h for 3 d, then 40 mg orally three times daily for 1 week) compared to 44 patients treated conventionally [Citation31].
Magnesium sulfate
Four papers reported a case series of four patients [Citation32] and three single patient case reports [Citation33–35] receiving this treatment. A small neurophysiological study of MgSO4 in four patients reported reversion of the NMJ effects of OP exposure, but no improvement in clinical condition [Citation32]. These patients were not treated soon after hospital presentation but were studied at a single time point between 2 and 9 d post-exposure. Three single case reports described the use of MgSO4 to manage cardiac dysrhythmias [Citation33,Citation34] and hypertonic uterine contractions [Citation35]. None of the case reports assessed the effect of MgSO4 on the OP poisoning in general.
Comparative clinical studies
We identified eight comparative clinical studies, four of which were randomized controlled trials (RCT) (). All studies were of MgSO4 after OP self-poisoning; we found no such studies of CCB. All studies were at risk of bias as per the Cochrane review recommendations (); a sample size power calculation was done for only one study which did not recruit to its target. They included three unpublished studies found by literature review and discussion with colleagues; we were sent the primary data for these studies. The studies included 441 patients, with 239 patients receiving MgSO4 and 202 receiving standard care (, ).
Figure 2. Assessment of sources of potential bias, using Cochrane Collaboration guidelines [Citation41]. All studies were considered at risk of “other sources of bias” due to their small size, variation in the effect of the variable OP insecticides ingested, and lack of identification of the responsible insecticide by laboratory analysis.
![Figure 2. Assessment of sources of potential bias, using Cochrane Collaboration guidelines [Citation41]. All studies were considered at risk of “other sources of bias” due to their small size, variation in the effect of the variable OP insecticides ingested, and lack of identification of the responsible insecticide by laboratory analysis.](/cms/asset/d6a4e660-6c21-4890-afa5-36225d673b27/ictx_a_1446532_f0002_c.jpg)
Figure 3. Forest plots indicating impact of treatment with magnesium sulfate on (A) mortality and (B) need for intubation/ventilation in OP insecticide poisoned patients. Odds ratios with 95% confidence interval. The studies are ordered by their magnesium dose to allow visualization of a dose effect if one exists.
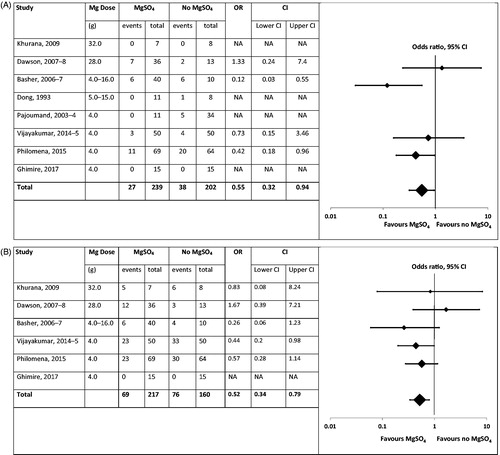
Table 2. Clinical studies of intravenous MgSO4 treatment for acute organophosphorus insecticide poisoning.
In 1993, a small case series reported treatment of 11 of 19 OP insecticide poisoned patients with MgSO4 (2.5–5 g daily for 2–3 d) [Citation36]. The authors reported shorter time to atropinization, shorter durations of muscular fasciculation and respiratory distress, and reduced arrhythmias, gastrointestinal bleeding, and mortality in the intervention group (). The basis of allocation is unclear.
A case series of 45 OP insecticide poisoned patients was reported in 2004; 1 in 4 (11) of these patients received 4 g of MgSO4 over 24 h [Citation37]. The authors reported that this treatment induced no change in atropine requirement or oxime dosage, but did reduce mortality and duration of hospitalization due to OP poisoning ().
A small phase II study testing four escalating doses of MgSO4 was reported in 2013 [Citation38]. The four doses (4 g over 1 h, every 4 h, for 1–4 doses) were compared in cohorts of 8–16 patients with a placebo control group that was integrated into each dose cohort. It found all four doses of MgSO4 to be well tolerated with no significant NMJ dysfunction or respiratory depression. Small differences in total atropine requirement were noted between MgSO4 and control arms, but no dose effect was apparent. A possible dose-response in effectiveness for mortality was reported since this reduced with increasing dose of magnesium (). However, due to the small size of the study, there was imbalance between groups with more severely ill patients in the control group.
A moderate-size phase III RCT (planned recruitment 300 patients) was set up in 2007 to compare MgSO4 4 g over 1 h, then 1 g per hour, up to a maximum of 28 g, vs. no MgSO4 (Dawson et al, unpublished). Staffing reasons caused it to stop after recruiting only 49 patients and the data were not published. No significant difference in atropine requirement, need for intubation and ventilation, duration of hospital stay, and mortality was noted between arms (), but the analysis was under-powered. Transient hypotension was noted in at least four patients treated with MgSO4.
In 2009, a small RCT compared treatment with MgSO4 4 g on presentation and every 6 h for 48 h in seven patients with standard treatment given to eight patients (Khurana, unpublished data). No difference was observed in the need for, and duration of, ventilation, and for mortality between arms ().
A phase III RCT in 100 patients compared a single dose of MgSO4 (4 g over 30 min) vs. no MgSO4 [Citation39]. It found a reduced length of ICU stay and need for atropine and ventilation in patients receiving MgSO4 but no effect on mortality ().
An observational clinical study compared the outcome of 69 patients presenting over three months who received MgSO4 4 g within 24 h after admission with 64 control patients admitted over the previous three months who received only standard therapy [Citation40]. This study reported reduced mortality and reduced need for mechanical ventilation in patients receiving MgSO4 ().
A small RCT compared 15 OP poisoned patients treated with MgSO4 4 g within the first 24 h of admission vs. a control group of 15 OP poisoned patients treated with standard therapy (Ghimire et al., unpublished). Atropine requirement, need for ventilation, duration of hospital stay, and mortality did not differ between groups ().
Adverse effects of magnesium therapy
Transient hypotension which resolved after stopping the infusion was noted in four (11.1%) patients receiving MgSO4 (1g per h) in one study (Dawson et al, unpublished). No adverse effects of MgSO4 therapy were noted in any of the other clinical trials () although this may be due to the level of observation and monitoring possible in the study hospitals. Closer monitoring might have identified adverse effects. It is also possible that such effects were noted in some studies but not reported.
Serum Mg2+ concentrations were measured in five studies (). In three studies, the Mg2+ concentration measured 24 h after the first MgSO4 dose was higher in the MgSO4 arm than the control arm. However, all concentrations were lower than 2 mmol/L.
Table 3. Serum Mg2+ concentrations in the clinical studies were reported.
Meta-analysis of outcomes
We produced forest plots for the impact of MgSO4 on mortality and need for intubation and ventilation (). The pooled odds ratios for mortality and need for intubation and ventilation were 0.55 (95% confidence interval [CI] 0.32–0.94) and 0.52 (95% CI 0.34–0.79), respectively. However, all the studies were small and at risk of bias (); there was also heterogeneity in the results of higher quality phase III RCTs providing more conservative estimates. There was no apparent evidence of a dose effect.
Discussion
In this systematic review, we found both preclinical and clinical data which suggested that calcium channel blockade, in addition to atropine and standard clinical care might be beneficial in acute OP insecticide toxicity. Reductions were noted in both mortality and need for intubation and ventilation in clinical trials. However, the trials were all of MgSO4 and generally small, poor in quality, and with risk of bias, thereby providing no definite answer on whether such treatment will benefit poisoned humans. The wide variety of insecticides ingested, treatments given, time to hospital presentation might conceal effectiveness amongst all patients or a sub-population. Large definitive phase III studies testing MgSO4 and/or a CCB, probably nimodipine (since it can be administered by the intravenous route to poisoned patients) are required to establish effectiveness.
The trials of MgSO4 were small to moderate in size and performed at single study sites across South Asia where OP insecticide poisoning is a major clinical problem. We were able to find three unpublished studies which showed either no effect on mortality (due to size and patient severity) or a negative effect, compared to the more positive studies reported in the published literature, raising the possibility of publication bias. The study with the greatest beneficial effect was a “before and after” study with high risk of bias. The studies with better design (e.g., randomized with allocation concealment () provided more conservative estimates of effect.
Risk of bias analysis showed that all the clinical studies were at risk of bias, in addition to the lack of randomization in four (50%) and performance of a sample size power calculation for only one study (which did not recruit to target). Half of the studies reported no allocation concealment or blinding of participants, researchers or statistical analysts to allocation, increasing the risk of bias. The animal studies were of modest clinical relevance since the drugs were given in non-clinical doses before or soon after the exposure rather than at a clinically possible time point. They also provided little mechanistic information. Such problems with the clinical translation of animal model to humans have been identified before [Citation42].
The most common dose of MgSO4 studied was 4 g, since this dose is routinely administered to prevent cardiac dysrhythmias and does not need intensive monitoring of Mg2+ concentration. However, this may be an inadequate dose – the dose escalation RCT [Citation38] suggested that MgSO4 doses such as 4 g every 4 h might offer greater benefit. An RCT was subsequently set up to test a bolus dose of 4 g over 1 h followed by an infusion of 1 g/h for 24 h – doses recommended in obstetric medicine [Citation43,Citation44]. The study was too small to assess effectiveness but toxic concentrations of Mg2+ (>2.5 mmol/L with severe effects occurring at concentrations >7 mmol/L [Citation45,Citation46]) were not noted. Eleven percent of patients in this study had to have their magnesium infusions stopped due to hypotension, which settled thereafter. A major advantage of infusions over intermittent bolus doses is the ability to stop the infusion if hypotension or bradycardia occur and, in the case of serious side effects, to administer calcium gluconate.
We found no comparative study of CCB as an antidote in OP-poisoned patients, despite positive results in pre-clinical studies. Nimodipine, the dihydropyridine CCB most commonly used in these animal studies, is used in humans to prevent arterial spasm after sub-arachnoid hemorrhage, often as an intravenous infusion of 1–2 mg/h. Possible side effects of nimodipine, such as low blood pressure, can again be reversed by stopping the infusion and administering calcium gluconate. The relevance of the pre-clinical rodent studies to clinical care is likely to be low due to the very early timing of treatment, sometimes before OP exposure.
The mechanism of an effect for MgSO4 and CCB is unclear. Pre-clinical studies suggest that any benefit of CCB is additive to atropine. However, the voltage-dependent Ca2+ channels inhibited by CCB appear not to be involved in increasing cytosolic calcium level after OP exposure [Citation47]. OP compounds may increase intracellular Ca2+ concentrations (and thereby ACh release at the NMJ) by inhibiting Ca2+ ATPase, the enzyme responsible for removing cytosolic Ca2+ by sequestrating it into intracellular stores such as mitochondria or pumping it to the extracellular medium [Citation47]. In rats, nimodipine restored Ca2+ ATPase activity reduced by dichlorvos, decreasing intra-cellular Ca2+ concentrations [Citation47]. Treatment with MgSO4 was also able to reduce inhibition of Ca2+ ATPase in rodent skeletal muscle after OP poisoning [Citation25].
Remarkably, the mechanism of the well-established antagonism of Ca2+-dependent exocytosis of acetylcholine at NMJs remains unclear. A rise of serum Mg2+ concentration from 1 to 4 mM would be expected to reduce the amount of evoked neurotransmitter release at NMJs by about 10–20% [Citation48,Citation49], but this should not substantially reduce functional neuromuscular transmission and muscle contractility because of the high “safety factor” for transmitter release and its effects on endplate depolarization at NMJs [Citation50]. Mg2+ ions have a dual effect on L-type (and possibly N-type) Ca channels, facilitating them at low (sub-micromolar) Mg2+ concentration but blocking them at millimolar concentration [Citation51–53]. But these Ca channel sub-types are not thought to be significantly involved in fast nerve-evoked transmitter release, whereas P/Q type Ca channels (CaV2.1 channels), which are responsible and are blocked by divalent cations, such as Cd2+ or Co2+, are probably not blocked by Mg2+, although this appears to be surprisingly under-researched [Citation54–56]. Mg2+ may compete with Ca2+ for binding sites on the Ca sensor synaptotagmin intracellularly, or it may act extracellularly to reduce the efficacy of intracellular Ca on release probability at presynaptic active zones [Citation57–59]. Further basic research is required to establish unequivocally how MgSO4 blocks transmitter release.
Limitations
In addition to the limitations to the identified studies, as pointed out above, the review was limited by our ability to identify relevant studies. We did find three unpublished studies, but more might exist that we are unaware of, although most patients present in China and India and publications from both countries were covered in the search. We were unable to get primary data from the authors of all the studies; however, primary data on mortality was available from all eight controlled clinical studies (and intubation/ventilation data from 6/8 studies).
Conclusions
This systematic review found both pre-clinical and clinical data which suggest that MgSO4 and CCB may be beneficial adjuncts to standard therapy after OP insecticide poisoning. Both are inexpensive, widely available, and can be administered by intravenous infusion to unconscious patients. However, this evidence is inadequate to recommend their use in clinical practice. Mechanistic studies and large adequately powered phase III RCTs, assessing mortality, and intubation/ventilation, are required. Due to marked variation between the effect of different OP insecticides in poisoning [Citation60,Citation61], it will be important to identify the insecticide ingested by each patient, to follow surrogate markers of poisoning, including BuChE activity and in a sub-group neurophysiological testing of NMJ function, and measure the serum concentration of free magnesium.
Acknowledgments
We are extremely grateful to Prof. Dheraj Khurana and Dr. Rakesh Ghimire for providing the primary data from their unpublished RCTs, Prof. Devika Rani Duggappa and Dr. Ariful Basher for providing additional data for their published studies, and Prof. Abdollahi and Prof. Ashish Bhalla for their help in seeking primary data. No funding was received for this work.
Disclosure statement
AHD was an investigator on two of the clinical trials included within the systematic review. The other authors report no declarations of interest.
References
- Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43:139–144.
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–731.
- Lamb T, Selvarajah LR, Mohamed F, et al. High lethality and minimal variation after acute self-poisoning with carbamate insecticides in Sri Lanka – implications for global suicide prevention. Clin Toxicol. 2016;54:624–631.
- Mew EJ, Padmanathan P, Konradsen F, et al. The global burden of fatal self-poisoning with pesticides 2006–15: systematic review. J Affect Disord. 2017;219:93–104.
- Wesseling C, McConnell R, Partanen T, et al. Agricultural pesticide use in developing countries: health effects and research needs. Int J Health Serv. 1997;27:273–308.
- Candole CA, Douglas WW, Evans C, et al. The failure of respiration in death by anticholinesterase poisoning. Brit J Pharmacol. 1953;8:466–475.
- Lotti M, Clinical toxicology of anticholinesterase agents in humans. In: Krieger RI, Doull J, editors. Handbook of pesticide toxicology Volume 2 Agents. San Diego (CA): Academic Press; 2001. p. 1043–1085.
- Eddleston M, Mohamed F, Davies JOJ, et al. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2006;99:513–522.
- Hulse EJ, Davies JO, Simpson AJ, et al. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med. 2014;190:1342–1354.
- Wadia RS, Sadagopan C, Amin RB, et al. Neurological manifestations of organophosphate insecticide poisoning. J Neurol Neurosurg Psych. 1974;37:841–847.
- Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med. 1987;316:761–763.
- Karalliedde L, Baker D, Marrs TC. Organophosphate-induced intermediate syndrome: aetiology and relationships with myopathy. Toxicol Rev. 2006;25:1–14.
- Eddleston M, Chowdhury FR. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br J Clin Pharmacol. 2016;81:462–470.
- Eddleston M, Dawson A, Karalliedde L, et al. Early management after self-poisoning with an organophosphorus or carbamate pesticide - a treatment protocol for junior doctors. Crit Care. 2004;8:R391–R397.
- Abedin MJ, Sayeed AA, Basher A, et al. Open-label randomized clinical trial of atropine bolus injection versus incremental boluses plus infusion for organophosphate poisoning in Bangladesh. J Med Toxicol. 2012;8:108–117.
- Buckley NA, Eddleston M, Li Y, et al. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011;2:CD005085.
- Blumenberg A, Benabbas R, deSouza IS, et al. Utility of 2-pyridine aldoxime methyl chloride (2-PAM) for acute organophosphate poisoning: a systematic review and meta-analysis. J Med Toxicol. 2018 [cited 2017 Dec 11]; [8 p.]. DOI:10.1007/s13181-017-0636-2
- Balali-Mood M, Ayati MH, Ali-Akbarian H. Effects of high doses of sodium bicarbonate in acute organophosphate pesticide poisoning. J Toxicol Clin Toxicol. 2005;43:571–574.
- Perera PM, Jayamanna SF, Hettiarachchi R, et al. A phase II clinical trial to assess the safety of clonidine in acute organophosphorus pesticide poisoning. Trials. 2009;10:73.
- Emerick GL, Peccinini RG, de Oliveira GH. Organophosphorus-induced delayed neuropathy: a simple and efficient therapeutic strategy. Toxicol Lett. 2010;192:238–244.
- Albertson TE, Owen KP, Sutter ME, et al. Gastrointestinal decontamination in the acutely poisoned patient. Int J Emerg Med. 2011;4:65.
- Trouve R, Nahas G. Nimodipine in combination with atropine and diazepam as an antidote to lethal organophosphorus intoxication. Br J Pharmacol. 1989;96:19P.
- Li Y, Cai ZF, Zhao LH. Experimental treatment and protective effect of verapamil on organophosphate poisoning [in Chinese]. Occup Med. 1996;23:4–6.
- Petroianu G, Toomes LM, Petroianu A, et al. Control of blood pressure, heart rate and haematocrit during high-dose intravenous paraoxon exposure in mini pigs. J Appl Toxicol. 1998;18:293–298.
- Li C, Zhao M, Yang L, et al. ATPase activities in skeletal muscle tissue after acute organophosphorus poisoning and the effects of magnesium sulfate [in Chinese]. Chinese J Emerg Med. 2005;14:276–278.
- Gunay N, Kekec Z, Demiryurek S, et al. Cardiac effects of magnesium sulfate pretreatment on acute dichlorvos-induced organophosphate poisoning: an experimental study in rats. Biol Trace Elem Res. 2010;133:227–235.
- Fang JL, Shao QP, Qian XQ. Application of calcium channel blocker in rescue of severe organophosphate pesticide poisoning [in Chinese]. Chinese Med Fact Mine. 1997;3:201–202.
- Tao XS, Zheng JQ. The experience of using diltiazem hydrochloride for sinus tachycardia in nine cases of severe organophosphate pesticide poisoning [in Chinese]. Fujian Med J. 2010;32:138–139.
- Zhu YC, Ma XY. Resuscitation experience of sudden cardiac arrest during recovery phase of acute organophosphate poisoning - a report of 7 cases. Chinese J Indust Med. 1990;4:28–29.
- Zhang JY, Zhao JY, Zhao CL. Clinical observation of diazepam and verapamil in preventing heart damage caused by acute organophosphate poisoning [in Chinese]. J Occup Health Damage. 2001;16:13–14.
- Ru XW. Study on the protective effect of verapamil on myocardial injury induced by organophosphate pesticide poisoning [in Chinese]. Central Plains Med J. 2003;30:21–22.
- Singh G, Avasthi G, Khurana D, et al. Neurophysiological monitoring of pharmacological manipulation in acute organophosphate (OP) poisoning. The effects of pralidoxime, magnesium sulphate and pancuronium. Electroencephalogr Clin Neurophysiol. 1998;107:140–148.
- Nel L, Hatherill M, Davies J, et al. Organophosphate poisoning complicated by a tachyarrhythmia and acute respiratory distress syndrome in a child. J Paediatr Child Health. 2002;38:530–532.
- Wang MH, Tseng CD, Bair SY. Q-T interval prolongation and pleomorphic ventricular tachyarrhythmia (Torsade de Pointes) in organophosphate poisoning: report of a case. Hum Exp Toxicol. 1998;17:587–590.
- Sun L, Li GQ, Yan PB, et al. Clinical management of organophosphate poisoning in pregnancy. Am J Emerg Med. 2015;33:305.e1–305.e3.
- Dong X. Effect of magnesium sulfate on organophosphate pesticide poisoning in 11 cases [in Chinese]. Clin Focus. 1993;8:276–277.
- Pajoumand A, Shadnia A, Rezaie A, et al. Benefits of magnesium sulfate in the management of acute human poisoning by organophosphorus insecticides. Hum Exp Toxicol. 2004;23:565–569.
- Basher A, Rahman SH, Ghose A, et al. Phase II study of magnesium sulfate in acute organophosphate pesticide poisoning. Clin Toxicol. 2013;51:35–40.
- Vijayakumar HN, Kannan S, Tejasvi C, et al. Study of effect of magnesium sulphate in management of acute organophosphorous pesticide poisoning. Anesth Essays Res. 2017;11:192–196.
- Philomena J, Sathi V, Prathiba P. A case control study of intravenous magnesium sulphate in treatment of acute organophosphate poisoning. J Evol Med Dent Sci. 2016;5:2290–2294.
- Cochrane Collaboration. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Hoboken (NJ): John Wiley & Sons; 2011.
- van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245.
- Hull J, Rucklidge M. Management of severe pre-eclampsia and eclampsia. Update in Anaesthesia. San Francisco (CA): Scribd HQ; 2009.
- British National Formulary (BNF 72) (September 2016). London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2016.
- Parikh M, Webb ST. Cations: potassium, calcium, and magnesium. Contin Educ Anaesth Crit Care Pain. 2012;12:195–198.
- Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth. 1999;83:302–320.
- Choudhary S, Gill KD. Protective effect of nimodipine on dichlorvos-induced delayed neurotoxicity in rat brain. Biochem Pharmacol. 2001;62:1265–1272.
- Elmqvist D, Quastel DM. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965;178:505–529.
- Hubbard JI, Jones SF, Landau EM. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968;196:75–86.
- Wood SJ, Slater CR. The contribution of postsynaptic folds to the safety factor for neuromuscular transmission in rat fast- and slow-twitch muscles. J Physiol. 1997;500:165–176.
- Hess P, Lansman JB, Tsien RW. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986;88:293–319.
- Shimosawa T, Takano K, Ando K, et al. Magnesium inhibits norepinephrine release by blocking N-type calcium channels at peripheral sympathetic nerve endings. Hypertension. 2004;44:897–902.
- Zhao M, Feng R, Shao D, et al. Mg(2+)-dependent facilitation and inactivation of L-type Ca(2+) channels in guinea pig ventricular myocytes. J Pharmacol Sci. 2015;129:143–149.
- Nachshen DA. Selectivity of the Ca binding site in synaptosome Ca channels. Inhibition of Ca influx by multivalent metal cations. J Gen Physiol. 1984;83:941–967.
- Porter VA, Wray D. Relative potencies of metal ions on transmitter release at mouse motor nerve terminals. Br J Pharmacol. 1996;118:27–32.
- Urbano FJ, Piedras-Renteria ES, Jun K, et al. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci USA. 2003;100:3491–3496.
- Dascal N, Landau EM, Lass Y. Divalent cations and transmitter release at low concentration of tetrodotoxin. Biophys J. 1981;35:573–586.
- Kharasch ED, Mellow AM, Silinsky EM. Intracellular magnesium does not antagonize calcium-dependent acetylcholine secretion. J Physiol. 1981;314:255–263.
- Park Y, Seo JB, Fraind A, et al. Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat Struct Mol Biol. 2015;22:815–823.
- Wadia RS, Bhirud RH, Gulavani AV, et al. Neurological manifestations of three organophosphate poisons. Indian J Med Res. 1977;66:460–468.
- Eddleston M, Eyer P, Worek F, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–1459.
Appendix A
A full description of the search terms for PubMed and China academic journals full-text database - medicine/hygiene series.
PubMed
(((organophosphate OR organophosphorus) OR carbamate OR (cholinesterase AND inhibitor)) AND poisoning) AND ((calcium AND channel AND blocker) OR (magnesium))).
(((organophosphate OR organophosphorus) OR carbamate OR (cholinesterase AND inhibitor)) AND poisoning) AND (nifedipine OR nimodipine OR nitrendipine or verapamil)).
China Academic Journals Full-text Database – Medicine/Hygiene Series
((有机磷(1)(full text) OR 氨基甲酸酯(1)(full text) OR 膽鹼酯酶抑製劑(1)(full text)) AND 中毒(1)(full text)) AND (钙通道阻滞剂(1)(full text) OR 钙拮抗剂(1)(full text)) Precise search
((有机磷(1)(full text) OR 氨基甲酸酯(1)(full text) OR 膽鹼酯酶抑製劑(1)(full text)) AND 中毒(1)(full text)) AND ((维拉帕米(1)(full text) OR 異搏定(1)(full text)) OR 硝苯地平(1)(full text) OR 尼群地平(1)(full text) OR (尼莫地平(1)(full text)) Precise search
(氨基甲酸酯(1)(full text) OR 膽鹼酯酶抑製劑(1)(full text)) AND 中毒(1)(full text)) AND (硫酸鎂(1)(full text)) Precise search
(有机磷(1)(full text)) AND (中毒(1)(full text)) AND (硫酸鎂(1)(keyword)) → Precise search
