Abstract
Introduction
Enemas containing phosphate are widely prescribed and may cause important adverse effects. A systemic review published in 2007 reported the literature on the adverse effects of phosphate enemas from January 1957 to March 2007 and identified 12 deaths. These were thought due to electrolyte disturbances, heart failure and kidney injury. These data raised concerns about the use of phosphate enemas in routine practice. Newer osmotic-based enema alternatives are now available that do not contain absorbable ions. We sought to review the literature since this review and evaluate the latest data on the toxicity of phosphate-containing enemas. To gain a fuller picture we included case series and larger studies as well as case reports.
Objectives
To review the toxicity of phosphate enemas, particularly with respect to acute metabolic consequences and their associated clinical features. To identify risk factors for metabolic toxicity and consider whether phosphate enemas should be relatively contra-indicated in specific patient groups.
Methods
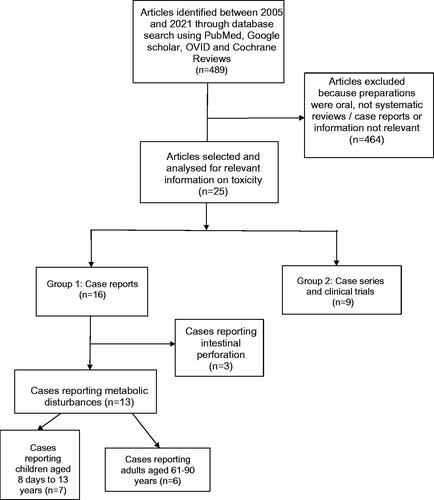
A systematic literature review was conducted in PubMed, Google Scholar, and Cochrane Reviews (2005–2021) using the search terms ‘phosphate enema or sodium phosphate enema’ or ‘phosphate-based enema’ or (phosphate AND enema) or (Fleet AND enema) or ‘sodium phosphate laxatives’ or ‘sodium phosphate catharsis’ or ‘sodium phosphate cathartic’. Relevant papers were read, and data were extracted.
Results
The searches identified 489 papers of which 25 were relevant: seven papers were case reports or small case series of metabolic abnormalities from the use of phosphate enemas in nine children, six were case reports on 16 adults. Nine papers were large case series or clinical studies that included data on systemic metabolic effects, of varying size from 24 healthy volunteers to a cohort of 70,499 patients. Case reports identified seven adult deaths but none in children. Children most often presented with decreased consciousness (6/9), and tetany (4/9). In adults overall only five cases had clinical features reported, hypotension was seen in four and QT prolongation in two. Treatment was generally symptomatic, with intravenous fluid and calcium salts for electrolyte changes and hypocalcaemia, and vasopressors for severe hypotension. Haemodialysis was used in three children and peritoneal dialysis in one, all of whom survived. In adults, haemodialysis did not prevent death in two of four cases in whom it was used. Common factors underlying toxicity were inappropriately high phosphate dose, or enema retention, both resulting in greater absorption of phosphate. Associated pre-disposing conditions included Hirschsprung disease in children and co-morbidity and renal impairment (2/5) in older adults. Absolute reported changes in serum phosphate or calcium were not accurate indicators of outcome. Larger case series and clinical trials confirm an acute effect of phosphate enemas on serum phosphate, which was related to both dose and retention time. These effects were not seen with non-phosphate preparations. In these cases series, adverse events were rarely reported.
Conclusion
Phosphate enemas are potentially toxic, particularly in young children with Hirschsprung disease and in the elderly with co-morbidity. Raised awareness of the risk of phosphate enemas is still required. Other less toxic enema preparations are available and should be considered in patients at extremes of age. If phosphate enemas are the only clinical option careful monitoring of biochemical sequelae should be undertaken.
Introduction
In the UK and US, sodium phosphate enemas contain osmotically active phosphate. Commonly used preparations include Sodium Acid Phosphate with Sodium Phosphate (Fleet Enema®; dihydrogen phosphate dihydrate 12.8 g with disodium phosphate dodecahydrate 10.24 g, in water to 128 mL; ∼1400 mM with an osmolality of >2200) [Citation1] and Sodium Phosphates Enema (monobasic sodium phosphate 19 g with dibasic sodium phosphate 7 g in water to 118 mL), the amount prescribed varying slightly from product to product. The dose is usually prescribed as a volume and depends on patient age, 2–4 years 25% adult dose (∼30–35 mL); 5–11 years 50% adult dose (∼60–70mL); over 12 years full dose (118–128 mL). The phosphate solution has osmotic action and works by pulling water into the lower bowel, softening and expanding the stool, resulting in a build-up of pressure that triggers peristalsis and allows defaecation to take place, usually within only a few minutes. This rapid expulsion of an enema with the stool results in low exposure of the gut to the high phosphate dose. However, occasionally, the sodium phosphate solution is retained in the gut lumen and then absorbed, which can lead to water and electrolyte imbalances, and resultant clinical consequences [Citation2]. Adverse effects resulting from rapid increases in serum phosphate with resultant changes in calcium and magnesium, or effects from the excess movement of fluid into the bowel lumen from the blood.
Phosphate enemas are commonly used to treat constipation and to clean the lower intestinal tract before endoscopic and surgical procedures. Their use is generally considered safe in healthy adults and does not normally result in patients experiencing any adverse effects [Citation3]. However, many patients with constipation have associated comorbidity, including gastrointestinal and neurological disorders that slow gut motility, and/or are at the extremes of age [Citation3]. Over the past decade, the use of enemas in some clinical situations, such as parturition, has been shown to be unnecessary [Citation4].
The potential hazard of over-the-counter (OTC) phosphate products, including enemas, was highlighted by the US FDA in 2014 [Citation5]. The FDA warning advised against the use of OTC preparations in those two years or under and caution in those aged up to five years and in those older than 55 years, particularly if they had co-morbidity. It advised against more than one enema dose in 24 h and warnings in those who had significant kidney or cardiac disease, dehydration, bowel inflammation or bowel obstruction. The FDA warning also included co-medication warnings on diuretics, angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and nonsteroidal anti-inflammatory drugs (NSAIDs).
Similar limitations in use are in place elsewhere. For example, in the UK and US, phosphate enemas are licensed only for patients three years and over in similar doses, from 45 to 128 mL of the solution once daily, depending on age with similar warnings on co-morbidity and co-medication. In the UK the advised volumes once daily are for a child 3–6 years 45–65 mL, 7–11 years, 65–100 mL, 12–17 years 100–128 mL and for adults 128 mL [Citation6]. We were unable to find any data on absorption differences of phosphates in the colon with age, but as the surface area of a lumen is proportional to the square of its radius, one might postulate that absorption due to osmotic pressure would reflect this and doses be adjusted with that ratio in mind. This does not seem to be the case from the advised dosing schedules.
Adverse effects are due to local trauma, including bowel perforation, and toxicity due to shifts in electrolytes, particularly of phosphate into blood, with resultant cellular shifts in calcium and magnesium that have effects on other organ function, depending on the extent of change, including particularly the heart, brain and kidney. A key factor in the risk of changes in blood results is the duration of enema retention.
In a systemic review published in 2007 that evaluated the literature on the use of phosphate enemas from January 1957 to March 2007, complications in the 44 cases identified were electrolyte abnormalities, heart failure and kidney injury, and there were 12 deaths, four in those under 18 y [Citation7]. Two-thirds of cases (29) were 18 years or under, of whom nine were in young children aged two or less. Of 26 cases in adults, 11 were aged over 65 years [Citation7]. Predisposing factors noted were gut motility disorders, renal and heart disease. However, this systematic review did not present data to clarify how electrolytes changed in patients treated with phosphate enemas. Consecutive patient case series or clinical studies are required to provide this information.
Objectives
To review the toxicity from phosphate enemas, particularly with respect to specific metabolic consequences, and their relation to clinical outcome.
Methods
We conducted a systematic literature review in PubMed, Google scholar, OVID and Cochrane Reviews to identify reports of acute toxicity due to phosphate enemas. We used the keywords: ‘phosphate enema or sodium phosphate enema’ or ‘phosphate-based enema’ or (phosphate AND enema) or (Fleet AND enema) or ‘sodium phosphate laxatives’ or ‘sodium phosphate catharsis’ or ‘sodium phosphate cathartic’ between 2005 (Jan) and 2021 (July 31). The language was restricted to English or English abstracts. The 2005 start date was to ensure as full a data set of reports was obtained as possible since the previous review.
All titles were reviewed, and relevant papers were retrieved and searched for information on non-traumatic complications of phosphate enemas. We then hand-searched the reference list of these papers for information on toxicity, poisoning or reviews as well as cases missed in the initial search and references in the papers we found. Data extraction was conducted by one author and checked by a second. We excluded all reports that were related to oral preparations. Our search ended with a list of papers dealing with complications and toxicity from rectal phosphate enema products which then divided into case reports or larger series and clinical trials in children and adults. Normal ranges were taken from the individual reports, where provided, as they varied slightly, and all values given were converted into SI and mass units as necessary to allow comparison. We report values outside these normal ranges although toxicity usually required significant change from normal, so we also included reported symptoms of toxicity, such as those of hypocalcaemia, including arrhythmia and tetany, where recorded.
Results
We identified 489 papers published between 2005 and 2021 using the search terms listed. Sixteen case reports or small case series and nine large case series or clinical trials were considered relevant to the question of toxicity ().
Case reports of toxicity
There were sixteen publications reporting cases of acute toxicity due to phosphate enemas, of which thirteen reported acute metabolic complications () and three reported intestinal perforation due to a phosphate enema [Citation8–10]. Seven publications reported cases of metabolic complications in children, including nine patients aged 8 days to 13 years, none of whom died [Citation11–17]. Six publications reported cases of metabolic complications in adults, including 16 patients aged 61–90 years, of whom seven died [Citation18–23] although one of these was in a patient with a “do not resuscitate” request [Citation19]. Associated clinical features were not reported in detail by all authors.
Table 1. Case reports of adverse effects of phosphate enemas 2005–2021. A. Children and B Adults.
In children, a common feature was reduced consciousness, reported in six children [Citation11–13,Citation16,Citation17]. Features indicative of tetany, carpopedal spasm or other muscular spasms were found in four [Citation12,Citation13,Citation15,Citation16] and hypotension in three [Citation13,Citation16,Citation17]. Prolonged QT at 477 msec was reported in only one child [Citation17]. Serum phosphate concentrations in these children were all greater than twice the upper limit of normal, ranging from 4.76 to 19.95 mmol/L (14.7–61.8 mg/dL). Serum calcium concentrations ranged from 0.36 to 1.38 mmol/L (1.44–5.53 mg/dL) in children. In children, common themes in causation were iatrogenic overdose, excess dose per kg weight, and excess retention times associated with Hirschsprung disease [Citation14,Citation16,Citation24,Citation25]. These children all survived, although four received haemodialysis [Citation13,Citation14,Citation16,Citation17].
In adults clinical details were provided in only five cases, four had hypotension [Citation18,Citation19,Citation21,Citation23] and two prolonged QT [Citation21,Citation22] although the QT values were not reported. Tetany was only reported in one adult patient [Citation22]. In adults symptomatic hyperphosphataemia was associated with phosphate concentrations between 2.65 and 14.54 mmol/L (8.3–44.8 mg/dL) the highest upper limit of normal reported in the reports for adults being 1.55 mmol/L (4.8 mg/dL) (). Deaths occurred in patients with reported phosphate concentrations of 5.17, 4.26, 11.56, 14.54, 1.71, 3.07 and 9.69 mmol/L (16, 13.2, 38.8, 45.2, 5.3, 9.5 and 30 mg/dL respectively). Serum calcium concentrations ranged from 0.5 to 2.18 mmol/L (2–8.74 mg/dL) in adults, and deaths were in those with minimum calcium concentrations of 1.2, 1.1, 1.05, 0.5, 2.18, 2.08 and 0.93 mmol/L (4.81, 4.41, 4.21, 2, 8.74, 8.34 and 3.73 mg/dL) the lower limits for calcium quoted being 2–2.1 mmol/L (8–8.4 mg/dL) (). It seems from these data that neither absolute rise in phosphate nor fall in calcium are precise indicators of outcome.
In adults, we were unable to identify a common risk factor, other than age and comorbidity: all but two (aged 61 and 77 years) being over 80 years. As in children, excess doses were administered in several patients; Ori et al. [Citation20] reported that three of their 11 patients had received a greater dose than recommended; one patient received two enemas in 30 min [Citation19] and another multiple enema [Citation21]. In the case of Hsu and Wu [Citation22] the enema was retained and only released after a manual examination, thus exposure to phosphate was prolonged.
Observational studies and clinical trials of effects on electrolytes
We identified nine large case series and clinical trials evaluating the effects of phosphate enemas on serum electrolytes or calcium and magnesium published since January 2005 (). Only one of these was in children, by Anderson et al. who retrospectively assessed the efficacy of different enema preparations by case note review in 768 children in a paediatric emergency department [Citation26]. The median age was 6.2 years (IQ range 3.3–10.3 y), enemas used were sodium phosphate, phosphate content not specified (median 59 mL (3.1 mL/kg), n = 396), a docusate, magnesium citrate, mineral oil, and sodium phosphate mixture (286 mL (9.6 mL/kg), n = 198), soap suds (240 mL (7.5 mL/kg), n = 160), and other preparations (n = 14). No metabolic complications were reported, the only ADRs mentioned being rare cases of abdominal pain and vomiting.
Table 2. Clinical trials and large case series reporting use of phosphate enemas.
Ainley and colleagues [Citation27] prospectively studied 100 consecutive adult patients receiving phosphate (Fleet) enemas (two doses of 45 mL at a 12 h interval; precise phosphate dose/kg not stated) for colonoscopy procedures. 45% developed a raised phosphate (> 1.6 mmol/L) with seven >2.3 mmol/L (7.12 mg/dL) (maximum 2.81 mmol/L; 8.7 mg/dL), 26% hypokalaemia (< 3.5 mmol/L), and 16% hypocalcaemia (< 2.15 mmol/L; 8.62 mg/dL)) after the event. There were weak positive correlations of age (p < 0.01, r = 0.35) and creatinine (p < 0.01, r = 0.31) with phosphate rise, although relationships were stronger for those with phosphate concentration >2.3 mmol/L (7.12 mg/dL); there was also an increased risk of electrolyte derangements in those on ACE inhibitors, AT-2 antagonists and diuretics [Citation27]. Phosphate concentration differed according to the delay to blood sampling (afternoon procedure and sampling, 8.5 h since 2nd enema, mean serum phosphate 1.97 mmol/L (6.1 mg/dL) versus morning procedure/sampling, 16.5 h since 2nd enema, mean phosphate 1.44 mmol/L (4.46 mg/dL); p < 0.001). Six of the seven patients with very high phosphates had afternoon procedures. The authors suggested that transient hyperphosphatemia might usually be early and short-lived, and therefore missed in some of the patients undergoing morning procedures. None of these patients were reported to develop symptoms associated with the electrolyte changes observed.
A letter reported 100 elderly patients, mean age 87.1 ± 5.8 years (range 71–100 years), with renal impairment (mean eGFR 40.8 ± 13.4 mL/min), treated with sodium phosphate enemas (133 mL, ∼184 mmol phosphates) [Citation28]. The patients were given from one to six doses of phosphate for faecal impaction. Individual patient data are not detailed in the report. Overall median serum calcium and phosphate did not change in the 4 h following the enema (although the period over which patients received the 1–6 enemas is not given). Before enema: median [IQR] (Units mmol/L): calcium 2.32 [0.12], phosphate 1.14 [0.39]; after enema: calcium 2.34 [0.13], phosphate 1.16 [0.42]. However, 6% developed hyperphosphatemia and 2% hypocalcaemia (values not reported). The authors indicated that using backward linear regression (Wald) there was a significant correlation with lower eGFR and risk of hyperphosphataemia (b = −0.08, OR = 0.92, 95% CI 0.88–0.94; p < .001). The estimated odds of post-enema hyperphosphatemia decreased 9% for every increase in eGFR value and those who developed hyperphosphatemia had a higher phosphate at baseline than those who did not. Any relationship with the dose of phosphate administered was not reported. The authors stated that three patients died; they had lower abbreviated mental test scores and Barthel indices than the main cohort (p < 0.001) but the cause of death was not reported.
Two studies [Citation29,Citation30] evaluated a Spanish phosphate enema preparation, Enema Casen (250 mL, containing 35.5 g monobasic sodium phosphate monohydrate and 17.8 g dibasic sodium phosphate dodecahydrate; a total of 304 mmol phosphate). The first compared the effects of Enema Casen with no treatment in a cross-over study in 24 volunteers aged 35–70 years [Citation29]. The median retention time was not provided by the authors, but the range was 4–40 min. Twelve hours after administration of the Casen 250 mL enema, mean serum phosphate and sodium concentrations increased by a mean of 1.18 mg/dL (0.3 mmol/L) and 1.32 mmol/L, respectively, compared to no treatment (both p < 0.001). Mean serum phosphate was above the upper limit of normal (5 mg/dL, 1.64 mmol/L) for 14 of the 24 subjects between 10 min and 2 h min after enema administration, returning to normal concentrations within 4 h in all cases, indicating an early rise that was short-lived in this cohort. Four subjects (16.7%) had more than one phosphate concentration considered as serious hyperphosphatemia, over 7 mg/dL (2.26 mmol/L) in this time interval. A significant correlation was found between maximum serum phosphate concentration (Cmax) and enema retention time (r2 = 0.452; p < 0.001). In the cases with a phosphate concentration over 7 mg/dL (2.26 mmol/L) the enema retention times were 15 min, 20–25 min, over 30 min and over 40 min. Phosphate AUC-12h was increased 86% (p < 0.001) but no serious adverse effects were observed. None of the changes in serum electrolyte concentrations were associated with clinical symptoms. No other electrolytes measured were outside normal ranges.
The second study on the Spanish product [Citation30] compared the effect of two phosphate enema formulations, a standard monobasic sodium phosphate monohydrate (133 mL of Fleet enema, containing ∼184 mmol phosphate) and Enema Casen [Citation30] in an open-label study. These enemas contain approximately the same quantity of total anhydrous phosphate per mL. Twenty volunteers aged over 50 y received a single Fleet (118 mL) or a Casen enema (232 mL). Each had a similar mean (± SD) retention time: Fleet 6.0 ± 3.2 min; Casen 5.7 ± 3.0 min; ranges <1–10 min). Asymptomatic transient hyperphosphatemia (> 1.45 mmol/L; 4.5 mg/dL) and change in phosphate AUC were significantly associated with an increase in retention time (p = 0.032), although the times only ranged between 0.5 and 10 min. The volume and formulation of enema had no additional impact on this relationship. All serum calcium concentrations remained in the normal range (2.12–2.64 mmol/L; 8.5–10.6 mg/dL) [Citation30].
A US study [Citation31] retrospectively compared a milk and molasses enema (n = 47) with a phosphate enema in children (n = 49); most were aged 2–11 years with only eight below 2 years. They study did not include measurement of changes in electrolytes and no major clinical adverse effects were reported, the only adverse effects noted being abdominal discomfort.
Niv et al. [Citation32] compared complications resulting from the use of the phosphate-containing Fleet enema in 269 adult patients versus the use of a phosphate-free enema (Easy Go) in 286 patients in two cohorts with acute constipation; over 50% of participants were aged 65 years and older. Their study focussed on bowel perforation and did not evaluate electrolyte changes formally. However, they identified lethal hyperphosphatemia in an 86-year-old patient within one day of a phosphate enema. In the same phosphate enema cohort, three deaths were associated with bowel perforation, in patients aged 72, 86 and 93 years [Citation32].
A large North American cohort study assessed renal function in a cohort of 70,499 outpatients aged 50–89 years receiving phosphate or polyethylene glycol enemas studied over a period of 15 months [Citation33]. A decline in renal function that was greater in those receiving phosphate as opposed to polyethylene glycol measured as any (p = 0.001) or long-term (p = 0.003) reduction in eGFR was observed. Odds ratios were 1.3 (95% CI, 1.2–1.5) for any or 1.4 (95% CI, 1.1–1.8) for long-term eGFR decline over this period. Other risk factors for eGFR decline in these two cohorts included diabetes and non-iron-deficient anaemia.
Dagan et al. [Citation34] examined the effects of phosphate enemas on long-term renal function in a retrospective age and renal function matched case-control study of 416 patients of whom 206 were treated with a single enema (300 mmol/L; 15 mL vial) and studied the effect on creatinine before and after the treatment. Cases and controls were matched by baseline creatinine > 1.5 mg/dL (132.6 mmol/L; 108 patients) and baseline creatinine > 2 mg/dL (177 µmol/L; 58 patients). Electrolytes, including phosphate, calcium and creatinine were measured daily, and no changes were observed, but acute changes in values would have been missed in this methodology. The mean (± SD) age of the cohort of the phosphate group was 75.7 ± 17.6 years. No information on enema retention time is provided. Those with impaired creatinine at baseline were modestly older, with means (± SD) being 82.8 ± 9.6 years and 83.3 ± 6.1 years in the two groups with mild and more severe renal function changes [Citation34].
Discussion
Our objective was to investigate the nature and pattern of reports of acute metabolic adverse effects of phosphate enemas, the toxicities most often seen by or referred to clinical toxicologists. The last review of this topic in 2005 [Citation7], covering the period 1957–2005, identified 44 cases, 15 in children 2 years and below, 14 in children aged 3–18 years, 15 aged 18–65 years (mean age 52 years), and 11 over 65 years (mean age 81 years). The authors identified 12 fatalities, 11 associated with co-morbidity as well as water and electrolyte disturbance. Four deaths were in cases aged <18 years, two in those aged 18–65 years, and six in patients over 65 years. This report included no details of precise magnitude in changes in phosphate, calcium or other electrolytes. The US FDA issued a warning in 2014 regarding OTC phosphate preparations, oral and enema, and their use in young children, older adults, those with renal, cardiac and lower GI disease, and those on some medications [Citation5]. This warning did not discuss in-hospital use, nor were details of the magnitude of potential changes in blood results provided. The UK MHRA Data Analysis Printouts of adverse effects to pharmaceuticals marketed in the UK contain data on sodium acid phosphate enemas (https://yellowcard.mhra.gov.uk/iDAP/). Since 1966 the database includes 2 deaths in 39 overall reports, but all preceded 2005. No specific warnings have been issued by the MHRA.
We found a lower proportion of children than adults in our review (nine children, 16 adults) than that in 2005 (29 under 18 years, 26 over 18 years) [Citation7]. We also found no reported deaths in children, as opposed to four previously, three of those being less than 1 year and one 3- years-old. These findings suggest that phosphate enemas may be being used less, or more safely, in young children, but as they are not usually licensed or contraindicated in those 2 years and younger [Citation5,Citation24,Citation35] there is obviously an issue with use in children. While some case reports in children and adults relate to out-of-hospital use, some are related to the use of enemas in a hospital environment, especially the larger case series. The warning messages from the FDA [Citation5] on OTC phosphate enemas may therefore not be sufficiently comprehensive.
Clinical features were often initially non-specific in children, as the decreased conscious level was the most frequent sign reported (6/9 cases). Tetany or symptoms suggestive as due to hypocalcaemia was seen is 4/9, and hypotension in three. In adults only five of the 15 cases were clinical features reported, and in them, hypotension was seen in four. Certain important adverse effects were seen more frequently in different patient age cohorts. Overt signs of clinical hypocalcaemia occurred in 4/9 child cases but only adult case; kidney injury was seen in both age cohorts. QT prolongation is being reported in only one child but two of five adults. We are unsure if this was due to a lack of routine 12 lead ECG measurements in children. Along with extremities of age, comorbidities including CKD, coronary artery disease and, in children, Hirschsprung's disease were more commonly associated factors for phosphate poisoning. Important common themes were the use of excessive doses of phosphate in children [Citation16,Citation24,Citation25] and in the elderly with renal impairment, and an association with longer enema retention. The reported changes in serum phosphate and calcium were not predictive of outcome, making decisions on precise clinical management more difficult.
The previous review did not identify any large case series or trials prior to 2007 [Citation7]. The larger series and comparative studies now reported show how frequently early post-enema rises in serum phosphate occur, even in those without symptoms. An association of large rises in serum phosphate with higher pre-treatment concentrations in one case series [Citation28] was not tested in others. In addition, there are asymptomatic short-term dose-response effects for phosphate dose in the clinical trials and large case series we report.
There are several types of toxicity from enemas, and in some age groups, metabolic effects may not be the most frequent. Thus, in a case series from Israel, four deaths were associated with Fleet enema use in the elderly, three from perforation, but only one hyperphosphatemia [Citation32]. As recently as 2021 a case of perforation was reported in an elderly patient [Citation8].
Other papers we identified on phosphate enemas included a review of ADRs in children in Germany which mentioned the potential hazard of phosphate enemas in children if dosage advice was not followed [Citation35]. A review in the Cochrane database showed enemas were not useful in labour but did not address systemic adverse effects [Citation4]. This large evidence-based review suggested that the evidence base for the use of phosphate was less than for other, less toxic, enemas. More detailed analysis on phosphate homeostasis provides a background on the handling of phosphate in man but no case data [Citation2].
We had hoped to draw up defined dosage limits for toxicity but as a major factor in toxicity risk was a retention of the enema beyond a few minutes, and clear enema dosing regimens were not always provided this was not possible. Other than repeated dosing outside the data sheet recommendation retention seemed the major factor in toxicity. Although co-prescription of drugs such as diuretics and ACE inhibitors are known risk factors these were not well enough itemised to identify cases where they were a key risk factor in toxic effect.
A potential weakness of all the reports we identified is the lack of data on serum magnesium, which is likely to be depressed by excess phosphate. There was no systematic attempt to assess treatment for the metabolic changes observed and all patients were treated based on their clinical and biochemical features. The blood results we report were those observed and reported but may not precisely coincide with features of acute toxicity. From a treatment perspective, haemodialysis tended to be reserved for severe cases but was not always successful in preventing death, 2 of the 4 patients haemodialyzed died [Citation19,Citation20]. Thus, apart from standard care with fluid and intravenous calcium as indicated on blood results or clinical and ECG features, and haemodialysis in those with associated severe renal impairment, no firm treatment recommendations can be clearly derived.
Conclusions
Phosphate enemas continue to be associated with severe adverse metabolic effects in some patients, particularly at extremes of age. A frequent risk factor in young children is Hirschsprung disease and in the elderly co-morbidity, including renal failure. There was no clear relationship between reported changes in serum phosphate and calcium and clinical outcome. No deaths were identified in children since the 2007 review [Citation7]. Important identifiable risk factors are ‘off licence’ use in very young children, excess phosphate dose, retention time and comorbidity. Larger case series and clinical trials show how frequently hyperphosphataemia occurs (), but this seems rarely to be associated with major systemic acute toxicity. Other enema products not containing phosphate are also available and seem a better option for ‘at risk’ patients, particularly at extremes of age [Citation3]. If phosphate enemas are an essential treatment consideration should be given to careful monitoring of electrolytes, particularly phosphate and calcium in those ‘at risk’ or with longer enema retention times.
Disclosure statement
The authors declare they have no conflicts of interest.
References
- Maisel K, Chattopadhyay S, Moench T, et al. Enema ion compositions for enhancing colorectal drug delivery. J Control Release. 2015;209:280–287.
- Razzaque MS. Phosphate toxicity: new insights into an old problem. Clin Sci. 2011;120(3):91–97.
- Muller-Lissner SA, Wald A. Constipation in adults. BMJ Clin Evid. 2010;07–413:1–25.
- Reveiz L, Gaitán HG, Cuervo LG. Enemas during labour. Cochrane Database Syst Rev. 2013;1–33.
- FDA. FDA Drug Safety Communication FDA warns of possible harm from exceeding recommended dose of over-the-counter sodium phosphate products to treat constipation [Internet]. FDA Drug Saf. Commun. FDA Warn. possible harm from Exceed. Recomm. dose over-the-counter sodium phosphate Prod. to treat constipation. 2014. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM381084.pdf.
- Anon. Sodium Acid Phosphate with Sodium Phosphate; 2021. https://bnf.nice.org.uk/drug/sodium-acid-phosphate-with-sodium-phosphate.html.
- Mendoza J, Legido J, Rubio S, et al. Systematic review: the adverse effects of sodium phosphate enema. Aliment Pharmacol Ther. 2007;26(1):9–20.
- Tsang CH, Baker J, Cheong JU, et al. Rectal perforation and necrosis associated with fleet enema. ANZ J Surg. 2021;91(5):E338–E339.
- Yu M, Tadin D, Conrad EJ, et al. A 48-year-old man with fever and abdominal pain of one day duration. J Louisiana State Med Soc. 2015;167:237–240.
- Viel G, Cecchetto G, Fabbri LD, et al. Forensic application of ESEM and XRF-EDS techniques to a fatal case of sodium phosphate enema intoxication. Int J Legal Med. 2009;123(4):345–350.
- Biebl A, Grillenberger A, Schmitt K. Enema-induced severe hyperphosphatemia in children. Eur J Pediatr. 2009;168(1):111–112.
- Ladenhauf HN, Stundner O, Spreitzhofer F, et al. Severe hyperphosphatemia after administration of sodium-phosphate containing laxatives in children: case series and systematic review of literature. Pediatr Surg Int. 2012;28(8):805–814.
- Becknell B, Smoyer WE, O’Brien NF. Hemodialysis for near-fatal sodium phosphate toxicity in a child receiving sodium phosphate enemas. Pediatr Emerg Care. 2014;30:814–817.
- Marek I, Benz K, Kusnik S, et al. [Phosphate intoxication after application of enema–a life-threatening iatrogenic complication]. Klin Padiatr. 2015;227:235–238.
- Núñez Sánchez MJ, Leighton Swaneck S, Díaz F. [Tetany secondary to phosphate enema toxicity, case report]. Rev Chil Pediatr. 2017;88(3):383–387.
- Kostic D, Rodrigues ABD, Leal A, et al. Flow-through peritoneal dialysis in neonatal enema-induced hyperphosphatemia. Pediatr Nephrol. 2010;25(10):2183–2186.
- Butani L. Life-threatening hyperphosphatemia and hypocalcemia from inappropriate use of fleet enemas. Clin Pediatr (Phila)). 2005;44(1):93.
- Carl I, Mitchell M. Symptomatic hyperphosphataemia following phosphate enema in a healthy adult [4]. Ulster Med J. 2007;76:172–173.
- Kosseifi S, Nassour D, Byrd RPJ, et al. Fatal iatrogenic hyperphosphatemia. J Ky Med Assoc. 2008;106(9):431–434.
- Ori Y, Rozen-Zvi B, Chagnac A, et al. Fatalities and severe metabolic disorders associated with the use of sodium phosphate enemas: a single center's experience. Arch Intern Med. 2012;172(3):263–265.
- Szoke D, Dolci A, Genderini A, et al. Fatal electrolyte abnormalities following enema administration. Clin Chem. 2012;58(11):1515–1518.
- Hsu HJ, Wu MS. Extreme hyperphosphatemia and hypocalcemic coma associated with phosphate enema. Intern Med. 2008;47(7):643–646.
- Di Mario F, Peyronel F, Greco P, et al. A case of extreme hyperphosphatemia due to sodium phosphate enemas successfully treated with sustained low efficiency dialysis. Clin Nephrol. 2021;95(1):62–64.
- Caswell M. Re: enema-induced severe hyperphosphatemia in children. Eur J Pediatr. 2009;168(8):1023.
- Caswell M. Fatal latrogenic hyperphosphatemia. J Ky Med Assoc. 2009;107(1):28.
- Anderson J, Furnival RA, Zhang L, et al. A comparison of the efficacy of enema solutions in pediatric emergency department Patients. J Emerg Med. 2019;57(4):461–468.
- Ainley EJ, Winwood PJ, Begley JP. Measurement of serum electrolytes and phosphate after sodium phosphate colonoscopy bowel preparation: an evaluation. Dig Dis Sci. 2005;50(7):1319–1323.
- Alami NF, Lim JKH, Goh KS, et al. Effect of sodium phosphate enemas on serum calcium and phosphate concentrations in older adult inpatients. J Am Geriatr Soc. 2015;63(8):1704–1705.
- Sédaba B, Azanza JR, Campanero MA, et al. Effects of a 250-mL enema containing sodium phosphate on electrolyte concentrations in healthy volunteers: an open-label, randomized, controlled, two-period, crossover clinical trial. Curr Ther Res – Clin Exp. 2006;67(5):334–349.
- Jacobson RM, Peery J, Thompson WO, et al. Serum electrolyte shifts following administration of sodium phosphates enema. Gastroenterol Nurs. 2010;33(3):191–201.
- Hansen SE, Whitehill JL, Goto CS, et al. Safety and efficacy of milk and molasses enemas compared with sodium phosphate enemas for the treatment of constipation in a pediatric emergency department. Pediatr Emerg Care. 2011;27:1118–1120.
- Niv Y, Niv G, Grinberg T, et al. Perforation and mortality after cleansing enema for acute constipation are not rare but are preventable. Int J Gen Med. 2013;6:323–328.
- Schaefer M, Littrell E, Khan A, et al. Estimated GFR decline following sodium phosphate enemas versus polyethylene glycol for screening colonoscopy: a retrospective cohort study. Am J Kidney Dis. 2016;67(4):609–616.
- Dagan A, Stein GY, Winter S, et al. Sodium phosphate enemas do not worsen renal function among hospitalized patients with mild to moderate renal failure: a matched, case-control study. QJM. 2017;110(12):803–806.
- Wimmer S, Neubert A, Rascher W. The safety of drug therapy in children. Dtsch Arztebl Int. 2015. 112:781–787.