Abstract
Introduction: Fasting, as well as a high-fat diet, might increase the risk on acetaminophen-induced toxicity after an acute overdose. Therefore, it has been suggested to lower the threshold for acetylcysteine treatment to prevent liver injury in case of fasting. This study aims to investigate the effects of 36 hours of fasting and three days of a hypercaloric high-fat diet on acetaminophen measurement and exposure.
Methods: Nine healthy male subjects were enrolled in a randomized crossover intervention study. Subjects received 1000mg oral acetaminophen after an overnight fast following: (1) regular diet,(2) 36h of fasting and (3) three days of a hypercaloric high-fat diet consisting of 500ml of cream (1715 kcal) supplemented to their regular diet. Pharmacokinetic parameters were determined by non-compartmental analysis. Samples were analyzed by an enzymatic colorimetric method used in routine practice and by LC-MS/MS being the gold standard. Agreement between these methods was assessed by the Bland–Altman method.
Results: Short-term fasting increased acetaminophen exposure by 20% (ΔAUC0-8 hours, p = .04) in comparison with the control diet. Three days of hypercaloric high-fat diet did not affect acetaminophen exposure (ΔAUC0–8 hours= 9%, p = .67). The intraclass correlation coefficient between the enzymatic assay and LC-MS/MS methods of the fasting samples was 0.46 (0.28–0.61), compared to 0.87 (0.81–0.92) and 0.87 (0.79–0.91) in the control and high-fat samples respectively.
Conclusions: Short-term fasting increases acetaminophen exposure in healthy subjects, whereas no effect is observed after a high-fat diet. Furthermore, short-term fasting decreases the accuracy of the enzymatic colorimetric method when measuring relatively low acetaminophen concentrations. This suggests considering nutritional status when assessing the risk of acetaminophen-induced toxicity, although further research at toxic doses is needed.
Introduction
Acetaminophen is a widely used and effective painkiller. However, an acetaminophen overdose is the most common cause of acute liver injury [Citation1]. Subjects at risk for the hepatotoxic effects of an acute overdose are treated with n-acetylcysteine in order to prevent liver damage [Citation2]. The Rumack–Matthew nomogram is used in routine practice to predict the risk for hepatotoxicity. It indicates that patients are at risk for hepatic toxicity when the serum acetaminophen concentration is greater than 150 µg/mL at 4 hours after an acute overdose [Citation3]. However, debate is going on the threshold for starting n-acetylcysteine therapy and about the use of other biomarkers to predict hepatotoxicity, as under- and overtreatment currently occur [Citation4–6]. Suggestions have been made to lower the threshold to 100 µg/mL at 4 hours after an overdose in specific high-risk clinical circumstances such as alcoholism or prolonged fasting [Citation7].
Fasting or malnutrition is considered as a risk factor for acetaminophen-induced hepatotoxicity [Citation8]. Several pathophysiological mechanisms might be affected. Hepatotoxicity is not caused by acetaminophen itself but by its metabolite N-acetyl-p-benzoquinone imine (NAPQI). Most acetaminophen is eliminated via glucuronidation (UGT1A1) and sulfation as non-toxic substances, but CYP2E1 and other P450 enzymes convert a small amount of acetaminophen into the toxic NAPQI, which is then detoxified by conjugation with glutathione (GST) [Citation9]. Fasting depletes glycogen stores. Glucose is required for the uridine diphosphate (UDP) glucuronic acid formation of acetaminophen. In rat studies, fasting actually decreases acetaminophen glucuronidation and as a consequence acetaminophen half-life increases and more acetaminophen is eliminated via the toxic oxidation route [Citation10]. Another explanation is that fasting reduces hepatic glutathione stores as described in several animal studies [Citation11,Citation12]. The shortage of glutathione allows NAPQI to form protein adducts on mitochondrial proteins and induces mitochondrial oxidative stress leading to hepatic necrosis. Furthermore, fasting readily induces biochemical alterations [Citation8] that cause steatotic alterations of the liver that might affect hepatic drug enzyme activity.
Analogous to fasting, a short-term high-fat diet induces steatotic alterations of the liver [Citation13,Citation14]. Non-alcoholic fatty liver disease (NAFLD) has been associated with an increased risk of acetaminophen-induced liver injury [Citation15]. The dietary-induced steatotic alterations of the liver might alter hepatic drug enzyme activity [Citation16,Citation17] and, consequently, alter exposure to acetaminophen.
These mainly preclinical studies suggest an effect of nutritional status on acetaminophen-induced toxicity. Moreover, in patients, hepatotoxicity seemed to occur more often after recent fasting, although alcohol abuse was a potential confounder [Citation9]. Therefore, suggestions have been made to lower the threshold for starting acetylcysteine treatment after an overdose in case of fasting [Citation8]. However, the effects of nutritional status on acetaminophen concentrations in humans have not been described.
In routine clinical toxicology, the enzymatic colorimetric method is used for measurement of acetaminophen concentrations, because of its rapid results. The effects of nutritional status on the accuracy of acetaminophen assays have not previously been described. These enzymatic assays are prone to interference by various substances that may be released in response to dietary interventions [Citation18]. The purpose of this study was to assess the effects of short-term fasting and short-term high-fat diet on exposure to acetaminophen in healthy subjects. In this study acetaminophen concentrations were initially assessed by an enzymatic colorimetric method as is used in clinical routine. In addition, samples were analyzed with liquid chromatography coupled to mass spectrometric (LC-MS/MS) detection, being the analytical gold standard.
Methods
Subjects
Nine male healthy non-smoking volunteers aged 18 years or older were enrolled after giving written informed consent. They had no major illness or surgery in the past, liver and kidney function were normal and they did not take any prescribed or non-prescribed drugs, herbal or dietary supplements. The study was approved by the human research ethics committee of the AMC and conducted in accordance with the Declaration of Helsinki.
Design
In order to assess acetaminophen exposure in two different nutritional conditions, we performed a single-dose crossover intervention study in which each subject was served as its own control. Subjects were randomly assigned to the sequence in which they received a single oral administration of 1000 mg acetaminophen (tablet) on three occasions with a wash-out period of at least 3 days: (1) after an overnight fast (control), (2) after 36hrs of fasting and (3) after 3 days of a high-fat diet.
In the control situation, subjects were instructed to have their regular diet. During the 36-hour period of starvation they were only allowed to drink water. The high-fat diet consisted of their regular diet supplemented with 500 ml cream (1715 kcal) after dinner for three days. During the three days before each intervention, subjects were not allowed to perform strenuous exercise, to drink coffee or alcohol or to consume grapefruits. Subjects were restricted to the protocol by food diaries and phone calls. Furthermore, we measured baseline biochemical parameters at the morning of each visit (free fatty acids, acetoacetate and 3-hydroxybutyric acid, gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), post-absorptive glucose and C-reactive protein (CRP).
Plasma acetaminophen concentrations were measured before the administration and at t = 0.25, t = 0.5, t = 0.75, t = 1, t = 2, t = 3, t = 4, t = 8 and t = 24 hours after drug ingestion. To exclude the influence of diet on drug absorption, subjects were fasting for 12–14 hours, at the start of each study day (except for the fasting study, in which they had been fasting for 36 hours) and they received a standard fluid mixed meal containing 25% of their estimated daily energy expenditure (25 kcal/kg/day) 4 hours after drug ingestion.
Plasma concentration analysis
Plasma samples were analyzed by a validated enzymatic colorimetric method using a COBAS® INTEGRA 400 (Roche Diagnostics Ltd, Rotkreuz, Switzerland) according to current routine practice of measuring acetaminophen levels after an acute acetaminophen overdose. The lower level of quantification (LLOQ) was 2 mg L−1 and the upper level of quantification (ULOQ) was 300 mg L−1. The accuracy was between 88.9% and 89.3% and the within- and between-run imprecision for the lower limit of quantification, the middle level and upper limit of quantification were <7.5% and <10%, respectively. All samples from the three interventions from one subject were analyzed in the same run to avoid inter-assay variation. Blood samples were both drawn and handled in a controlled setting. No hemolytic or lipemic samples were detected either at blood sampling or sample preparation.
As a gold standard the plasma concentrations of APAP were determined using a validated liquid chromatography/tandem mass spectrometry (LC-MS/MS) method as well. Samples were deproteinized and spiked with internal standards (APAP-d4). A LC-30 Nexera (Shimadzu, Kyoto, Japan) system using a CT020AC column oven was used for chromatography which was coupled to a QTrap 5500mass spectrometer (ABsciex, Concord, Canada). For chromatographic separation a ternary gradient was applied using two eluents: (1) 2% formic acid/1% ammonium formate in water (Merck/Fluka) and (2) 2% formic acid/1% ammonium formate in CAN:MeOH 70:25 (vol/vol) (Merck/Fluka/Biosolve). The lower and upper limits of quantification (LLOQ and ULOQ) were 0.10 and 80 mg L−1 for acetaminophen. Furthermore, the accuracy was between 96.8% and 100% and the within- and between-run imprecision were <5% for the lower limit of quantification, the middle level and upper limit of quantification.
Pharmacokinetic analysis
We have plotted the individual plasma concentration time curves for each individual at each intervention. Hereafter, we performed a non-compartmental analysis via PK solver [Citation19] using the log linear trapezoidal setting to calculate the acetaminophen pharmacokinetic parameters of each subject in each condition. The primary endpoint was the difference between the area under the plasma concentration versus time curve from 0 to 8 hours (AUC0–8 hrs) of acetaminophen after the control situation versus the short-term fasting or short-term high-fat diet intervention. Oral clearance was estimated by the formula: dose/AUC0–>inf with the latter parameter being the AUC from 0 to infinity.
For both the LC-MS/MS and enzymatic colorimetric assay methods, the acetaminophen concentrations at t = 24 h were occasionally below the level of quantification. Since a value of 0 or just above 0 makes a large difference for the estimation of the AUC0–>inf in non-compartmental analysis, we decided to exclude all t = 24 h concentrations from the pharmacokinetic analysis. As a result, AUC0–>inf was calculated by extrapolation of concentration from t = 8 hours. Oral volume of distribution was obtained by the following formula: plasma half-life*oral clearance/0.693.
Statistical analysis
Baseline biochemical and pharmacokinetic parameters were compared between control and 36h fasting intervention and between control and three day high-fat diet intervention by non-parametric Wilcoxon’s sign rank tests for paired samples. In this test each subject serves as its own control and it tests whether the difference between two observations has a mean signed rank of 0. Thus, it is robust against outliers. The supposed biological effect of the nutritional intervention on acetaminophen exposure would occur in every subject in the same direction – which would result in a high rank correlation (r = the sum of ranks/total rank sum) – and consequently allows for a sample size of nine patients to detect clinical significant differences. Based on the results, a post hoc power analysis for the primary outcome measure (AUC0–8) was performed using a 0.05 two-sided significance level (nQuery Advisor version 7.0).
The extent of agreement between the enzymatic colorimetric assay and the LC-MS/MS was assessed by Bland–Altman plots, based on evaluation of the difference between the methods against their mean concentration. The limits of agreement are determined by the mean ±1.96 × standard deviation. In addition, the single measure intraclass correlation coefficients were calculated by a two-way mixed effects model. IBM SPSS statistics version 23 was used.
Results
Nutritional effect on biochemical parameters
All subjects completed the study without side effects. shows the effects of the nutritional intervention on biochemical parameters after an overnight fast. As expected 36 h of fasting increased plasma levels of free fatty acids, acetoacetate and 3-hydroxybutyric acid, and, interestingly, also bilirubin and gamma glutamyltransferase (GGT). In addition, 36 h of fasting decreased post-absorptive glucose levels compared to an overnight fast. A three-day hypercaloric high-fat diet increased post-absorptive plasma levels of glucose, alkaline phosphatase and GGT and decreased bilirubin levels.
Table 1. Baseline biochemical values (median (25th–75th)).
Nutritional effect on acetaminophen enzymatic colorimetric assay
After plotting of the enzymatic colorimetric assay concentration–time curves, it appeared incidentally that a concentration was, physiologically based, impossibly high or low in relation to the other concentrations within the same plasma concentration–time curve. An example is shown in .
Figure 1. Example of a plasma concentration time curve in one subject after fasting. Samples were measured by (A) the enzymatic colorimetric assay method or by (B) LC-MS/MS.
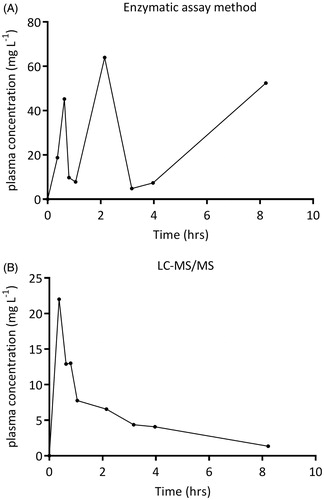
As these artefacts influenced the assessment of the pharmacokinetic parameters, samples were re-analyzed by the gold standard LC-MS/MS. This method produced well-shaped and pharmacokinetically more plausible curves.
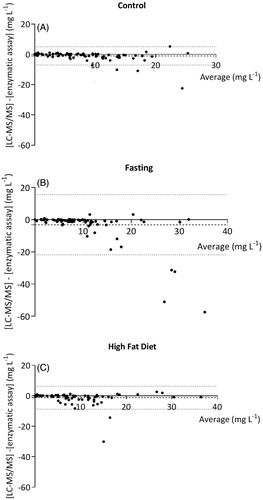
The differences between the concentrations measured by LC-MS/MS and the enzymatic colorimetric assay are demonstrated in the Bland–Altman plots given in . For the samples collected during the control intervention (n = 90), the mean difference between the concentrations as assessed by the two methods was −1.0 (95%CI: −1.6, −0.4) mg L−1 with limits of agreement from −7.1 to 5.1 mg L−1. For the high-fat samples, the mean difference was −1.2 (95%CI: −2.0, −0.4) mg L−1 and limits of agreement ranged from −8.9 to 6.5 mg L−1. After fasting, the mean difference was −3.2 (95%CI: −5.1, −1.1) mg L−1 and limits of agreement were wider ranging from −22.2 to 16.0 mg L−1. Accordingly, the intraclass correlation coefficient of the fasting samples was 0.46 (0.28–0.61), compared to 0.87 (0.81–0.92) and 0.87 (0.79–0.91) of the control and high-fat samples, respectively. shows that differences between both methods occur mainly at higher concentrations.
Figure 2. Bland–Altman plots of differences between the LC-MS/MS method and the enzymatic colorimetric assay in (A) control samples, (B) after fasting and (C) after a high-fat diet. The dashed line represents the mean difference between the methods, and the dotted line indicates the upper and lower limit of agreement, set to ±2SD of the mean difference.
Nutritional effect on acetaminophen exposure
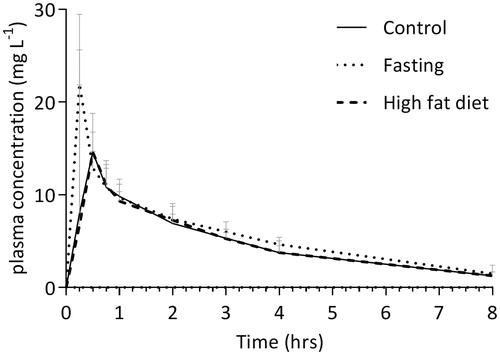
The median (interquartile range) plasma concentration–time curves for acetaminophen as determined by LC-MS/MS for the three different nutritional conditions are demonstrated in .
Figure 3. The median (interquartile range) plasma concentration versus time curve of acetaminophen in three different nutritional conditions. The closed line represents control samples, the dotted line represents fasting samples and the dashed line represents the high-fat diet samples. Concentrations were measured by LC-MS/MS.
Short-term fasting
The median AUC0–8 of acetaminophen in control situation was 36.4 mg L−1 min−1. Thirty-six hours of fasting significantly increased exposure to acetaminophen by ∼20% to 43.7 mg L−1 min−1 (p = .008). The individual changes in AUC0 − 8 are represented in . Fasting increased AUC0−>inf from 41.3 to 48.9 mg L−1 min−1 (p = .008) and plasma half-life by ∼14% (p = .03). Accordingly, fasting significantly decreased oral clearance by ∼15% (p = .008). The interquartile range of Cmax was 12.9 − 22.2 mg L−1 in the control situation and 11.2 − 25.7 mg L−1 during fasting. Median Tmax, Cmax and oral volume of distribution were not affected by fasting. The pharmacokinetic parameters are summarized in .
Figure 4. Individual AUC0–8h of acetaminophen in subjects receiving oral dose of 1000 mg acetaminophen after (A) fasting or after (B) a high-fat diet, compared to the AUC0–8h of acetaminophen after the regular control diet.
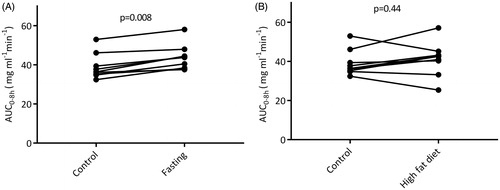
Table 2. Pharmacokinetic parameters of acetaminophen in the three different nutritional conditions based on LC-MS/MS measurements.
Based on these results, a post hoc power analysis was performed. For the AUC0−8, the standard deviation of the differences between control and fasting was 2.0 mg L−1 min−1. This means that a sample size of nine subjects will have 80% power to detect a difference of at least 2.1 mg L−1h−1 (= ∼ 5%) between both interventions.
Short-term high-fat diet
The median AUC 0−>8 hrs of acetaminophen after a short-term high-fat diet was 42.3 mg L−1 min−1, compared to 36.4 mg L−1 min−1 in the control situation (p = .44), as presented in . The median AUC0−inf after a high-fat diet was 46.1 mg L−1 min−1, which is not significantly different from 41.3 mg L−1 min−1 in the control situation. The high-fat diet neither affected acetaminophen exposure nor other pharmacokinetic parameters, summarized in .
Discussion
This study demonstrates that short-term fasting increases exposure to acetaminophen by approximately 20%, whereas a short-term hypercaloric high-fat diet does not affect acetaminophen exposure. Furthermore, the accuracy of the routinely applied enzymatic colorimetric method to measure therapeutic and sub-therapeutic serum acetaminophen concentrations is diminished when samples are obtained during fasting. Although previous studies have hypothesized about altered acetaminophen exposure and toxicity after fasting or in case of hepatic steatosis, this is the first study that assessed the effects of different nutritional conditions on the pharmacokinetics of acetaminophen in healthy subjects. shows that the nutritional interventions used in this study induce rapid biochemical changes and these results point to good protocol adherence. Fasting decreases post-absorptive glucose and increases ketone bodies and total bilirubin levels as expected [Citation20,Citation21], but interestingly also GGT was significantly increased which refers to the process of hepatic steatosis [Citation14]. The three-day high-fat diet increased post-absorptive glucose, GGT and alkaline phosphatase levels.
In the initial analysis with the enzymatic colorimetric method irregular plasma concentration–time profiles were obtained, especially when concentrations were measured after fasting. Measurements were repeated by the enzymatic colorimetric assay method but remained deviant. In order to evaluate the possible consequences for clinical practice, all samples were re-analyzed by the LC-MS/MS method. Differences between the methods occurred in all subjects, but there were two subjects with several extreme differences between both methods in the fasting state that did not occur in the control situation. We did not find any specific circumstances in these subjects to explain the outliers. Enzymatic colorimetric assays are more sensitive to interference with plasma proteins or salts, especially if the analytes are present at low concentrations [Citation18]. The acetaminophen levels in this study were relatively low and it is likely that substances released by the metabolic response of fasting interfere with binding of the analyte to the antibody. One of these substances could be bilirubin as this parameter was increased by fasting in the present study and it has been suggested that increased levels cause interference with the assay [Citation22–24]. However, the bilirubin concentrations in this study were well below the concentrations known to interfere with the assay. Therefore, other, yet unidentified, components of the metabolic response are likely to be responsible for the effect.
It is interesting that throughout all interventions the enzymatic colorimetric assay method seems to overestimate rather than underestimate the plasma acetaminophen values. At higher (toxic) acetaminophen concentrations the competitive binding of metabolic by-products or other endogenous components may be less. Nevertheless, the question arises whether in routine clinical practice overestimation of the risk of hepatotoxicity may occur, especially in case of fasting. The extra costs of the analysis by LC-MS/MS may then be compensated by a reduction in the number of patients treated with intravenous acetylcysteine. Further studies on differences between LC-MS/MS and the enzymatic colorimetric assay in the setting of an acute overdose – especially the setting of fasting or starvation – are needed to test this hypothesis.
The increased exposure to acetaminophen after fasting can be caused by altered metabolism of acetaminophen. Fasting decreased hepatic glycogen stores and this may limit acetaminophen glucuronidation [Citation25]. In addition, the increase in bilirubin levels after fasting indicates that fasting may limit glucuronidation, since bilirubin is eliminated via UGT1A1. Alternatively, P450 activity may be decreased after fasting. This has been previously described in healthy young men [Citation26]. However, only a small part (5–10%) of acetaminophen is metabolized via P450. Further studies into the effect of nutritional status on the differential production of various acetaminophen metabolites may address which hepatic enzymes are most impacted by fasting. Other theoretical possibilities for this increased exposure of acetaminophen are an increase in bioavailability or a change in the fraction unbound to protein. The bioavailability is determined by the rate of absorption and by the first-pass effect of the liver. Since absorption of acetaminophen is almost 100% [Citation27], a further increase in absorption is unlikely. Furthermore, the trial was designed to exclude the potential effects of food on drug absorption, because acetaminophen was administered after an overnight fast in two interventions and after 36 hours of fasting in the other intervention. The first-pass effect of the liver could be decreased by a decrease in hepatic drug enzyme activity. An effect on protein binding is unlikely since acetaminophen hardly binds to plasma proteins (10–20%) [Citation28].
These observations in healthy young men limit our ability to draw conclusions on a more diverse population including women, children, chronic alcohol consumption or chronic disease. Furthermore, the number of subjects entered in this study may appear rather small. However, fasting-induced changes in exposure (AUC) in the same direction in each subject (), strengthening the case for a statistically significant effect on acetaminophen exposure. Accordingly, the post hoc power analysis demonstrated enough power to detect even small differences in exposure. A limitation of this approach could be that the power of the study highly depends on the rank correlation. If more than two out of nine ranks are negative instead of positive (or vice versa), then the rank correlation decreases and the power to detect differences smaller than 30% significantly decreases. On the other hand, in case of opposite ranks an effect is unlikely to occur.
Since this study was in healthy subjects with a non-toxic dose, we cannot conclude with clear recommendations for routine practice. Nevertheless, acetaminophen toxicity has been described even at subtherapeutic doses in specific cases of fasting or malnourished states [Citation29–32]. This study demonstrates that altered pharmacokinetics of acetaminophen in fasting conditions may be (one of) the explanation(s). Moreover, extrapolation of our observation to the situation of supratherapeutic doses and the nomogram is interesting. Since fasting already increases acetaminophen concentrations, the suggestion of lowering the threshold to start acetylcysteine treatment may not be valid. Further research on the effects on acetaminophen metabolites is needed to determine whether the increased acetaminophen exposure due to fasting leads to increased toxicity. Furthermore, the study provides a rationale to assess whether acetaminophen exposure is increased in patients in clinical situations comparable to fasting such as anorexic, cachectic or cancer patients. This could result in lower dosing recommendations in these vulnerable patient groups.
The high-fat diet induced biochemical symptoms of hepatic steatosis by increasing gamma-GT and alkaline phosphatase levels. However, we did not find any effect of the high-fat diet on acetaminophen exposure. Preclinical studies have found decreased UGT1A1 expression in obese and steatotic rats [Citation33]. Accordingly, in a previous clinical study we found decreased hepatic drug enzyme activity (i.e., CYP3A4 and CYP2C19) after this short-term high-fat diet in healthy subjects [Citation34]. A reason for the current finding could be opposing effects on glucuronidation and on P450 enzyme activity, resulting in a final net lack of effect on acetaminophen exposure. Analogous to the arguments used for the fasting intervention, an interacting effect with bioavailability or protein binding is less likely.
In conclusion, we have demonstrated proof of principle that short-term fasting increases acetaminophen exposure by ∼20%. This result supports increased awareness for toxicity in fasted patients, although additional research into the effect of fasting with supra-therapeutic doses and repeat dosing is necessary, as is research into the effect of fasting on the generation of various acetaminophen metabolites. Moreover, we have demonstrated that fasting decreases the accuracy of the enzymatic colorimetric assays when measuring relatively low acetaminophen concentrations. The current development of rapid LC-MS/MS methods [Citation35,Citation36], could be an argument to investigate the benefit of LC-MS/MS over the enzymatic colorimetric method in routine practice. In an actual overdose situation, one can usually only estimate the patient’s nutritional status and its impact will be influenced by whether the acetaminophen ingestion was chronic, acute or subacute. Nevertheless, this study suggests that nutritional status is a factor that should be considered.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ostapowicz G, Fontana RJ, Schiødt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954.
- Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med. 1988;319:1557–1562.
- Rumack BH, Peterson RC, Koch GG, et al. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380–385.
- Bateman DN. Paracetamol poisoning: beyond the nomogram. Br J Clin Pharmacol. 2015;80:45–50.
- Koppen A, van Riel A, de Vries I, et al. Recommendations for the paracetamol treatment nomogram and side effects of N-acetylcysteine. Neth J Med. 2014;72:251–257.
- Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin. Toxicol. 2017;1–14.
- Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28:499–516.
- Kalsi SS, Dargan PI, Waring WS, et al. A review of the evidence concerning hepatic glutathione depletion and susceptibility to hepatotoxicity after paracetamol overdose. Open Access Emerg Med OAEM. 2011;3:87–96.
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;55:879–892.
- Price VF, Miller MG, Jollow DJ. Mechanisms of fasting-induced potentiation of acetaminophen hepatotoxicity in the rat. Biochem Pharmacol. 1987;36:427–433.
- Walker RM, Massey TE, McElligott TF, et al. Acetaminophen toxicity in fed and fasted mice. Can J Physiol Pharmacol. 1982;60:399–404.
- Langley SC, Kelly FJ. Differing response of the glutathione system to fasting in neonatal and adult guinea pigs. Biochem Pharmacol. 1992;44:1489–1494.
- Hernández EÁ, Kahl S, Seelig A, et al. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J Clin Invest. 2017;127:695–708.
- van der Meer RW, Hammer S, Lamb HJ, et al. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab. 2008;93:2702–2708.
- Michaut A, Moreau C, Robin M-A, et al. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34:e171–e179.
- Murray M. Altered CYP expression and function in response to dietary factors: potential roles in disease pathogenesis. Curr Drug Metab. 2006;7:67–81.
- Xu J, Kulkarni SR, Li L, et al. UDP-glucuronosyltransferase expression in mouse liver is increased in obesity and fasting-induced steatosis. Drug Metab Dispos. 2012;40:259–266.
- Darwish IA. Immunoassay methods and their applications in pharmaceutical analysis: basic methodology and recent advances. Int J Biomed Sci IJBS. 2006;2:217–235.
- Zhang Y, Huo M, Zhou J, et al. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–314.
- Soeters MR, Sauerwein HP, Faas L, et al. Effects of insulin on ketogenesis following fasting in lean and obese men. Obesity (Silver Spring). 2009;17:1326–1331.
- Meyer BH, Scholtz HE, Schall R, et al. The effect of fasting on total serum bilirubin concentrations. Br J Clin Pharmacol. 1995;39:169–171.
- Roche. User info Cobas Integra 400/800 Acetaminophen-bilirubin interferenz. 2010.
- Bertholf RL, Johannsen LM, Bazooband A, et al. False-positive acetaminophen results in a hyperbilirubinemic patient. Clin Chem. 2003;49:695–698.
- Fong BM, Siu TS, Tam S. Persistently increased acetaminophen concentrations in a patient with acute liver failure. Clin Chem. 2011;57:9–11.
- Price VF, Schulte JM, Spaethe SM, et al. Mechanism of fasting-induced suppression of acetaminophen glucuronidation in the rat. Adv Exp Med Biol. 1986;197:697–706.
- Lammers LA, Achterbergh R, van Schaik RHN, et al. Effect of short-term fasting on systemic cytochrome p450-mediated drug metabolism in healthy subjects: a randomized, controlled, crossover study using a cocktail approach. Clin Pharmacokinet. 2017;56:1231–1244.
- Browne TR, Szabo GK, Ajami A, et al. Performance of human mass balance studies with stable isotope-labeled drug and continuous flow-isotope ratio mass spectrometry: a progress report. J Clin Pharmacol. 1998;38:309–314.
- Milligan TP, Morris HC, Hammond PM, et al. Studies on paracetamol binding to serum proteins. Ann Clin Biochem. 1994;31:492–496.
- Ceelie I, James LP, Gijsen V, et al. Acute liver failure after recommended doses of acetaminophen in patients with myopathies. Crit Care Med. 2011;39:678–682.
- Pearce B, Grant IS. Acute liver failure following therapeutic paracetamol administration in patients with muscular dystrophies. Anaesthesia. 2008;63:89–91.
- Seifert SA, Kovnat D, Anderson VE, et al. Acute hepatotoxicity associated with therapeutic doses of intravenous acetaminophen. Clin Toxicol. 2016;54:282–285.
- Claridge LC, Eksteen B, Smith A, et al. Acute liver failure after administration of paracetamol at the maximum recommended daily dose in adults. BMJ. 2010;341:c6764.
- Ghose R, Omoluabi O, Gandhi A, et al. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011;89:57–64.
- Achterbergh R, Lammers LA, van Nierop S, et al. A short-term high fat diet increases exposure to midazolam and omeprazole in healthy subjects. Expert Opin Drug Metab Toxicol. 2016;12:715–720.
- Lammers LA, Achterbergh R, Pistorius MCM, et al. Quantitative method for simultaneous analysis of acetaminophen and 6 metabolites. Ther Drug Monit. 2017;39:172–179.
- Flint RB, Mian P, van der Nagel B, et al. Quantification of acetaminophen and its metabolites in plasma using uplc-ms: doors open to therapeutic drug monitoring in special patient populations. Ther Drug Monit. 2017;39:164–171.
