Abstract
Background
Snakebite-associated thrombotic microangiopathy (TMA) occurs in a subset of patients with venom-induced consumption coagulopathy (VICC) following snakebite. Acute kidney injury (AKI) is the commonest end-organ manifestation of TMA. The epidemiology, diagnostic features, outcomes, and effectiveness of interventions including therapeutic plasma-exchange (TPE), in snakebite-associated TMA are poorly understood.
Methods
We reviewed all patients with suspected or confirmed snakebite recruited to the Australian Snakebite Project (2004-2018 inclusive), a prospective cohort study, from 202 participating Australian hospitals across the country. TMA was defined as anemia with schistocytosis.
Results
2069 patients with suspected snakebite were enrolled, with 1158 (56.0%) systemically envenomed, of which 842 (72.7%) developed VICC, from which 104 (12.4%) developed TMA. Of those systemically envenomed, TMA occurred in 26% (13/50) taipan, 17% (60/351) brown, and 8% (16/197) tiger snakebites. Thrombocytopenia was present in 90% (94/104) of TMA cases, and a further eight (8%) had a > 25% decrease in platelets from the presentation. Patients with TMA were significantly older than non-TMA patients with VICC (53 [35–61] versus 41 [24–55] years, median [IQR], p < 0.0001). AKI developed in 94% (98/104) of TMA patients, with 34% (33/98) requiring dialysis (D-AKI). There were four deaths. In D-AKI TMA cases, eventual dialysis-free survival (DFS) was 97% (32/33). TPE was used in five D-AKI cases, with no significant difference in DFS or time to independence from dialysis. >90-day follow-up for 25 D-AKI cases (130 person-years) showed no end-stage kidney disease but 52% (13/25) had ≥ stage 3 chronic kidney disease (CKD).
Conclusion
Our findings support a definition of snakebite-associated TMA as anemia with schistocytosis and either thrombocytopenia or >25% drop in platelet count. AKI occurring with snakebite-associated TMA varied in severity, with most achieving DFS, but with a risk of long-term CKD in half. We found no evidence of benefit for TPE in D-AKI.
1. Introduction
Bites from Australian venomous snakes are associated with a range of different snake toxin syndromes, including hemotoxicity, neurotoxicity and myotoxicity [Citation1]. There are seven genera of medically important snakes in Australia: brown snakes (Pseudonaja spp.), taipans (Oxyuranus spp.), tiger snakes (Notechis spp.), rough-scale snakes (Tropidechis carinatus), broad-headed snakes (Hoplocephalus spp.), death adders (Acanthophis spp.), and black snakes (Pseudechis spp.) [Citation1]. Different snake types produce differing clinical envenoming syndromes, depending on the toxin composition of the venom. These syndromes include hemotoxicity, myotoxicity and neurotoxicity [Citation2]. Brown snakes, tiger snakes, taipans, broad-headed snakes, and rough scaled snakes are associated with hemotoxicity due to procoagulant toxins in their venom, which cause a venom-induced consumption coagulopathy (VICC). VICC poses a risk of hemorrhage which can be fatal [Citation3,Citation4].
A subset of patients with VICC develop thrombotic microangiopathy (TMA), which is associated with acute kidney injury (AKI) [Citation5,Citation6]. Snakebite-associated TMA presents with microangiopathic hemolytic anemia (MAHA) evidenced by red cell fragments (schistocytes) on the blood film [Citation7]. Thrombocytopenia is typically present [Citation8–10]. Most studies on snakebite-associated TMA report a high prevalence of AKI, with a proportion requiring dialysis (D-AKI) [Citation8,Citation9,Citation11–13].
Snakebite-associated TMA has been likened to other TMAs, most notably thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) [Citation12,Citation14–16]. Comparisons with HUS have arisen due to their shared predominant renal end-organ damage. HUS is typically associated with Shiga-toxin diarrheal illnesses in children; and in both adults and children with complement dysregulation, with numerous predisposing genetic mutations in complement genes identified [Citation17]. TTP is a life-threatening TMA that results from acquired, or rarely hereditary, deficiency of disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13) [Citation18]. Untreated, TTP has a high fatality rate, and treatment with therapeutic plasma exchange (TPE) with fresh frozen plasma (FFP) volume replacement is lifesaving [Citation19].
Our current understanding of the epidemiology, clinical and laboratory features and treatment of snakebite-associated TMA are limited. Most existing studies are case reports and case series [Citation6]. The few cohort studies available are typically small, single centre, and/or based in highly selected settings such as tertiary hospital renal specialist centres [Citation8,Citation9]. Long-term follow-up has been limited, particularly with respect to renal outcomes [Citation6,Citation8,Citation9]. Any association between TMA secondary to snakebite, and treatment based on approaches used in either HUS or TTP, is unsubstantiated [Citation6]. TPE has been used for the treatment of D-AKI due to snakebite-associated TMA, with little supporting evidence [Citation8–10,Citation20]. Similarly, the role of antivenom in the prevention of TMA or AKI in TMA is uncertain. Whilst antivenom is the standard of care in snake envenoming, there is no clear evidence supporting its efficacy in VICC [Citation21], nor TMA specifically [Citation6].
We aimed to investigate the epidemiology, presenting features, prevalence and severity of AKI, the effectiveness of intervention with antivenom and TPE, and long-term renal outcomes in snakebite-associated TMA secondary to Australian snakebite.
2. Methods
2.1. Study design, setting and participants
We undertook a review of all patients with snakebite-associated TMA recruited to the Australian Snakebite Project (ASP), a multicentre prospective cohort of patients with suspected or definite snakebite. ASP design, patient recruitment, informed consent and data collection have been previously described [Citation1]. ASP has Human Research and Ethics Committee approval covering all involved institutions, including from the Human Research Ethics Committees (HRECs) of the Northern Territory Department of Health (04/08), Royal Perth Hospital and South Metro Area Health Service (RA-08/003), Western Australian Country Health Service (2008:03), Newcastle Hunter New England Health (07/11/21/3.06), Tasmania Network (H00109965), Gold Coast Health Service District (200835), and an additional ten HRECs.
Patients are recruited to ASP on presentation from over 200 Australian hospitals, via referrals from emergency departments, clinical toxicology units and the Australian Poisons Information Centre Network. Patients are enrolled to ASP subject to informed consent from the patient, or a parent/guardian for children less than 18 years of age. Children less than two years old are not eligible for enrollment. Patient information sheets, consent forms, and purpose-designed datasheets are completed by the referring clinicians. Data collected include baseline demographics, circumstances of the bite, clinical effects, laboratory investigations, treatment (antivenom, dialysis and plasmapheresis), complications (hemorrhage, AKI), and outcomes (hospital length of stay [LOS], intensive care unit [ICU] admission, and death). At enrollment serial blood samples are taken including a full blood count, peripheral blood film, coagulation studies and biochemistry at initial presentation, two hours, six hours and then 24-hourly until hospital discharge. These laboratory results and samples are sent to the ASP chief investigators. An additional serum sample is also collected, frozen and stored for venom assays. All data are entered in a relational database and missing data are manually extracted from patient medical records.
2.2. Data extraction
We included all snakebites from ASP for the analysis between January 2004 and December 2018. Patient baseline characteristics, snake-type based on expert snake identification or venom specific enzyme immunoassay [Citation22], presenting features of envenoming, coagulation studies (INR, fibrinogen and D-dimer), hemoglobin nadir, platelet nadir, time to platelet normalisation, peak lactate dehydrogenase (LDH), serial creatinine and liver enzymes (AST and ALT), creatine kinase (CK) (to exclude the possibility of concurrent myotoxicity and rhabdomyolysis as a cause of AKI), time to hospital presentation, antivenom administration, hospital length of stay and dialysis use, for all ASP cases were extracted from the ASP database. Blood films for the confirmation of schistocytosis were examined where available by the International Council for Standardization in Haematology method [Citation23]. If blood films were not available, referring centre laboratory reports were used to confirm the presence of schistocytosis for categorization as TMA.
In addition to prospectively collected data for ASP, medical records, inpatient and outpatient laboratory reports were retrieved with patient consent from treating centres and manually reviewed for all TMA cases. From these records, data on comorbidities; ICU admission, ventilator, and inotrope requirements; urine output; complications including acute coronary syndrome (ACS), cardiac arrest, ventricular arrhythmia, stroke, respiratory failure, hypoxic brain injury, liver failure, and pancreatitis; timepoints for dialysis; cause of death where relevant; intervention with TPE; and eGFR at last point of contact were extracted. Available specialised test results of relevance to the potential etiology of TMA were also extracted, including complement and ADAMTS-13 testing.
2.3. Case definitions
Cases were classified as non-envenomed or envenomed and envenomed cases further classified into clinical syndromes, including partial and complete VICC as previously described [Citation1] (Supplemental material, Table S1). For this analysis envenomed patients were grouped as envenomed without VICC; envenomed with partial VICC and no TMA; envenomed with complete VICC and no TMA; and envenomed/VICC with TMA. TMA was defined as MAHA (anemia <120 g/L for females and <135 g/L for males, with schistocytosis on blood film) (Supplemental material, Table S1). AKI was classified by the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) stage [Citation24], and requirement for dialysis (D-AKI). Long-term (>90-day) renal outcomes were classified by chronic kidney disease (CKD) stage (Supplemental material, Table S2), including end-stage kidney disease (ESKD). eGFR was calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [Citation25].
2.4. Outcomes and interventions
Outcomes of interest for the analysis were the prevalence of TMA; development of TMA in patients with VICC versus intervention with antivenom; and within the TMA group, the prevalence of D-AKI; overall survival; dialysis free survival (DFS) for D-AKI cases; the long-term prevalence of chronic kidney disease (CKD), including ESKD; and effectiveness of TPE intervention in the D-AKI group for the primary outcome of DFS and secondary outcome of days to dialysis independence.
2.5. Statistical analysis
Continuous variables were displayed as the median and interquartile range (IQR), and categorical variables as frequencies and percentages. Comparisons between the two groups of non-TMA VICC versus TMA were compared using Mann–Whitney U and Fisher’s exact test for non-parametric data, for continuous variables and categorical variables respectively. Kaplan Meier and log-rank (Mantel–Cox) analyses were performed for D-AKI TMA cases versus intervention with TPE, for the primary outcome of DFS, and secondary outcome of days to dialysis independence. Statistical analysis was conducted by GraphPad Prism version 8.4.2 for Windows, GraphPad Software, La Jolla California USA.
3. Results
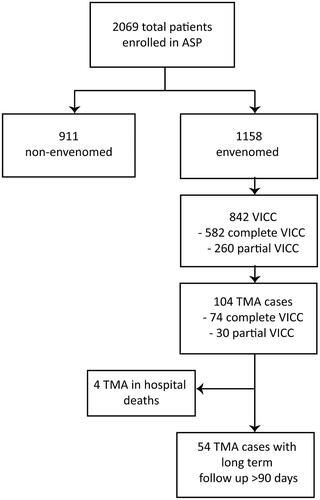
From 2004-2018 inclusive, 2069 suspected or confirmed snakebite cases were included (). Of these, 1158 were systemically envenomed, and 842 developed VICC. There were 104 TMA cases, representing 9.0% (104/1158) of envenomings and 12.4% (104/842) of all VICC cases. All patients with TMA had a preceding partial or complete VICC. Of those systemically envenomed, TMA occurred in 26% (13/50) of taipan bites, 17% (60/351) of brown snakebites, and 8% (16/197) of tiger snakebites (). Brown snakes were the most common snake species causing TMA (58% of TMA cases).
Table 1. Clinical envenoming syndromes including VICC and TMA, versus snake type for ASP prospective cohort 2004–2018 inclusive.
Seventy-two (69%) of TMA cases were male. TMA cases were significantly older compared to cases with VICC who did not develop TMA (53 [35–61] versus 41 [24–55] years, median [IQR], p < 0.0001) (). The median time to hospital presentation for the TMA group was 1.3 (IQR:0.7–3.0) hours, which was not significantly different from the VICC without the TMA group (median 1.2; IQR:0.7–2.2 h). In TMA versus VICC non-TMA groups, preceding hypotension/prior collapse was present in 9% versus 20% (p = 0.003), and major haemorrhage 3% versus 2% (p = 0.44) (). Antivenom was given to 96% (100/104) TMA cases versus 94% (692/738) of the VICC without the TMA group (). Initial treatment with FFP infusion was given to 25% (26/104) of TMA cases, compared to 12% (88/738) non-TMA VICC cases.
Table 2. Characteristics of TMA versus non-TMA VICC patient groups.
TMA cases had a median hemoglobin nadir of 95 (IQR:71–116) g/L (). The median time to hemoglobin nadir was 4.1 d (IQR:1.9–6.5 d; N = 103). Thrombocytopenia (<150 × 109/L) was present in 90% (94/104) of TMA cases. A further eight cases had a > 25% decrease in platelets from presentation and two had neither. Median platelet nadir for TMA cases was 55 (IQR:25–110) ×109/L (). Median time to platelet nadir was median 2.3 d (IQR:1.5–3.4 d). The median time to platelet recovery (>150 × 109/L) was 7.7 d (IQR:5.9–9.0 d; N = 42). The median peak LDH for the TMA group was 1305 U/L (IQR:608–2960 U/L; N = 87) (). Ten TMA cases had complement (C3 C4) testing, and five had ADAMTS13 testing, all of which were normal.
Figure 2. Laboratory parameters for TMA versus other clinical toxin syndromes. Tukey plot with median, interquartile range, 5 and 95% percentiles, and outliers. Env: envenomed; P-VICC: partial venom-induced consumption coagulopathy; C-VICC: complete VICC; TMA: thrombotic microangiopathy.
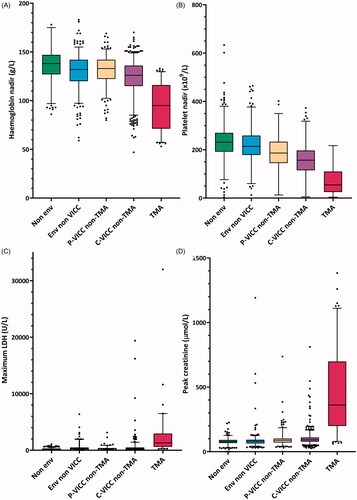
Peak creatinine for TMA was median 362 (IQR: 200–699) µmol/L (normal reference range <110 µmol/L). This compared to a peak creatinine for the non-TMA complete and partial VICC groups of 93 (78–110) µmol/L and 88 (71–102) µmol/L (median [IQR]) respectively (). AKI was present in 94% (98/104) of TMA cases, of which 34% (33/98) were D-AKI. This compared to an AKI prevalence of 6% (32/508) in the complete VICC non-TMA; 4% (9/230) in the partial VICC non-TMA; 1% (3/316) in the envenomed non VICC; and 0% (0/911) in the non-envenomed groups.
Six (6%) TMA cases had no AKI; 11 (11%) KDIGO Stage 1 AKI; 20 (19%) KDIGO Stage 2 AKI; and 67 (64%) KDIGO Stage 3 AKI, of which 33/67 (49%) were D-AKI (, Table S3). The median age for the D-AKI group was 58 (IQR:46–76) years. There was a trend to more comorbidities in the KDIGO Stage 3 group compared to other TMA cases, but comorbidity data were incomplete. Six (4%) TMA cases had a history of CKD. Of these, one did not develop AKI, one had a background of Stage 2 CKD and developed Stage 2 KDIGO AKI, two developed Stage 3 KDIGO non-D-AKI on a background of Stage 2 and Stage 3A CKD, and two developed D-AKI on a background of Stage 2 and Stage 3A CKD.
Table 3. Characteristics and outcomes for TMA cases versus acute kidney injury KDIGO stage.
Time to hospital presentation was similar between the AKI stages. In the D-AKI TMA group, four (12%) cases had hypotension/prior collapse at presentation, two (6%) had major haemorrhage (one intracranial and one gastrointestinal), and four (12%) had clinically relevant non-major bleeding at predominantly skin, gastrointestinal and other mucocutaneous sites. Six (18%) of D-AKI TMA cases had concurrent mild myotoxicity with a peak CK 1000–10,000; and 1/33 (3%) had severe myotoxicity evidenced by a peak CK >10,000. The median peak CK for the whole TMA group was 327 (IQR:183–767) U/L ().
There were four (4%) deaths in the TMA group (supplemental material, Table S4). All had complete VICC, received antivenom 0.7–3 h post-bite, and developed AKI, one of which was D-AKI (). The cause of death was intracranial hemorrhage (n = 1), and pre-hospital cardiac arrest with prolonged time before return of circulation, and secondary ischemic multiorgan failure leading to death 5–8 d post-bite (n = 3). This compared with 29 (1.4%) deaths in the whole ASP cohort, of which 21 (4.1% mortality) were in the complete VICC non-TMA group.
Of TMA survivors, all 32 D-AKI cases achieved independence from dialysis. >90-day follow-up eGFR was available for 54% (54/100) cases, totalling 301 person-years (). Of these, there was no Stage 5 ESKD. Stage ≥3 CKD occurred in 52% (13/25) of the KDIGO Stage 3 D-AKI group, compared to 0% (0/19) of the KDIGO Stage 3 non-D-AKI, 14% (1/7) of the KDIGO Stage 2, and 0% (0/3) of the KDIGO Stage 1 group (). Of the seven D-AKI TMA cases lost to follow-up <90 d, eGFR at last point of contact was median 58 (IQR: 16–86, range: 10–88) ml/min/1.73m2 at a median follow up of 38 (IQR:14–52, range: 13–58) days. Two of these cases had an eGFR <20 (10 and 16 ml/min/1.73m2), both of which had the shortest term follow up (13 and 14 d respectively), and an improving creatinine at the time of loss to follow up.
Of 33 TMA D-AKI patients, five received TPE. The TPE group had more severe hemoglobin and platelet nadirs than the non-TPE group. There was one death in the non-TPE group, compared to no deaths in the TPE group. DFS and time to independence from dialysis were not statistically significant between TPE versus non-TPE treated groups (Supplemental material, Table S5, Figure S1).
ICU admission data were available for 88 TMA cases, of which 66 (75%) required ICU support. Eight (10%) cases required inotropes, and 16 (24%) invasive ventilation, including five cases with concurrent severe neurotoxicity and bulbar palsy requiring prolonged ventilation.
Non-renal end-organ damage potentially attributable to TMA was uncommon; and occurred in the setting of concurrent other potential causes, such as cardiovascular collapse and prolonged hypotension. There was no documented liver failure; median AST for the TMA group was 92 (IQR: 49-181) U/L and median ALT 48 (IQR: 29-103) U/L. Cardiac event data were available for 90/104 TMA cases, with one non-ST elevation myocardial infarction, and five cases of ACS presenting with ECG changes and/or troponin elevations. One case developed pancreatitis and an ileus.
4. Discussion
We found that snakebite-associated TMA developed in a subset of patients with VICC, presenting with MAHA, and typically delayed thrombocytopenia. Whilst our study is confined to Australian snakebites alone, our findings, together with the few international cohort studies available, support the premise that TMA occurs in a diverse range of venomous snake types worldwide, but exclusively in association with VICC [Citation5,Citation6,Citation8,Citation9]. Increasing age was clearly associated with an increased likelihood of developing TMA. Consistent with previous studies, AKI occurred in most TMA cases, and a reasonable subset of D-AKI patients developed CKD, although long-term follow-up was incomplete [Citation6]. Other end-organ damage was uncommon. Cardiac events occurred in a minority and were mainly attributable to other causes.
Whilst past studies have defined TMA in snakebite as MAHA plus thrombocytopenia, we did not require thrombocytopenia in the context of other TMAs such as HUS occasionally reported without thrombocytopenia [Citation17]. Our findings support a definition of snakebite-associated TMA as suspected or confirmed snakebite with MAHA and thrombocytopenia (<150 × 109/L) or a > 25% drop in platelets from baseline, as recommended by Australian consensus guidelines for TMA more broadly [Citation26].
Our findings differed from some previous studies, with respect to both the prevalence of D-AKI and renal outcomes (DFS). We found a spectrum of severity, with a third requiring dialysis support. Previous studies have reported a much higher prevalence of D-AKI [Citation8,Citation9,Citation27]. We attribute this to selection bias in previous studies which often recruited from renal referral centres or included AKI as a diagnostic criterion for TMA. Additionally, in more resource-limited settings globally, where snakebite is a significant public health issue, it is unlikely all patients presenting with snakebite have serial creatinine testing performed to enable detection of less overt AKI. There were few deaths and no ESKD in our cohort. Deaths, when they occurred, were attributable to other etiologies: major hemorrhage, and pre-hospital cardiac arrest. Our finding that most patients survive and achieve DFS is consistent with recent smaller prospective and retrospective cohort studies from Sri Lanka and India [Citation8,Citation9,Citation27].
Over half of TMA D-AKI patients with long-term follow-up had ≥ stage 3 CKD at the point of the last contact. This compares to a recent Sri Lankan prospective cohort study based in a renal tertiary referral hospital, which found that amongst patients with AKI due to snakebite-associated TMA, ≥stage 3 CKD was present in 81% (21/26) cases amongst survivors at 3 months follow up [Citation8]. An Indian study of ≥3 month renal outcomes in snakebite with hemotoxicity and all-cause AKI found 19% (14/73) had an eGFR <60ml/min/1.73m2 at last follow up, with a median follow up time of 15.5 (IQR 6–21) months [Citation28]. Another Sri Lankan study of all-cause AKI in snakebite reported a 37% (20/54) prevalence of CKD at one year [Citation29]. Other studies on renal outcomes have been limited by retrospective designs, lack of long-term follow-up, and inconsistencies or unclear case definitions regarding renal outcomes [Citation6,Citation28]. Outside of snakebite specifically, AKI is epidemiologically linked to a significant risk of CKD [Citation30]. This additional risk of CKD following all-cause AKI has been estimated as an extra 10 per 100 person-years and for end-stage kidney disease (ESKD) as an extra 0.4 cases per 100 person-years [Citation31]. Of existing studies on snakebite-associated TMA, our study reports the longest follow-up in person-years on snakebite-associated TMA and CKD outcomes. Our findings, together with the existing limited data on long-term renal outcomes, indicate that patients with snakebite-associated TMA and AKI are at significant risk of long-term CKD.
There was no evidence of benefit for antivenom with respect to preventing TMA in VICC. We cannot make a definitive conclusion about any benefit of antivenom in preventing TMA. Most cases received antivenom, and antivenom administration was not significantly associated with the development of TMA in patients with VICC. This finding is in keeping with the existing lack of evidence for the efficacy of antivenom in VICC more broadly in Australian snakebite [Citation21]. Time to hospitalisation versus the development of TMA in VICC cases was also not significantly different. Irrespective of our data, early intervention with antivenom and emergency access to hospitalisation and best supportive medical care is the standard of care in snakebite envenoming more broadly. Moreover, hemodynamic instability including hypotension is a known contributor to the risk of AKI, and immediate management in patients at risk of snakebite-associated TMA is therefore imperative.
We found no evidence to support the use of TPE in TMA with D-AKI, with respect to DFS or time to independence from dialysis. TPE is resource-intensive, uses significant blood donor plasma products, and is associated with risks including hypotension and allergic reactions [Citation32]. Our findings, together with our previously published systematic review showing no evidence for TPE in improving renal outcomes [Citation6], provide further evidence supporting the recommendation for no role for TPE in the management of snakebite-associated TMA.
The etiology of snakebite-associated TMA remains unclear. ADAMTS-13 and complement testing were normal in the few patients tested, which is concordant with existing studies [Citation33–36]. It is likely snakebite-associated TMA is more akin to drug and other toxin-mediated TMAs, rather than either TTP or complement-mediated HUS.
The main limitations of our study include its size, loss to long-term follow-up, and restriction to Australian snakebite. We report 104 TMA cases from a cohort of 2069 suspected and confirmed snakebites. This compares to a conservative estimate of 1.8–2.7 million snake envenomings per year globally [Citation2]. Snakebite is classified as a Neglected Tropical Disease by the World Health Organisation, defined as a diverse range of tropical conditions which receive inequitable research funding and investment [Citation37]. Larger scale studies are therefore challenging, and our study is the largest and only national cohort published to date [Citation6]. Our detailed manual review of TMA case medical records partially relied on routinely collected clinical information. Whilst possible confounders for AKI such as concurrent nephrotoxic medicines are not able to be completely ruled out, (e.g., anti-inflammatory medicines), our findings, together with existing studies on snakebite-associated TMA support TMA being the predominant cause of AKI in snakebite [Citation5,Citation7]. Hypotension and prior collapse occurred in only a minority of AKI patients and indeed was less common in those with TMA than in those with VICC but not TMA, a finding which we cannot explain. We were unable to explore associations between TMA and geographic or interspecific venom composition variability, given the limited TMA sample size and difficulties in accurate taxonomic identification of most snake bites. Loss to long-term follow-up is typical of prospective cohort studies. Given that our cases lost to follow up were predominantly stage 1 and 2 KDIGO AKI, and the likelihood that patients with increasing age and other comorbidities are more likely to undergo long term surveillance with serum creatinine testing, it is likely that our data have a selective inclusion of patients at higher risk of long term CKD. Nevertheless, at least 39% (13/33) of TMA patients with initial D-AKI later developed ≥ stage 3 CKD.
5. Conclusion
We recommend that for snake envenoming with VICC and suspected TMA, best practice includes early intervention with antivenom, which is the standard of care in all snake envenoming. Patients presenting with snakebite-associated TMA are at high risk of AKI. Our findings highlight the importance of best supportive care, including high-level ICU care where needed and available, and dialysis when required for AKI. Patients with snakebite-associated TMA commonly present with concurrent major haemorrhage, associated hypovolaemia, and hypotension. These are well-described risk factors for renal injury and should be judiciously managed [Citation30]. Intervention with TPE is not recommended. Follow-up for CKD surveillance, detection and management of renal risk factors is critically important in the long-term medical care of these patients.
Author contributions
T. Noutsos conceptualized and designed the research under the general direction of G.K. Isbister. G.K. Isbister, S.G. Brown and K.Z. Isoardi recruited patients and coordinated data collection. T. Noutsos performed manual case note reviews for all TMA cases, analyzed results and made figures and tables. T. Noutsos primarily interpreted findings and wrote the paper under the general direction or G.K. Isbister and B.J. Currie. All authors substantially reviewed, commented on, and approved the final version of the submitted manuscript.
Supplemental Material
Download MS Word (186.7 KB)Acknowledgments
We acknowledge the assistance of the Australian Snakebite Project Research database administrators, S. Jenkins and K. Tape, and all local co-investigators, medical, nursing and laboratory staff at referring centers around Australia, who assisted in patient recruitment and data collation for the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Johnston CI, Ryan NM, Page CB, et al. The Australian Snakebite Project, 2005–2015 (ASP-20). Med J Aust. 2017;207(3):119–125.
- Gutiérrez JM, Calvete JJ, Habib AG, et al. Snakebite envenoming. Nat Rev Dis Primers. 2017;3(1):17063.
- Isbister GK, Brown SGA, Page CB, et al. Snakebite in Australia: a practical approach to diagnosis and treatment. Med J Aust. 2013;199(11):763–768.
- Isbister GK, Scorgie FE, O’Leary MA, et al. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10)). J Thromb Haemost. 2010;8(11):2504–2513.
- Wijewickrama ES, Gooneratne LV, Gnanathasan A, et al. Severe acute kidney injury following Sri Lankan Hypnale spp. envenoming is associated with thrombotic microangiopathy. Clin Toxicol. 2021;59(4):296–302.
- Noutsos T, Currie BJ, Lek RA, et al. Snakebite associated thrombotic microangiopathy: a systematic review of clinical features, outcomes, and evidence for interventions including plasmapheresis. PLOS Negl Trop Dis. 2020;14(12):e0008936.
- Noutsos T, Currie BJ, Brown SG, et al. Schistocyte quantitation, thrombotic microangiopathy and acute kidney injury in Australian snakebite coagulopathy [ASP28]. Int J Labor Hematol. 2021;1–7.
- Wijewickrama ES, Gooneratne LV, Gnanathasan A, et al. Thrombotic microangiopathy and acute kidney injury following Sri Lankan Daboia Russelii and Hypnale species envenoming. Clin Toxicol. 2020;58(10):997–1003.
- Rao IR, Prabhu AR, Nagaraju SP, et al. Thrombotic microangiopathy: an under-recognised cause of snake-bite-related acute kidney injury. Indian J Nephrol. 2019;29(5):324–328.
- Isbister GK, Little M, Cull G, et al. Thrombotic microangiopathy from Australian brown snake (Pseudonaja) envenoming. Intern Med J. 2007;37(8):523–528.
- Chugh KS. Snake-bite-induced acute renal failure in India. Kidney Int. 1989;35(3):891–907.
- Date A, Pulimood R, Jacob CK, et al. Haemolytic-uraemic syndrome complicating snake bite. Nephron. 1986;42(1):89–90.
- Mittal BV, Kinare SG, Acharya VN. Renal lesions following viper bites – a study of 14 years. Indian J Med Res. 1986;83:642–651.
- Namal Rathnayaka R, Ranathunga PAN, Kularatne SA. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura following Hump-nosed Pit Viper (genus: Hypnale) envenoming in Sri Lanka. Wilderness Environ Med. 2019;30(1):66–78.
- Mitrakrishnan JY, Bandula CW, Mitrakrishnan CS, et al. Haemolytic uremic syndrome a hitherto unreported complication of humpnosed viper envenomation. Indian J Hematol Blood Transfus. 2013;29(2):116–118.
- Godavari KSV. Hemolytic uremic syndrome – an unusual complication of snake envenomation. Univ J Med Med Sci. 2016;2(2).
- Fakhouri F, Zuber J, Frémeaux-Bacchi V, et al. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681–696.
- Sadler JE. Pathophysiology of thrombotic thrombocytopenic purpura. Blood. 2017;130(10):1181–1188.
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836–2846.
- Mohan G, Guduri PR, Shastry S. Role of therapeutic plasma exchange in snake bite associated thrombotic microangiopathy – a case report with review of literature. J Clin Apher. 2019;34(4):507–509.
- Isbister GK, Duffull SB, Brown SGA. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM. 2009;102(8):563–568.
- Maduwage K, O’Leary M, Silva A, et al. Detection of snake venom in post-antivenom samples by dissociation treatment followed by enzyme immunoassay. Toxins. 2016;8(5):130.
- Zini G, D’Onofrio G, Briggs C, et al. ICSH recommendations for identification, diagnostic value, and quantitation of schistocytes. Int J Lab Hematol. 2012;34(2):107–116.
- Kidney disease: improving global outcomes acute kidney injury working group. Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Fox LC, Cohney SJ, Kausman JY, et al. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Intern Med J. 2018;48(6):624–636.
- Mohan G, Guduri PR, Shastry S, et al. Thrombotic microangiopathy in hematotoxic snakebites and its impact on the prognosis: an entity often overlooked. J Thromb Thrombolysis. 2019;48(3):475–482.
- Priyamvada PS, Jaswanth C, Zachariah B, et al. Prognosis and long-term outcomes of acute kidney injury due to snake envenomation. Clin Kidney J. 2020;13(4):564–570.
- Herath HMNJ, Wazil AWM, Abeysekara DTDJ, et al. Chronic kidney disease in snake envenomed patients with acute kidney injury in Sri Lanka: a descriptive study. Postgrad Med J. 2012;88(1037):138–142.
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964.
- See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172.
- Szczeklik W, Wawrzycka K, Włudarczyk A, et al. Complications in patients treated with plasmapheresis in the intensive care unit. Anaesthesiol Intensive Ther. 2013;45(1):7–13.
- Casamento AJ, Isbister GK. Thrombotic microangiopathy in two tiger snake envenomations. Anaesth Intensive Care. 2011;39(6):1124–1127.
- Dineshkumar T, Dhanapriya J, Sakthirajan R, et al. Thrombotic microangiopathy due to Viperidae bite: two case reports. Indian J Nephrol. 2017;27(2):161–164.
- Bucaretchi F, Pimenta MMB, Borrasca-Fernandes CF, et al. Thrombotic microangiopathy following Bothrops jararaca snakebite: case report. Clin Toxicol. 2019;57(4):294–299.
- Malaque CMS, Duayer IF, Santoro ML. Acute kidney injury induced by thrombotic microangiopathy in two cases of Bothrops envenomation. Clin Toxicol. 2019;57(3):213–216.
- Williams DJ, Faiz MA, Abela-Ridder B, et al. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLOS Negl Trop Dis. 2019;13(2):e0007059.