Abstract
Background
The bipyridyl herbicide paraquat was first introduced into agriculture in the 1960s by Imperial Chemical Industries. Due to issues with unintentional poisoning, the centrally acting emetic PP796 was added in 1976 to the company’s 20% paraquat ion soluble liquid (SL20) formulations (Gramoxone®) at a concentration of 0.5 g/L or 0.05% (equivalent to 0.071 mg/kg in a 70 kg adult ingesting a minimum lethal dose of 10 mL) to induce early vomiting (within 30 min), reduce paraquat absorption from the gut, and prevent deaths. Its presence in paraquat products was subsequently mandated by the Food and Agriculture Organization Committee of Experts on Pesticides in Agriculture (predecessor to the current FAO/WHO Joint Meeting on Pesticide Specifications). However, no primary pre-clinical or clinical data have been published regarding the effectiveness of PP796. We reviewed the published literature and unpublished company reports for data on the effectiveness of PP796.
Methods
PubMed and Google were searched for published studies on the emetic using the search terms “paraquat” and [“emetic” or “PP796”]. Company documents reporting pre-clinical and clinical studies were accessed at the website of U.S. Right to Know (https://usrtk.org/pesticides/paraquat-papers/). Primary study reports were sought as well as overviews written by company toxicologists.
Results
Pre-clinical dog and monkey studies indicated that the PP796 EC50 dose for vomiting was around 0.5–2 mg/kg. Further increasing the PP796 concentration speeded up the time to first vomit and reduced the amount of paraquat absorbed (as assessed by the 0–24 h plasma area-under-the-curve) 100-fold compared to a control group receiving no PP796. However, the dose selected for paraquat SL20 formulations by the company (0.5 g/L or 0.05%) was based exclusively on a phase II study in the early 1970s involving five volunteers receiving 3 different doses, with only two individuals actually vomiting, supplemented by data from 37 patients taking 2 mg in clinical trials. A UK-mandated toxicovigilance study in the 1980s identified only 21 patients ingesting paraquat SL20 with PP796 for whom data on time to vomit was available; of these patients, 11 vomited within 30 min (52.4%, 95%CI 31–73.7%). No effect on mortality could be identified from any study of paraquat SL containing 0.05% PP796. A clinical study in Sri Lanka 30 years after the emetic was first introduced, of a revised formulation (Gramoxone® Inteon) containing a three-fold higher amount of PP796, as well as MgSO4 and an alginate, showed increased rates of early vomiting and modestly reduced mortality for patients ingesting up to 100 mL.
Conclusion
Pre-clinical studies showed a clear dose response for PP796 to cause early vomiting, with effective doses in the 0.5–20 mg/kg range. A too low concentration of PP796 was selected for paraquat formulations based on an inadequate phase II study. Currently, evidence that PP796 at 0.05% in paraquat SL20 causes more rapid vomiting after ingestion is weak or unpublished; no evidence of clinical benefit or fewer deaths has been identified. There is no evidence to support the FAO/WHO Joint Meeting on Pesticide Specifications mandate to include PP796 or any other emetic in paraquat products. Products with higher emetic concentrations have been developed but are not widely used; it is possible they may prevent deaths.
Keywords:
Introduction
Paraquat dichloride is a bipyridyl compound that has been widely used as a rapid-acting non-selective contact herbicide since 1962 [Citation1–3]. It exerts its herbicidal activity by interfering with electron transfer, inhibiting the reduction of nicotinamide adenine dinucleotide phosphate (NADP) to nicotinamide adenine dinucleotide phosphate (NADPH) during photosynthesis (PS I electron diversion, HRAC MoA classification 22) [Citation4]. Unfortunately, despite being of moderate acute toxicity to rodents (rat oral LD50 150 mg/kg, WHO hazard class II [Citation5]), it is highly toxic to humans, with deaths after ingestion of small amounts being reported soon after its introduction into agricultural practice [Citation6,Citation7]. Tens of thousands of deaths have occurred from self-poisoning since its introduction [Citation8–11]. Toxicity results from direct corrosive effects on the gut and from oxidative damage, through redox cycling, causing multi-organ failure at high doses of paraquat and lung injury and fibrosis at lower doses [Citation4,Citation12]. Case fatality is often over 50% with liquid 20% paraquat ion (SL20) formulations [Citation10,Citation13]. Treatment is generally ineffective [Citation14].
In the 1970s, the main manufacturer Imperial Chemical Industries (ICI) Agrochemicals added stenching agents and a blue colour to its paraquat SL20 products (often called ‘Gramoxone®’) in some markets to reduce the risk of unintentional poisoning [Citation15]. In 1976, a cAMP phosphodiesterase inhibitor (initially called ICI 63197, then PP796) was added as an emetic agent, aiming to cause people drinking paraquat to vomit soon after ingestion, reducing absorption and deaths, especially after unintentional poisoning [Citation15]. It was introduced rapidly into important markets such as the UK, Ireland, and France in western Europe, as well as Western Samoa where there was a problem with suicide [Citation16]. In 1983, a decision was made to introduce it into all markets worldwide [Citation17].
The concentration of emetic selected was 5 mg of PP796 per 10 mL of product (equivalent to 0.5 g in 1 L, 0.05%) or a PP796:paraquat ratio of 1:400. This provided a dose of 5 mg PP796 to anyone ingesting the estimated minimal lethal paraquat SL20 dose of 10 mL (equivalent to 0.071 mg/kg PP796 in a 70 kg adult, 0.167 mg/kg PP796 in a 30 kg child); higher PP796 doses would be consumed with larger ingestions.
The formulation was subsequently recommended by the manufacturer [Citation18] to the Committee of Experts on Pesticides in Agriculture of the Food and Agriculture Organization of the United Nations (FAO) (the predecessor to the current FAO/World Health Organization (WHO) Joint Meeting on Pesticide Specifications (JMPS) as the global quality specification for paraquat (Box 1) [Citation19,Citation20]; a standard that was confirmed in 2021 [Citation21] and is still used worldwide (see for example [Citation22]). The FAO specifications state that a rapidly absorbed and effective emetic, causing emesis in about half an hour in at least 50% of cases, must be incorporated into the SL formulation. The 2008 specifications further state: “To date, the only compound found to meet these requirements is 2-amino-4,5-dihydro-6-methyl-4-propyl-s-triazole-(1,5a)pyrimidin-5-one (PP796)” [Citation20]. Of note, no evidence was provided in the specifications that this emetic does cause 50% of patients to vomit within 30 min, or that its presence reduces deaths. Furthermore, there is no such emetic requirement for SL formulations of the related herbicide, diquat [Citation23]. Naming of PP796 in the specification allowed ICI to sell its patented product to other companies who wished to manufacture paraquat, giving it a commercial advantage [Citation16,Citation24].
Over the last few years, court cases in the USA have resulted in documents from ICI and its successor companies, Zeneca and Syngenta, being made public as part of the discovery process. This offers the opportunity to review unpublished clinical and pre-clinical studies alongside the published literature for information on the dose and efficacy of PP796 in different paraquat products. There is wide precedent for review and analysis of such unpublished corporate documents, for example from the tobacco and food industries, for a better understanding of corporate influence on policy and public health [Citation25–27]. The purpose of this review is to identify the evidence that drove the use of PP796 in paraquat SL20 and the evidence for efficacy as an emetic and in saving lives.
Methods
PubMed and Google were searched for published studies on the PP796 emetic using the search terms “paraquat” and [“emetic” or “PP796”] (final search date 06 Jan 2022).
During a court case in the State of Illinois, IL (https://usrtk.org/wp-content/uploads/2021/03/Hoffmann-v.-Syngenta-Crop-Illinois-Tillery-3.pdf), many documents were turned in by Syngenta as part of the discovery process. This included documents from the former companies that became part of Syngenta plus other companies such as Chevron in the USA which marketed paraquat there on ICI’s behalf. A petition was made by the claimant’s lawyer that the documents were not confidential and should be released to the public. This was agreed by Syngenta (personal communication, Mr Stephen Tillery of Korein Tillery, LLC, St. Louis, MO) and the documents then made available in March 2021 on the U.S. Right to Know website (https://usrtk.org/pesticides/paraquat-papers/).
Documents on the website were reviewed, seeking reports of clinical and pre-clinical studies relevant to PP796 and the emetic-containing paraquat SL20 formulations, and of internal reports about these studies. The results are presented here according to study type. More detailed study and publication descriptions are provided in an Online supplement.
Results
A search of PubMed revealed only one primary publication reporting a pre-clinical study of PP796 in rats addressing cardiotoxicity [Citation28] but no clinical studies of PP796. The search did reveal five relevant studies: a retrospective review of 27 clinical cases with and without the emetic [Citation29], a pre-clinical dog study [Citation30] and two clinical study reports [Citation31,Citation32] with a revised formulation (Gramoxone® Inteon), and a review on the subject [Citation15]. A search of Google revealed multiple relevant company documents on the U.S. Right to Know website (see below) as well as a pre-clinical dog study of PP796 [Citation33], a United States Environmental Protection Agency (US EPA) report of four pre-clinical dog studies with the Inteon formulation [Citation34], and two reviews or commentaries [Citation35,Citation36].
On the U.S. Right to Know website, 35 documents were identified that contained information about initial clinical studies of PP796, pre-clinical dose-finding studies of PP796 in dogs and primates, a phase II efficacy study of PP796 in humans, a UK-based observational clinical study comparing paraquat herbicide formulations with and without PP796, and development of further formulations with a higher PP796 concentration.
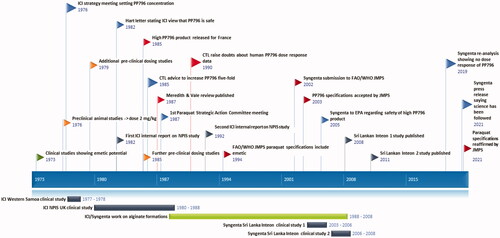
A timeline for key meetings, studies, and documents is presented in .
Early clinical studies
Human phase I volunteer study with ICI 63197/PP796
PP796 was first developed by ICI Pharmaceuticals as compound ICI 63197. In an initial healthy volunteer study designed to assess safety in humans, 12 participants (weight 50.5–82.5 kg) received single doses of ICI 63197 from 0.25 mg to 8 mg [Citation37,Citation38], at seven dose levels (with 1–3 volunteers receiving each dose). Seven reported nausea, including the single volunteer dosed with 0.5 mg. Two volunteers vomited, one 30 min after being dosed with 4 mg (0.048 mg/kg) and the other 2 h after being dosed with 8 mg (0.1 mg/kg). Only one volunteer received the top 8 mg dose; one of two volunteers receiving 4 mg (0.05 mg/kg) showed neither nausea nor vomiting. No pharmacokinetic data have been published.
Clinical studies
ICI 63197 was found to have a bronchodilator effect in animals and consequently moved into clinical development [Citation37]. Clinical trials took place in a number of conditions (online supplement Table 1); however, studies soon showed it had adverse effects, including nausea, vomiting, dizziness and flushing, at low doses (1–4 mg). Due to a lack of apparent benefit and to adverse effects, trials were stopped, and it was not developed any further for clinical use by ICI Pharmaceuticals.
These studies of ICI 63197 at 2 mg (single doses up to a maximum of 126 doses) included a total of 46 participants of whom four (8.7%) vomited [Citation37]. Accounting for study drop-outs, 1 mg and 2 mg doses were administered on a total of approximately 84 and 601 occasions, respectively, giving an estimated incidence of zero vomits per 1 mg dose and 4/601 (0.7%) vomits per 2 mg dose [Citation37]. Unfortunately, data on the number of individuals vomiting at first exposure, which might be most relevant, were not available.
Selection of the emetic dose for paraquat formulations
A dose response curve was created for PP796 in humans and presented in the ICI research report CTL/R/390(R) in 1977 [Citation38]. However, instead of using all 12 volunteers from the safety study, it selected five at three dose levels (0.015 mg/kg [n = 2], 0.06 mg/kg [n = 2] and 0.11 mg/kg [n = 1]) and replaced the three volunteers in the largest group (0.03 mg/kg, n = 3), none of whom vomited, with 37 participants from the clinical studies who had received a dose of about 0.03 mg/kg. Doses were not adjusted for participants’ weight and were rounded up by varying degrees. The 0.03 mg/kg dose group appears to represent a dose of 2 mg divided by an average adult weight (70 kg, 0.029 mg/kg), despite the weight of participants being highly variable in the original studies.
This flawed dose response curve [Citation16,Citation38] was reported as showing rates of 0/2 (0%) at 0.015 mg/kg, 4/37 (10.8%) at 0.03 mg/kg, 1/2 (50%) at 0.06 mg/kg and 1/1 (100%) at 0.11 mg/kg, allowing a classical sigmoidal curve to be drawn through the data and a ED50 of around 0.05 mg/kg estimated. However, the four vomits in participants receiving 2 mg occurred after 601 dose administrations, producing an incidence of 4/601 (0.07%) not 10.8% as used in the dose response calculation. In addition, the vomiting at the highest dose occurred at 2 h post-dose, not the 30 min required for vomiting post-paraquat exposure.
As a result of these data, a dose of 5 mg PP796 (equivalent to 0.071 mg/kg in a 70 kg adult, 0.167 mg/kg in a 30 kg child) in a minimal lethal dose (10 mL of 20% paraquat) was selected as the appropriate dose for formulation with the stated expectation that “the majority of those ingesting 10 mL of this formulation will vomit within an hour” [Citation16,Citation38]. The scientist who authored the report stated that this dose would produce vomiting within one hour for the majority [Citation38], or 15 min for 75–85% [Citation39], of those ingesting a minimum lethal dose. No data to support these statements was presented. Syngenta reanalysed the human data in 2019 and concluded that a dose-response relationship could not be established [Citation57].
Pre-clinical studies of PP796
Dose-finding studies of PP796
A series of acute oral administration studies of PP796 was carried out in three vomiting animal species (dogs, pigs and primates) over the dose range 0.05–1.5 mg/kg body weight [Citation38] (). In this report, no mention is made of the time to vomiting [Citation38]. As a result of the studies, a dose of 2 mg/kg was chosen by ICI as one that would ensure vomiting in dogs and primates and that should be used for further pre-clinical studies on the effect of emesis on paraquat toxicity [Citation16,Citation38].
Table 1. Initial preclinical vomiting studies with PP796.
Subsequent studies of PP796 in dogs and primates
After the emeticised paraquat SL20 formulation was agreed in 1976, further pre-clinical studies were performed to assess the effects of PP796 both alone and in combination with paraquat (Online supplement). These studies tested PP796 alone in doses of 1–30 mg/kg, and 100 mg/kg. Overall, up to 30 mg/kg, vomiting occurred rapidly, but with no apparent dose response [Citation41]. Vomiting motions continued for only a few minutes after which the animals became lethargic and, at doses ≥10 mg/kg, developed reduced consciousness by about 30 min after dosing. They remained obtunded for 1 h, before making a gradual and complete recovery. Four out of ten animals receiving 100 mg/kg died [Citation42].
To test efficacy of the emetic against paraquat poisoning, two groups of eight primates were orally dosed (20 mL) with Gramoxone® W (containing paraquat ion 100 mg/kg) with or without PP796 (2 mg/kg) [Citation41]. All eight animals receiving paraquat alone died (median [IQR] time to death 28 [range 5–52] h) while all but two animals receiving paraquat + PP796 survived to 14 days (two dying after 48 and 312 h). The animals receiving emetic vomited much earlier than those not receiving emetic: median (IQR) time to vomit 29 (range 20–455) min versus 291 (range 225–300) min. The two animals in the emetic group that died vomited after 290 min and 455 min, significantly later than the surviving animals.
Further primate studies with higher dose paraquat/PP796 combinations (250 or 500 mg/kg paraquat ion with 2.5 or 5 mg/kg PP796, respectively) did not show markedly improved outcome. Increasing the PP796 concentration eight-fold, from 0.05% to 0.4%, reduced toxicity with the LD50 increased from 40–100 mg/kg to 250–350 mg/kg paraquat [Citation41].
In 1985, additional animal studies were performed by ICI to further assess PP796’s dose response (Online supplement). A dose response study of PP796 in paraquat treated dogs tested 0.5, 3.0 or 20 mg/kg PP796 or control against 20 mg/kg paraquat ion. Control animals vomited after 29–360 min while animals receiving 0.5, 3.0 and 20 mg/kg vomiting after 8–26 min, 4–6 min, and 1.5–6 min, respectively (). The plasma paraquat AUC (0–24 h) were 10.5 and 32.5-fold lower at 0.5 and 3.0 mg/kg PP796 doses compared to no PP796. The authors concluded that the effective dose range of PP796 in dogs lay between 0.5 and 3.0 mg/kg and that doses above 3.0 mg/kg provided no advantages over 3.0 mg/kg [Citation43]. As a result of these studies, the Head of ICI’s biochemical toxicology department recommended the company increase the PP796 concentration five-fold in paraquat SL20 [Citation44].
Table 2. Summary of results from an unpublished pre-clinical dose-response study of PP796 with 20 mg/kg paraquat ion (ref [Citation43,p.296]).
Pre-clinical studies of novel formulations
French formulation of paraquat SL20
In the mid-1980s, a high-emetic, low paraquat ion concentration product was developed by ICI for its French market as a result of national pesticide regulator pressure [Citation45]. This product (Gramoxone Plus®) had three times the PP796 (1.5 g/L) and half the paraquat (10% ion) concentration, giving a higher PP796:paraquat ratio of 1:67. Dog studies were set up using as control a similar two-fold lower paraquat concentration product (SL10, containing 0.25 g/L PP796 per 100 g paraquat ion); they showed a reduced paraquat AUC with this preparation, contrasting an AUC of 70 mcg/L.h for 16 mg/kg paraquat SL10 with 13 mcg/L.h for 64 mg/kg French paraquat – a 5.4-fold reduction in AUC with a 4-times higher dose of paraquat. Vomiting occurred as early as 15 min with this dose and formulation [Citation45].
Development of Magnoxone
At the end of 1980s, ICI started carrying out extensive testing of novel emulsion liquid formulations that contained 100 g/L paraquat ion and 3 times the level of PP796 [Citation46,Citation47]. A new paraquat SL20 formulation known as Magnoxone was developed and patented [Citation40,Citation47,Citation49]. It was stated to reduce the oral toxicity of Gramoxone® 15-fold in dogs [Citation48] (although primary data are unavailable). Magnoxone contained the pharmaceutical antacid gelling agent, magnesium trisilicate (100 g/L), to reduce paraquat-induced gastric mucosal injury and increase viscosity of gastric contents, slowing gastric emptying. It also had a three-fold increase in PP796 concentration, magnesium sulphate (100 mg/L) as an osmotic purgative, and xanthan gum (3 g/L) to suspend the insoluble magnesium trisilicate.
Development of Gramoxone® Inteon
An SL20 formulation similar to Magnoxone, registered as Gramoxone® Inteon, was developed by Syngenta with the aim of reducing acute oral toxicity following ingestion [Citation50]. It contained a polysaccharide alginate isolated from seaweed [Citation30] that gelled on contact with acid (pH ≤3) in the stomach, slowing gastric emptying and delivery to the small bowel. It also contained a three-fold higher concentration of PP796 and magnesium sulphate (100 mg/L) [Citation50]. Studies in rabbits [Citation30] and dogs [Citation50] showed much reduced toxicity, with the authors estimating a ten-fold reduction in toxicity [Citation50].
Overview of PP796’s pre-clinical toxicity
Acute oral toxicity was evaluated in rats, with administration of single oral doses of 100, 150 and 200 mg/kg PP796. Moderate signs of toxicity were seen at 100 mg/kg, but all animals recovered by day 7. Marked signs of toxicity were seen at both 150 and 200 mg/kg, with 90% and 80% lethality, respectively, at day 2 [Citation51]. Dogs receiving a single oral 20 mg/kg dose of PP796 vomited within 5 min of ingestion, became subdued, but had fully recovered by 6 h [Citation52]. Primates receiving 30 mg/kg PP796 vomited rapidly, became lethargic and then sedated for one hour before recovering fully over the following hours [Citation41]. Primates receiving 100 mg/kg became unconscious, with 4/10 dying [Citation42].
Observational clinical studies with the paraquat SL20 formulation
Observational clinical studies were initiated to assess the effectiveness of emeticised formulations.
Western Samoa
Hospital records were retrospectively reviewed in Western Samoa from January 1977 to May 1978, after introduction of the emeticised formulation in May and August 1977, identifying 21 paraquat poisoning cases [Citation53]. Thirteen (61.9%) died. Clinical data suggested that nine cases had ingested the emeticised preparation, due to extensive and prolonged vomiting. Of these nine cases, six died (66.7%) including all ingesting 3 g or more paraquat ion. Amongst patients ingesting non-emeticised preparations, there were seven deaths (58.3%) – all of whom had ingested more than 6 g paraquat ion.
France
A retrospective study by the Poison Control Center of Paris identified 27 cases of acute paraquat ingestion in 1981, of whom 23 took paraquat SL20 [Citation29]. The presence of PP796 was known for only three cases, meaning that no conclusions about efficacy could be drawn [Citation29]. A second paper reported 28 cases of whom 20 ingested SL20 products; six were noted to have ingested emeticised products, of whom four (66.7%) died [Citation54].
United Kingdom
In 1980, ICI established a study of paraquat poisoning with the National Poisons Information Service at Guy’s Hospital, London, as part of a mandated toxicovigilance study. Interim results on 262 patients were first reported in abstract form at a 1983 meeting [Citation55]. No study methodology and few data are presented in the abstract (see Online supplement). A second short abstract was published in 1985, reporting 500 cases up to March 1984. Again no data were presented in this abstract [Citation56].
Some data were presented as unpublished data in a 1987 review [Citation15]. It reported that the timing of vomiting and the presence/absence of an emetic could be identified for 61 of 262 patients (40 with emetic, 21 without emetic) (). Sixty-five percent of patients ingesting emeticised formulations vomited within 30 min vs 19.0% of patients ingesting non-emeticised formulations (p < 0.005 according to the review). However, the number ingesting liquid SL vs granular SG products was not presented (important due to the much lower toxicity and higher PP796:paraquat ratio of the SG products). This article was used by the manufacturers to support claims that the addition of PP796 to paraquat SL20 increased the incidence of early vomiting and made the emeticised paraquat product safer [Citation40,Citation57,Citation58].
Table 3. Time of spontaneous vomiting after ingestion of emetic/non-emetic formulations of paraquat.
An unpublished report [Citation59] clarified the study methodology. Initial data collection involved recording only the hospital, patient name, and symptoms present. A follow-up phone call was made 2–7 days after the poisoning to the treating doctor for more information on symptoms, treatment, lab analyses and outcome. A questionnaire was then sent for a complete case history for the patient. Data was mostly obtained by questionnaire (70% of cases) or telephone (15% of cases) [Citation59]. It is unclear that accurate data on the time of pre-hospital vomiting could be collected with a retrospective methodology relying on a doctor’s memory several days after the event (due to information bias [Citation60]). Unfortunately, neither Bramley’s abstract [Citation55] nor the review [Citation15] presented the methodological limitations of the study.
A second unpublished company report presents an updated analysis of the UK NPIS study including 93 paraquat SL20 poisoning cases for which the emetic status was known (51 with, 42 without), collected during 1980–1988 [Citation61]. The relatively small number of confirmed emeticised paraquat SL20 cases reported here after eight years indicates that the great majority of cases ingesting emeticised formulations in the earlier analysis [Citation15] had ingested granular formulations.
Time of vomiting was known for only a small sub-sample of patients ingesting emeticised paraquat SL20 (21/51, 41.2%). The number vomiting within 30 min was 11/21 (52.4%), just exceeding the FAO’s paraquat specification. The 95% confidence intervals for this estimate were not presented but are 31– 73.7%. Five (31.3%) of 16 patients ingesting paraquat SL20 without emetic also vomited within 30 min; a difference of 21.1% (95% confidence interval −10% to 52.3%). The validity of these estimates is affected by both the small sample size (n = 37 with known formulation and timing of vomiting, 61.3% of cases not used) and the study’s methodology, which remained retrospective and prone to information bias.
Clinical studies of Gramoxone® Inteon in Sri Lanka
The Gramoxone® Inteon formulation was introduced into agricultural practice in Sri Lanka in October 2004 as part of a company-funded observational study to assess its effect on mortality at three months after ingestion in collaboration with the South Asian Clinical Toxicology Research Collaboration (SACTRC) [Citation31]. The study was conducted in nine large hospitals from before its introduction in 2003 until 2006; patients were recruited into the study if they reported ingesting products containing paraquat or, if the pesticide ingested was unknown, had clinical signs typical of paraquat poisoning. Packaging was similar to the standard products, but the product included a tracer compound (500 ppm diquat dibromide) that could be detected in blood/urine following oral ingestions. Data on product and volume ingested, and the timing of vomit, were collected prospectively by trained researchers soon after presentation to hospital, a median of 3–4 h post-ingestion. Plasma and/or urine samples were analysed for paraquat concentration and the presence of diquat to classify the case as standard formulation or Inteon.
Data were collected on 774 patients. The primary analysis included 297 confirmed cases of standard formulation ingestion and 289 confirmed, probable and possible cases of Inteon ingestion [Citation31]. There was an increase in estimated 3-months survival (Kaplan–Meier estimates) among Inteon patients from 27.1% to 36.7% (difference 9.6%; 95% CI 2.0– 17.1%; p = 0.002). Median survival time increased from 2.3 (95% CI 1.2–3.4) days with the standard formulation to 6.9 (95% CI 3.3–10.7) days with Inteon (p = 0.002).
Time to vomiting was collected in this study but only data on vomiting within 15 min presented [Citation31]. Thirty-eight percent and 54.7% of patients ingesting standard formulation or Inteon, respectively, vomited within 15 min. No data were provided for 30 min.
The Inteon study was continued further with a different version of the Inteon formulation, lacking built-in surfactants (wetters) [Citation32,Citation50]. This showed that 49.2% and 42.5% of patients ingesting standard formulation or Gramoxone® Inteon without wetters, respectively, vomited within 15 min. No data were provided for 30 min.
The revised Inteon® formulation was also introduced into agricultural practice for a short time in South Korea [Citation62]. The effect of its introduction has not been reported; however, a review of paraquat cases in 2010–2012 showed few cases with the Inteon formulation and a high case fatality of 75% [Citation63].
Discussion
This review of the published and unpublished literature on PP796 indicates that the standard dose (0.5 g/L of paraquat SL20, 0.05%; 0.071 mg/kg in a 70 kg adult drinking the minimum lethal 10 mL dose) is not supported by the animal or human literature. It was selected on the basis of a single inadequate human study that did not show 50% of recipients vomiting within 30 min. Animal studies clearly demonstrate that a higher dose (at least 0.5–2 mg/kg) is needed for efficacy in both dog and primates, and that higher doses (20–30 mg/kg) induced more rapid vomiting while being well tolerated. Humans were considered to be more sensitive to PP796 as reported in 1977 [Citation38], but this was not shown by the primary data and no mechanistic explanation was given for why PP796 would be markedly more emetogenic in humans than the three other vomiting animal species studied.
Observational clinical studies that attempted to demonstrate effectiveness at inducing early vomiting mixed up the less hazardous low-dose granular formulations with more concentrated liquid paraquat SL20 product (with its five-fold higher case fatality) and had weak retrospective designs. An unpublished analysis of a small subset of these patients suggested that emeticised paraquat did cause vomiting in 52% of cases, but the confidence intervals were wide. Paraquat SL20 formulations with higher PP796:paraquat ratios were developed for important markets where there was pressure from regulators to ban the herbicide, but not made available worldwide despite impressive pre-clinical studies. New formulations including gelling agents and three-fold higher PP796 concentrations were also developed and one of these, the Gramoxone® Inteon formulation, introduced in Sri Lanka. It was associated with increased vomiting within 15 min and moderately improved outcomes. Unfortunately, the first formulation was withdrawn due to stability issues. The second study could not be completed according to plan because of the parallel introduction of a low-strength product required by the national pesticide regulator. An insufficient improvement in safety was concluded based on a large number of patients that could not be reliably categorised; the registration of paraquat products was then cancelled in Sri Lanka. The company did not continue with the product in other countries other than the Republic of (South) Korea.
In the early 1990s, ICI successfully requested the FAO Committee of Experts on Pesticides in Agriculture to mandate the inclusion of an emetic in all paraquat formulations. In 2002–2003, data were provided that resulted in PP796 being named as the only effective emetic [Citation20]. As a result, all manufacturers had to use the ICI-patented PP796 at 0.05% in their products. This was done, despite a lack of robust evidence that 0.05% PP796 actually causes effective vomiting within 30 min and no evidence that it improves outcome. The current specifications still require the incorporation of an emetic despite the lack of data for clinical benefit [Citation21].
Human data used to select the PP796 dose
The phase I safety study on which the dose response was assessed was far too small for purpose [Citation64–66], as confirmed by Syngenta’s recent review [Citation40]. It is standard practice in phase II human studies assessing effectiveness (as retrospectively done here) to test a variety of doses in groups of 6–8 participants to find an effective dose, creating a robust dose response curve [Citation66]. Such a study would have provided good evidence for an effective dose in humans or, in this case, a dose that clearly and reproducibly caused vomiting as a side effect. It was not done for PP796. Remarkably, three patients who received a middle range dose (3 mg) but did not vomit were excluded from the dose response curve.
Of note, in a memo summarising the situation with PP796 in January 1984, the Senior Products Medical Adviser raised the issue of whether the optimum dose had been selected to cause the earliest possible vomiting [Citation67]. He noted that he and a colleague had approached ICI Pharmaceuticals about additional healthy volunteer studies to address this question but had been told such studies would be unethical. It indicates that at least two of the company’s doctors realised that better data were needed, shortly before the company’s senior toxicologist called for a five-fold increase in PP796 concentration. The weakness of the human data appears to have been well recognised by ICI in 1987, when its Paraquat Strategic Action Committee described the human data as “flimsy” [Citation68]; the weaknesses were also explicitly reported to ICI management in 1990 by a formulation toxicologist [Citation69].
Evidence that emeticised paraquat SL20 is effective at rapidly inducing vomiting/saving lives
The 1987 paper [Citation15] has long been used (and is still be used [Citation18,Citation70]) to support the idea that emeticised paraquat SL20 induces rapid vomiting [Citation40,Citation57,58]. However, the data that it cited was predominantly from poisoning with low paraquat granular products (with a higher ratio of emetic to paraquat). This was not made clear in any publication, and the study’s results and methodology have still never been reported in full. Subsequent unpublished analyses did find evidence of 52% of patients vomiting within 30 min but the sample size was very small (n = 36) with large numbers of cases not used due to missing data.
The methodology used for this study contrasts strongly with the methodology for the Inteon studies. In these, data on timing of vomiting was collected prospectively and directly from the patient or family at their first presentation to hospital, a median of 3–4 h post-ingestion [Citation31]. The studies were also much larger, with hundreds of patients in each group with complete data.
Unfortunately, none of the studies provide sufficient data to address whether the dose of PP796 in standard paraquat SL20 saves lives. In the first Inteon study, 55/221 patients (for whom the dose ingested was known) reported ingesting a dose of standard formulation paraquat SL20 in the low lethal range (10–30 mL). The emetic at its original 0.05% concentration should have caused vomiting (in at least 50% as per the FAO specifications), yet about 78% of these patients died [Citation31].
The first Inteon formulation, with its three-fold higher concentration of PP796 and built-in wetting agent, caused more patients to vomit after ingestion and had a lower-case fatality [Citation31]. It is not possible to be certain that it was the higher PP796 concentration that resulted in fewer deaths, rather than other novel aspects of its formulation because separate studies were not carried out. However, it is possible that the increased vomiting increased survival. Unfortunately, the improvement in outcome was disappointingly small [Citation35] and in the second smaller Inteon® study the proportion of patients vomiting within 15 min of ingestion was lower for Inteon® versus the standard formulation [Citation32].
Doses suggested by animal studies
The dog is considered a better model of human gastric function and vomiting than many other species [Citation50,Citation71–74]. It is not clear why ICI ignored its high-quality canine data when selecting a concentration of PP796 to add to its paraquat products, especially in light of the poor quality of the human data (as acknowledged at company meetings in 1976 [Citation16] and 1987 [Citation68]). The animal studies showed a dose response for both speed/severity of vomiting and reduced paraquat absorption; a dose of 2 mg/kg (compared to 0.071 mg/kg in adult humans) was chosen for further animal studies. Doses of 20–30 mg/kg caused adverse effects (sedation) but these were transient and resolved fully; there is no indication that any high dose adverse effect would have outweighed the case fatality of paraquat SL20 [Citation13].
Issues raised by the company with increasing the dose of PP796
Dose of paraquat ingested in self-poisoning
The company believes that emetics cannot save the majority of patients who ingest paraquat for self-harm because they ingest high doses of paraquat, far above the lethal dose, for which the emetic cannot be expected to work [Citation4,Citation75]. However, in clinical practice this is commonly not the case.
Many people, especially younger people, ingest small amounts of poison in what may be called “suicide gestures” – impulsive expressions of distress with their world and relationships [Citation76–78]. When these gestures do not cause death, the vast majority go on to leave productive lives [Citation79]. Many deaths in young people occur when they ingest small quantities of highly toxic poisons, such as paraquat [Citation80].
That such small doses of paraquat are regularly ingested was clearly shown in the Inteon studies. In the first study [Citation31], 55 and 57 patients reported ingesting less than 10 mL of paraquat SL20 or Inteon. Another 55 and 88 reported ingesting 10–30 mL of paraquat SL20 or Inteon – just 2 or 3 times the minimum lethal dose – giving a total of 255/586 (43.5%) reporting that they ingested such small volumes. These are not huge and certainly would contain an amount of paraquat for which an improvement would be expected if the emetic was effective.
The case fatality for these patients was improved by Inteon® – the case fatalities for <10 mL and 10–30 mL respectively were about 38% and 78% for Gramoxone® and 30% and 55% for Inteon® [Citation31]. About 24 patients ingesting low doses survived in this study associated with the potentially safer Inteon formulation, with its higher emetic concentration and other formulation changes.
Increasing the dose of PP796 will increase the toxicity of paraquat SL20
The company has stated that medical experts believe that increasing the dose of PP796 would increase toxicity to humans [Citation81]. However, paraquat SL20 is highly toxic, killing people who unintentionally ingest small amounts of the 20% solution [Citation4]. It also kills a very high number of people who ingest small amounts of paraquat in suicide gestures. In the 1st Inteon study [Citation31], the case fatality after proven “suicidal” exposure was 72.9% and 63.3% for Gramoxone® or Inteon formulations, respectively. High concentration (56%) 3 g aluminium phosphide tablets are the only other pesticide with similar case fatality [Citation13,Citation82,Citation83].
There is no greater risk or hazard than death. Therefore, the only way that increasing the emetic might result in more risk and injury is if it increases the case fatality yet further. Dogs and primates received 20–30 mg/kg PP796 – more than 100× higher than the dose in a minimal lethal paraquat dose – and recovered fully. These studies suggest that the PP796 concentration in paraquat SL20 formulations could be increased 10–20 fold, without any risk of harm that would outweigh the current risk of death from paraquat ingestion [Citation84]. No reports of harm from PP796 have occurred from human self-poisoning with emeticised paraquat SL20 formulations containing modestly higher doses of PP796 (Inteon and French formulations). ICI staff argued in 1982 that any toxic effects of a large dose of PP796 would be minor compared to the effect of the co-ingested paraquat [Citation84].
Changing clinical toxicology views on emetics
Syngenta argue that medical knowledge has moved on since 1970s and the use of emetic could be counter-productive in the treatment of ingestion [Citation18], stating concerns around: being unable to administer activated charcoal, damaging the oesophagus from vomiting paraquat, and reduced GCS in vomiting patients.
There is no good evidence that activated charcoal is effective at preventing deaths from paraquat self-poisoning or that it is superior to emesis [Citation4,Citation14,Citation85]. Charcoal is given on admission to hospital which is usually several hours after ingestion. Higher doses of PP796 (at least 2 mg/kg) caused rapid vomiting in primates and dogs within 15 min that was associated with improved outcome after oral gavage of paraquat SL20. It seems likely that emesis almost immediately post-ingestion from built-in emetics is more effective than activated charcoal several hours later.
Paraquat has corrosive effects on the oesophagus, but effects are mostly mild-moderate [Citation86] and perforation reported relatively rarely [Citation87–89]. Moreover, it takes time for the damage to occur. Effective vomiting within minutes that removes paraquat might save lives, as seen in the animal studies. A low risk of perforation is likely outweighed by the potential benefit from early vomiting.
Although poisoned patients do become sedated with some poisons, this is not a common feature of paraquat poisoning except in the very largest doses [Citation4]. Since the emeticised formulation was designed for low dose poisoning, at around the minimal lethal dose, this concern is not relevant (unless the dose of PP796 is increased 100-fold, at which dose PP796 does cause transient sedation in primates).
Would increasing the dose of emetic change the outcome?
The pre-clinical studies indicate that high doses of PP796 speed the onset of vomiting and reduce lethality from paraquat ingestion (gavage), with a dose-response. Company toxicologists often concluded that PP796 produced a > 10-fold reduction in toxicity. Increasing the PP796 dose at least 10-fold (to a dose of 0.7 mg/kg in a 70 kg adult ingesting 10 mL) would possibly increase the incidence of patients vomiting soon after ingesting a paraquat product, without an unacceptable increase in adverse effects. It is not possible to estimate how far this might reduce the case fatality.
Observational clinical data do not strongly support a beneficial effect of early intense vomiting. A small study of 30 Malaysian patients (nine unintentional) from 1978 to 1979, when Malaysian paraquat SL20 was not emeticised [Citation17], reported vomiting within 15 min for 24/30 (80.0%) patients [Citation90]. Despite this rapid vomiting, case fatality remained high at 27/30 (90%) [Citation90]. The Malaysian and West Samoa [Citation53] data suggest that rapid and/or intense vomiting is not associated with clinical benefit, at least without the other changes associated with the Inteon formulation.
FAO/WHO specifications for paraquat
At the beginning of the 1990s, ICI successfully requested that the FAO Committee of Experts on Pesticides in Agriculture [Citation91] mandate an emetic to be included in all paraquat products; since only PP796 apparently fulfilled their suggested criteria as listed in the footnotes of the 2008 specifications [Citation20], this patented emetic had to be added at ICI’s chosen concentration. Initially, the emetic was included as a specific “active ingredient” in the technical concentrate and aqueous solution specifications, with notes setting criteria for effectiveness [Citation19]. By 2008, this had been revised, with the emetic no longer listed as an active ingredient; instead the need for an “effective emetic” was now included in the initial description of the product with a footnote listing criteria that the emetic had to meet and that PP796 was the only compound known to meet these criteria [Citation20].
It is unclear why JMPS provides instructions about the emetic in its specifications for paraquat. The JMPS’s guiding manual states: “The term “pesticide” is considered to embrace active ingredients in any form, irrespective of whether, or to what extent, they have been formulated for application” and “The specifications do not encompass the chemical characteristics of the formulants, other than where they influence the physical characteristics (which are taken to include characteristics such as pH, acidity and alkalinity)” [Citation91]. The presence of an emetic (and its identity or concentration) is out of scope of the JMPS.
The emetic therefore cannot be part of the specification, the footnotes are present for information only, and the emetic cannot be ‘mandated’. However, the current wording in footnote 3 for the SL formulation is: “An effective emetic, having the following characteristics, must be incorporated into the SL” [Citation21] (emphasis added), which seems to preclude any doubt about its meaning.
Company documents [Citation16,Citation24] suggest that this FAO/WHO mandate to include PP796 in all paraquat products was a continuation of ICI’s business strategy. The company explicitly acknowledges [Citation40,Citation58,Citation70] that it used the 1987 review [Citation15] to support their claim of PP796’s efficacy, despite the published review being unable to show evidence of efficacy for the toxic paraquat SL20 products.
Conclusion
The emetic concentration included in paraquat SL20 formulations is almost certainly unable to improve the outcome of poisoning; until now there is no good evidence that it produces the desired, mandated rapid onset of effective vomiting. How the errors in data interpretation described in this review came into being and were used to support claims for efficacy of PP796 is outside the scope of this manuscript. However, while individuals within Syngenta and its predecessor companies recognised issues with the selected concentration, Syngenta continues to state that its decision is based on science, which seems hard to sustain in light of internal company discussion. To what extent these errors have contributed to deaths from paraquat poisoning is uncertain, but it seems at least likely that they led to some patients dying who might have survived if early vomiting after paraquat ingestion had been assured. This review illustrates the difficulties in adding an agent to a commercial preparation to reduce its systemic toxicity. It should serve as a lesson in the need to design products with minimal human toxicity if they are for wide-spread use in poor rural communities with no ability to use or store them safely and no possibility of preventing all acts of self-poisoning.
Supplemental Material
Download MS Word (150.7 KB)Acknowledgements
I am grateful to Nick Bateman, David Gunnell, Nick Buckley, Martin Wilks, Leah Utyasheva, Mark Davis and the reviewers for their critical comments on the document and to Dr M D Müller, of FAO/WHO JMPS, for his critique on that aspect of the document.
Disclosure statement
ME is a WHO member of the FAO/WHO Joint Meeting on Pesticide Management (JMPM). He received an unrestricted research grant from Cheminova (2012) and travel expenses from Syngenta to attend meetings for the first Inteon study (2005–2006), but no personal income. He wrote a scientific expert report on these documents for PublicEye and Unearthed/Greenpeace, for which he was not paid.
Box 1 Current FAO/WHO JMPS specifications for paraquat dichloride soluble concentrate [Citation21].
Description
“The material shall consist of technical paraquat dichloride, complying with the requirements of FAO Specification 56.302/TK (October 2021), in the form of an aqueous solution (Notes 1 and 2), together with any other necessary formulants, and must contain an effective emetic (Note 3). The material may also include colorants, olfactory alerting agents and thickeners. It shall contain not more than a trace of suspended matter, immiscible solvents and sediment.
“Note 3. An effective emetic, having the following characteristics, must be incorporated into the SL.
It must be rapidly absorbed (more rapidly than paraquat) and be quick acting. Emesis must occur in about half an hour in at least 50% of cases.
It must be an effective (strong) stimulant of the emetic centre of the brain, to produce effective emesis. The emetic effect should have a limited ‘action period’, of about 2–3 h, to allow effective treatment of poisoning.
It must act centrally on the emetic centre in the brain.
It must not be a gastric irritant because, as paraquat is itself an irritant, this could potentiate the toxicity of paraquat.
It must be toxicologically acceptable. It must have a short half-life in the body (to comply with the need for a limited action period).
It must be compatible with, and stable in, the paraquat formulation and not affect the herbicidal efficacy or occupational use of the product.
“The paraquat TK, SL and SG produced by the manufacturer mentioned in the evaluation reports 56.302/2003, 56.302/2020 and 56.302/2021 contain the emetic 2-amino-4,5-dihydro-6-methyl-4-propyl-s-triazole-(1,5-a)pyrimidin-5-one (PP796). The method for determination of PP796 content in TK and formulated products is provided in Appendix 1.
A previous version was more explicit about the need to use PP796: “To date, the only compound found to meet these requirements is 2-amino-4,5-dihydro-6-methyl-4-propyl-s-triazole-(1,5a)pyrimidin-5-one (PP796). PP796 must be present in the SL at not less than 0.23% of the paraquation content” [Citation20].
Additional information
Funding
References
- Calderbank A. The bipyridylium herbicides. Adv Pest Control Res. 1968;8:127–235.
- World Health Organization. Environmental health criteria # 39. Paraquat and diquat. Geneva: IPCS Inchem, WHO; 1984.
- Brown R, Clapp M, Dyson J, et al. Paraquat in perspective. Outlook Pest Man. 2004;15(6):259–267.
- Lock EA, Wilks MF. Paraquat. In: Krieger R, editor. Hayes’ handbook of pesticide toxicology. 3rd ed. New York: Elsevier; 2010. p. 1771–1827.
- World Health Organization. The WHO recommended classification of pesticides by hazard and guidelines to classification. 2019 ed. Geneva: WHO; 2020.
- Bullivant CM. Accidental poisoning by paraquat: report of two cases in man. Br Med J. 1966;1(5498):1272–1273.
- Campbell S. Death from paraquat in a child. Lancet. 1968;291(7534):144.
- Revkin AC. Paraquat - a potent weedkiller is killing people. Sci Digest. 1983;36–38(42):100–104.
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93(11):715–731.
- Huang J, Xuan D, Li X, et al. The value of APACHE II in predicting mortality after paraquat poisoning in Chinese and Korean population: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(30):e6838.
- Buckley NA, Fahim M, Raubenheimer J, et al. Case fatality of agricultural pesticides after self-poisoning in Sri Lanka: a prospective cohort study. Lancet Glob Health. 2021;9(6):e854–e862.
- World Health Organization. Poison information monograph 399. Paraquat. Geneva: IPCS Inchem, WHO; 1989.
- Dawson AH, Eddleston M, Senarathna L, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7(10):e1000357.
- Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745–757.
- Meredith TJ, Vale JA. Treatment of paraquat poisoning in man: methods to prevent absorption. Hum Toxicol. 1987;6(1):49–55.
- Slade P. Emetic formulation of paraquat: proposed strategy for introduction worldwide. EDC paper no 729. Haslemere, Surrey (UK): ICI Ltd; 1976. p. 391–418.
- Slade P. Paraquat: policy on inclusion of emetic (draft). ICI Agrochemicals; 1983. [cited 2022 Feb 14]. Available from:https://usrtk.org/wp-content/uploads/2021/03/1983.03.05-ICI-Draft-Paraquat-Policy-on-Inclusion-of-Emetic-SYNG-PQ-02451028_R.pdf.
- Syngenta AG. The evolution of medical opinion on the use of emetics. Basel: Syngenta; 2021. [cited 2022 Jan 6]. Available from: https://www.syngenta.com/sites/syngenta/files/docs/the-evolution-of-medical-opinion-on-the-use-of-emetics.pdf
- Food and Agricultural Organization. FAO specifications and evaluations for agricultural pesticides. Paraquat dichloride. Rome: FAO; 1996.
- Food and Agricultural Organization. FAO specifications and evaluations for agricultural pesticides. Paraquat dichloride. Rome: FAO; 2008. [cited 2021 Apr 4]. Available from: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/Paraquat08.pdf.
- Food and Agricultural Organization. FAO specifications and evaluations for agricultural pesticides. Paraquat dichloride. Rome: FAO; 2021. [cited 2022 Jan 6]. Available from: https://www.fao.org/3/ca9629en/ca9629en.pdf.
- Australian Pesticides and Veterinary Medicines Authority. Standard for paraquat dichloride technical concentrate active constituent. Caberra: Australian Government; 2011. [updated 2021; cited 2022 Jan 6]. Available from: https://apvma.gov.au/node/2594
- Food and Agricultural Organization. FAO specifications and evaluations for agricultural pesticides. Diquat dibromide. Rome: FAO; 2008. [cited 2022 Jan 61]. Available from: https://www.fao.org/3/ca9591en/ca9591en.pdf
- Slade P. Letter to Nils Ospenson, Chevron Chemical Company. Haslemere Surrey (UK): ICI Ltd; 1976. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1976.11.03-ICI-to-CCC-re-global-policy-decisions-on-emetic-formulation-CUSA-00088288-at-8290-8291.pdf.
- Bero L. Implications of the tobacco industry documents for public health and policy. Annu Rev Public Health. 2003;24:267–288.
- Collin J, Lee K, Gilmore AB. Unlocking the corporate documents of British American Tobacco: an invaluable global resource needs radically improved access. Lancet. 2004;363(9423):1746–1747.
- Barlow P, Serôdio P, Ruskin G, et al. Science organisations and Coca-Cola’s 'war' with the public health community: insights from an internal industry document. J Epidemiol Community Health. 2018;72(9):761–763.
- Noguchi N, Misawa S, Tsuchiya S, et al. Cardio-respiratory effects of paraquat with and without emetics on Wistar rats. Vet Hum Toxicol. 1985;27(6):508–510.
- Frelon JH, Merigot P, Garnier R[, et al. Prognostic factors in acute paraquat poisoning. A retrospective study of cases registered by the Poison Control Center of Paris in 1981. Toxicol Eur Res. 1983;5(4):163–169.
- Heylings JR, Farnworth MJ, Swain CM, et al. Identification of an alginate-based formulation of paraquat to reduce the exposure of the herbicide following oral ingestion. Toxicology. 2007;241(1–2):1–10.
- Wilks MF, Fernando R, Ariyananda PL, et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 2008;5(2):e49.
- Wilks MF, Tomenson JA, Fernando R, et al. Formulation changes and time trends in outcome following paraquat ingestion in Sri Lanka. Clin Toxicol (Phila). 2011;49(1):21–28.
- Kawai M, Koyama M, Kaneko Y, et al. The effects of administration of an emetic on paraquat toxicity. J J R M. 1983;32(4):887–892.
- Protzel A. Paraquat: toxicokinetics in dogs. Washington (DC): US EPA; 2005. [cited 2022 Jan 71]. Available from: https://archive.epa.gov/pesticides/chemicalsearch/chemical/foia/web/pdf/061601/061601-2005-07-29a.pdf.
- Bateman DN. New formulation of paraquat: a step forward but in the wrong direction. PLoS Med. 2008;5(2):e58.
- Onyon LJ, Volans GN. The epidemiology and prevention of paraquat poisoning. Hum Toxicol. 1987;6(1):19–29.
- Bayliss PFC. A summary of clinical results of the phosphodiesterase inhibitor ICI 63,197 in a variety of disease states. Alderley Park (UK): ICI Pharmaceuticals Division; 1973. [cited 2021 Apr 8]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1973.07.23-ICI-rpt-PH20992-Summary-of-clinical-results-of-PDE-inhibitor-63197-SYNG-PQ-14420786_R.pdf.
- Rose MS. The concentration of PP796 required to produce emesis in experimental animals and an estimation of the emetic dose in man. CTL/R/390(R). Macclesfield: Central Toxicology Laboratory, ICI Agrochemicals; 1976. revised 1977. [cited 2022 Feb 13]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1977.02.xx-ICI-rpt-CTL-R-390R-Concentration-of-PP796-required-to-produce-emesis-SYNG-PQ-00524793.pdf.
- Wiseman RD. An emetic formulation of ‘Gramoxone’. Haslemere (UK): ICI Plant Protection Division; 1976. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1976.10.06-ICI-memo-An-emetic-formulation-of-Gramoxone-SYNG-PQ-02450673_R.pdf.
- Travis KZ. A new analysis of the human emetic dose-response to PP796 based on clinical data for dosing of PP796 only. Jealott’s Hill (UK): Syngenta Ltd; 2019. [cited 2021 Apr 5]. Available from: https://usrtk.org/wp-content/uploads/2021/03/2019.04.12-SYN-Travis-new-dose-response-analysis-of-clinical-data-SYNG-PQ-29299971.pdf.
- HRC. The acute oral toxicity and mode of action of emetic PP796 in cynomolgus monkeys, and its effect upon the oral toxicity of several formulations of paraquat. HRC Report No. IC I 119/78556. Huntingdon (UK): HRC; 1979. [cited 2022 Jan 10]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1979.03.19-ICI-HRC-rpt-Acute-oral-tox-and-MOA-of-PP796-in-monkeys-effect-on-tox-of-PQ-SYNG-PQ-30880010.pdf.
- HRC. The toxicity of orally administered emetic PP796 in cynomolgus monkeys. HRC Report No ICI 171/78627. Huntingdon (UK): HRC; 1978. [cited 2022 Jan 10]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1978.11.08-ICI-HRC-rpt-Toxicity-of-orally-admin-PP796-in-cymologous-monkeys-SYNG-PQ-00527245.pdf
- Robinson M, Brammer A. PP796: emetic study in paraquat treated dogs. CTL/T/2471. Alderley Park (UK): ICI Plc Central Toxicology Laboratory; 1985. [cited 2021 Apr 15]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1995.04.19-ZEN-PQ-EEC-Review-Appendix-A-the-Emetic-PP796-SYNG-PQ-04262278_R-at-2282-2295-1.pdf
- Smith LL. Memo regarding concentration of PP796 in Gramoxone 04 Dec 1985. Ref LLS/SAB/146. Alderley Park (UK): Cheshire Central Toxicology Laboratory, ICI Plc; 1985.
- Heylings JR. Memo: French formulation of paraquat. Macclesfield (UK): ICI CTL; 1990. [cited 2022 Jan 20]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1987.03.30-1991.02.73-ICI-Paraquat-Safer-Formulations-file-SYNG-PQ-03709681_R.pdf
- Heylings JR, Smith LL. Toxicology of multiple emulsion formulations of paraquat. Alderley Park (UK): ICI Central Toxicology Laboratory; 1990. [cited 2021 Apr 15]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1990.02.19-ICI-Heylings-Smith-Toxicology-of-multiple-emulsion-formulations-of-PQ-SYNG-PQ-03709681_R-at-9742-9762.pdf.
- Shaunak R. Safer paraquat - a summary. Yalding (UK): Zeneca Agrochemicals; 1996. [cited 2022 Feb 13]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1996.05.29-Safer-paraquat-A-summary-SYNG-PQ-14416846_R.pdf
- Heylings JR. Memo: formulation additives and gastrointestinal toxicity 01 Jul 1991. Ref GI001/LCM. Alderley Park (UK): ICI Central Toxicology Laboratory; 1991. [cited 2021 Apr 15]. Available from:https://usrtk.org/wp-content/uploads/2021/03/1990.02.19-ICI-Heylings-Smith-Toxicology-of-multiple-emulsion-formulations-of-PQ-SYNG-PQ-03709681_R-at-9742-9762.pdf
- Anon. Summary of the development of Magnoxone. Haslemere, Surrey (UK): Clinical Toxicology Laboratory, ICI Plc; 1993. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1993.11.01-ZEN-Summary-of-development-of-magnoxone-SYNG-PQ-02768710-.pdf
- Elliott B, Clapp M. Gramoxone Inteon and improved safety. Haslemere, Surrey (UK): Syngenta; 2006. [cited 2022 Jan 19]. Available from: https://usrtk.org/wp-content/uploads/2021/03/2006.05.31-SYN-Gramoxone-Inteon-and-improved-safety-SYNG-PQ-22611082-.pdf
- Environmental Protection Agency. 2-amino-4,5-dihydro-6-methyl-4-propyls-triazolo(1,5-alpha)pyrimidin-5-one (PP796); notice of filing a pesticide petition to amend the existing tolerance exemption. Fed Regist. 2005;70:37847–37851.
- Brammer A, Robinson M. PP796: emetic dose response study in dogs. CTL/T/2459. Alderley Park (UK): ICI Plc Central Toxicology Laboratory; 1985. [cited 2021 Apr 15]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1995.04.19-ZEN-PQ-EEC-Review-Appendix-A-the-Emetic-PP796-SYNG-PQ-04262278_R-at-2282-2295-1.pdf.
- Howard JK. Paraquat poisoning in Western Samoa, 1977–78. A preliminary assessment of the effect of PP796; 1978. [cited 2022 06/01]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1978.09.30-ICI-rpt-PQ-poisonings-in-Western-Samoa-1977-78-preliminary-assessment-of-effect-of-PP796-SYNG-PQ-04263349_R.pdf.
- Bismuth C, Garnier R, Dally S, et al. Prognosis and treatment of paraquat poisoning: a review of 28 cases. J Toxicol Clin Toxicol. 1982;19(5):461–474.
- Bramley A, Hart TB. Paraquat poisoning in the United Kingdom (abstract). Human Toxicol. 1983;2:417.
- Denduyts-Whitehead AP, Hart TB, Volans GN. Effects of the addition of an emetic to paraquat formulations on acute poisoning in man (abstract). J Toxicol Clin Toxicol. 1985;23:422–423.
- Johnen BG. Memo: concerning the concentration of the emetic PP796 in liquid paraquat formulations. Haslemere (UK): Zeneca Agrochemicals; 1994. [cited 2022 Jan 21]. Available from https://usrtk.org/wp-content/uploads/2021/03/1995.01.23-ZEN-Memo-re-emetic-concentrations-in-liquid-paraquat-formulations-SYNG-PQ-33966318_R-1.pdf.
- French DA. Paraquat and PP796. Basel: Syngenta; 2019. [cited 2022 Feb 24]. Available from https://usrtk.org/wp-content/uploads/2021/03/2019.05.01-SYN-French-to-Heylings-may-be-draft-SYNG-PQ-25596243-.pdf.
- Bramley A, Hart TB. Paraquat poisoning in the United Kingdom (internal report). Fernhurst (UK): Plant Protection Division, ICI Ltd; 1982. [cited 2021 Apr 5]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1982.06.xx-ICI-rpt-Bramley-Hart-PQ-poisonings-in-the-UK-SYNG-PQ-03720006_R.pdf.
- Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359(9302):248–252.
- Imperial Chemical Industries PLC. ‘Gramoxone’ poisoning in the UK 1980–1988 and the role of the emetic. Fernhurst (UK): ICI; 1992. [cited 2021 Apr 5]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1992.10.31-ICI-rpt-Gramoxone-poisoning-in-the-UK-1980-1988-and-role-of-emetic-CHEV-SJ0014644-.pdf.
- Moon JM, Chun BJ. Acute intoxication with the adjuvant itself for gramoxone INTEON. Hum Exp Toxicol. 2012;31(1):18–23.
- Ko DR, Chung SP, You JS, et al. Effects of paraquat ban on herbicide poisoning-related mortality. Yonsei Med J. 2017;58(4):859–866.
- European Medicines Agency. Statistical principles for clinical trials. CPMP/ICH/363/96. London: EMA; 1998. [cited 2022 Feb 14]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-9-statistical-principles-clinical-trials-step-5_en.pdf.
- DeMets DL. Statistical issues in interpreting clinical trials. J Intern Med. 2004;255(5):529–537.
- European Medicines Agency. ICH Topic E 4. Dose response information to support drug registration. CPMP/ICH/378/95. London: EMA; 1994. [cited 2022 Feb 14]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-4-dose-response-information-support-drug-registration-step-5_en.pdf.
- Hart TB. Memo: the addition of emetic (PP796) to paraquat formulations. Haslemere (UK): ICI Plant Protection Division; 1984. p. 370–381. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1995.04.19-ZEN-PQ-EEC-Review-Appendix-A-the-Emetic-PP796-SYNG-PQ-04262278_R-at-2282-2295-1.pdf
- Paraquat Strategic Action Committee. Memo: increased emetic content for paraquat formulations. ICI Agrochemicals; 1987. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1987.12.31-ICI-Memo-re-CTL-recommendation-Increased-Emetic-Content-for-PQ-Formulations-SYNG-PQ-02451232_R.pdf.
- Heylings JR. Human data with the paraquat emetic (PP796). Macclesfield (UK): ICI Central Toxicology Laboratory; 1990. p. 345–346. [cited 2022 Feb 14]. Available from https://usrtk.org/wp-content/uploads/2021/03/1976.10.xx-ICI-rpt-Emetic-formulation-proposed-strategy-for-intro-worldwide-SYNG-PQ-04262278_R-at-2668-2695.pdf
- Anon. Draft discussion - Jon Heylings. Unstated: Syngenta; 2019. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/2019.04.09-SYN-Draft-dossier-re-Heylings-SYNG-PQ-23675404-.pdf
- Schein PS, Davis RD, Carter S, et al. The evaluation of anticancer drugs in dogs and monkeys for the prediction of qualitative toxicities in man. Clin Pharmacol Ther. 1970;11(1):3–40.
- Holmes AM, Rudd JA, Tattersall FD, et al. Opportunities for the replacement of animals in the study of nausea and vomiting. Br J Pharmacol. 2009;157(6):865–880.
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–380.
- Daly MJ, Humphray JM, Stables R. Inhibition of gastric acid secretion in the dog by the H2-receptor antagonists, ranitidine, cimetidine, and metiamide. Gut. 1980;21(5):408–412.
- Anon. Paraquat alternative formulations. Haslemere, Surrey (UK): ICI; 1987. [cited 2022 Jun 24]. Available from: https://www.documentcloud.org/documents/20521590-1987-memo-on-paraquat-alternative-formulations
- Hawton K, Cole D, O’Grady J, et al. Motivational aspects of deliberate self-poisoning in adolescents. Br J Psychiatry. 1982;141:286–291.
- Hawton K, van Heeringen K, editors. The international handbook of suicide and attempted suicide. Chichester (UK): John Wiley & Sons; 2002.
- Hawton K. Suicide and attempted suicide among children and adolescents. 0 ed. Newbury Park (CA): Sage Publications, Inc; 1986.
- Carroll R, Metcalfe C, Gunnell D. Hospital presenting self-harm and risk of fatal and non-fatal repetition: systematic review and meta-analysis. PLoS One. 2014;9(2):e89944.
- Eddleston M, Gunnell D, Karunaratne A, et al. Epidemiology of intentional self-poisoning in rural Sri Lanka. Br J Psychiatry. 2005;187:583–584.
- Syngenta AG. Paraquat in the media. Addition of the emetic and its level – Jon Heylings’ allegations. Basel: Syngenta; 2021. [cited 2022 May 26]. Available from: https://www.syngenta.com/en/paraquat-in-the-media.
- Gupta S, Ahlawat SK. Aluminium phosphide poisoning - a review. J Toxicol Clin Toxicol. 1995;33(1):19–24.
- Singh S, Singh D, Wig N, et al. Aluminium phosphide ingestion - a clinicopathological study. J Toxicol Clin Toxicol. 1996;34(6):703–706.
- Hart TB. Letter to Dr HAFM Custers. Haslemere (UK): ICI Plant Protection Division; 1982. [cited 2022 Feb 14]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1982.06.07-ICI-Hart-to-Custers-at-BV-Luxan-Netherlands-comments-on-report-Paraquat-and-the-Addition-of-an-Emetic-SYNG-PQ-03719883_R.pdf.
- Hoegberg LCG, Shepherd G, Wood DM, et al. Systematic review on the use of activated charcoal for gastrointestinal decontamination following acute oral overdose. Clin Toxicol (Phila). 2021;59(12):1196–1227.
- Chen HH, Lin JL, Huang WH, et al. Spectrum of corrosive esophageal injury after intentional paraquat or glyphosate-surfactant herbicide ingestion. Int J Gen Med. 2013;6:677–683.
- Ackrill P, Hasleton PS, Ralston AJ. Oesophageal perforation due to paraquat. Br Med J. 1978;1(6122):1252–1253.
- Lee SY, Shin, JH, Lee WG. Paraquat poisoning. J Korean Pediatr Soc. 1987;30:891–900.
- James N, Bakshi R, Rudresh SS, et al. Pneumoperitoneum from pneumomediastinum in paraquat poisoning. Trop Doct. 2021;51(2):241–242.
- Chan KW, Cheong IK. Paraquat poisoning: a clinical and epidemiological review of 30 cases. Med J Malaysia. 1982;37(3):227–230.
- Food and Agricultural Organization of the United Nations WHO. Manual on development and use of FAO and WHO specifications for pesticides. 1 ed–3rd revision ed. Rome: FAO; 2016.